Published online Oct 7, 2009. doi: 10.3748/wjg.15.4617
Revised: September 8, 2009
Accepted: September 15, 2009
Published online: October 7, 2009
Iron is an essential micronutrient, as it is required for adequate erythropoietic function, oxidative metabolism and cellular immune responses. Although the absorption of dietary iron (1-2 mg/d) is regulated tightly, it is just balanced with losses. Therefore, internal turnover of iron is essential to meet the requirements for erythropoiesis (20-30 mg/d). Increased iron requirements, limited external supply, and increased blood loss may lead to iron deficiency (ID) and iron-deficiency anemia. Hepcidin, which is made primarily in hepatocytes in response to liver iron levels, inflammation, hypoxia and anemia, is the main iron regulatory hormone. Once secreted into the circulation, hepcidin binds ferroportin on enterocytes and macrophages, which triggers its internalization and lysosomal degradation. Thus, in chronic inflammation, the excess of hepcidin decreases iron absorption and prevents iron recycling, which results in hypoferremia and iron-restricted erythropoiesis, despite normal iron stores (functional ID), and anemia of chronic disease (ACD), which can evolve to ACD plus true ID (ACD + ID). In contrast, low hepcidin expression may lead to iron overload, and vice versa. Laboratory tests provide evidence of iron depletion in the body, or reflect iron-deficient red cell production. The appropriate combination of these laboratory tests help to establish a correct diagnosis of ID status and anemia.
- Citation: Muñoz M, Villar I, García-Erce JA. An update on iron physiology. World J Gastroenterol 2009; 15(37): 4617-4626
- URL: https://www.wjgnet.com/1007-9327/full/v15/i37/4617.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4617
| Protein name (alternative name) | Acronyms | Function | Localization |
| Divalent metal transporter 1 (divalent cation transporter 1, NRAMP 1 and 2) | DMT1 | Traffics divalent metal ions such as iron, zinc, copper and cobalt across the membrane by a proton coupled mechanism | Enterocyte (apical membrane) |
| DCT1 | Erythroblast (siderosome) | ||
| NRAMP1* | Macrophage (plasma membrane, phagocytic vesicles*) | ||
| NRAMP2 | |||
| Hepatocyte | |||
| Kidney cells | |||
| Ferrireductase | Dcytb | Reduction of Fe3+ to Fe2+ | Enterocyte (apical membrane) |
| STEAP3* | Erythroblast (siderosome*) | ||
| Heme carrier protein 1 | HCP1 | Putative transporter that traffics heme across the membrane by an unknown mechanism | Enterocyte (apical membrane) |
| Hepatocyte | |||
| Kidney cells | |||
| Heme oxygenase | An enzyme that disassembles heme to liberate iron | Enterocyte (microsomal fraction) | |
| Macrophages | |||
| Ferroportin 1 (Iron regulatory protein 1) | FPN 1 | Transmembrane Fe2+ transporter (exporter) | Enterocyte (basolateral membrane) |
| Ireg1 | Macrophages | ||
| MTP1 | Hepatocytes | ||
| Hephaestin | Hp | Membrane-bound multicopper ferroxidase, similar to plasma ceruloplasmin, which oxidizes Fe2+ to Fe3+ to load it onto transferrin | Enterocyte (basolateral) |
| Maintenance of cell-surface localization of ferroportin | Macrophage | ||
| Hepatocyte? | |||
| Transferrin | Tf | Plasma Fe3+ binding protein | Plasma |
| Ligand for transferrin receptors 1 and 2 | |||
| Transferrin receptor 1 | TfR1 | Cellular uptake of transferrin bound iron | Ubiquitously expressed |
| Transferrin receptor 2 | TfR2 | Sensor for diferric transferrin; regulates hepcidin expression; may participate in a signaling complex with HFE | Enterocyte |
| Hepatocyte | |||
| Erythroblast | |||
| Mitoferrin | SLC25A37 | Mitochondrial iron importer that plays a critical role in supplying iron to ferrochelatase for insertion in protoporphyrin IX to form heme | Erythroblast (mitochondria) |
| Ferritin | Ft | Iron storage protein (H and L chains) | Enterocyte |
| Ferroxidase activity (H chain) | Erythroblast | ||
| Macrophage | |||
| Hepatocyte | |||
| Myocytes and cardiomyocytes | |||
| Hemosiderin | Iron storage protein; breakdown product of ferritin that occurs when iron levels are high | Macrophage (lysosomes) | |
| Hepatocytes (lysosomes) | |||
| Heme exporters | LFLVCR* | ATP-independent heme export at the cell membrane* ATP-dependent heme export at the cell membrane** and mitochondrial membrane*** | Erythroblast* |
| Bcrp/Abcg2** | Ubiquitously expressed**,*** | ||
| Abcb6*** | |||
| HFE | HFE | Regulates hepcidin expression, mechanism uncertain; may participate in a signaling complex with TfR2; interacts with TfR1 & β-2-microglobulin | Enterocyte |
| Macrophage | |||
| Hepatocyte | |||
| Hemojuvelin | HFE2 | Acts as a BMP co-receptor to stimulate hepcidin transcription | Hepatocytes |
| Lipocalin 2 | Mediates a siderophore-like iron uptake pathway. Its an innate immune response to bacterial infection by sequestrating iron, but its physiologic role in iron absorption is not fully worked out | Enterocytes (apical membrane) | |
| Macrophages | |||
| Adipocyte | |||
| Hepcidin | HEP | Iron regulatory hormones, binds ferroportin to cause its internalization and degradation | Hepatocytes |
| HAMP | Adipocytes (low secretion) | ||
| LEAP1 | Enterocytes? | ||
| Erythropoietin | EPO | Upregulates the expression ferroportin in macrophages, TfR1 in erythroblasts, and DMT1 and hephaestin in enterocytes | Kidney (interstitial peritubular cells) |
| Downregulates hepcidin expression in hepatocytes and DMT1 expression in macrophages | Hepatocytes (low secretion) |
| Laboratory test | Normal values, units | Conversion to SI units |
| Iron depletion in the body | ||
| Serum iron | 50-180 μg/dL | × 0.179 μmol/L |
| Transferrin | 200-360 mg/dL | × 0.01 g/L |
| TSAT | 20%-50% | |
| Ferritin | 30-300 ng/mL | × 2.247 pmol/L |
| sTfR | 0.76-1.76 mg/L | 6.4-25.7 nmol/L |
| Ratio of sTfR to serum ferritin (sTfR/log ferritin) | < 1 | |
| Iron deficient red cell production | ||
| Hemoglobin | 12-16 g/dL (female) | × 0.6206 mmol/L |
| 13-17 g/dL (male) | ||
| MCV | 80-100 fL | |
| RDW | 11-15 | |
| MCH | 28-35 pg | |
| HYPO | < 5% | |
| CHr | 28-35 pg |
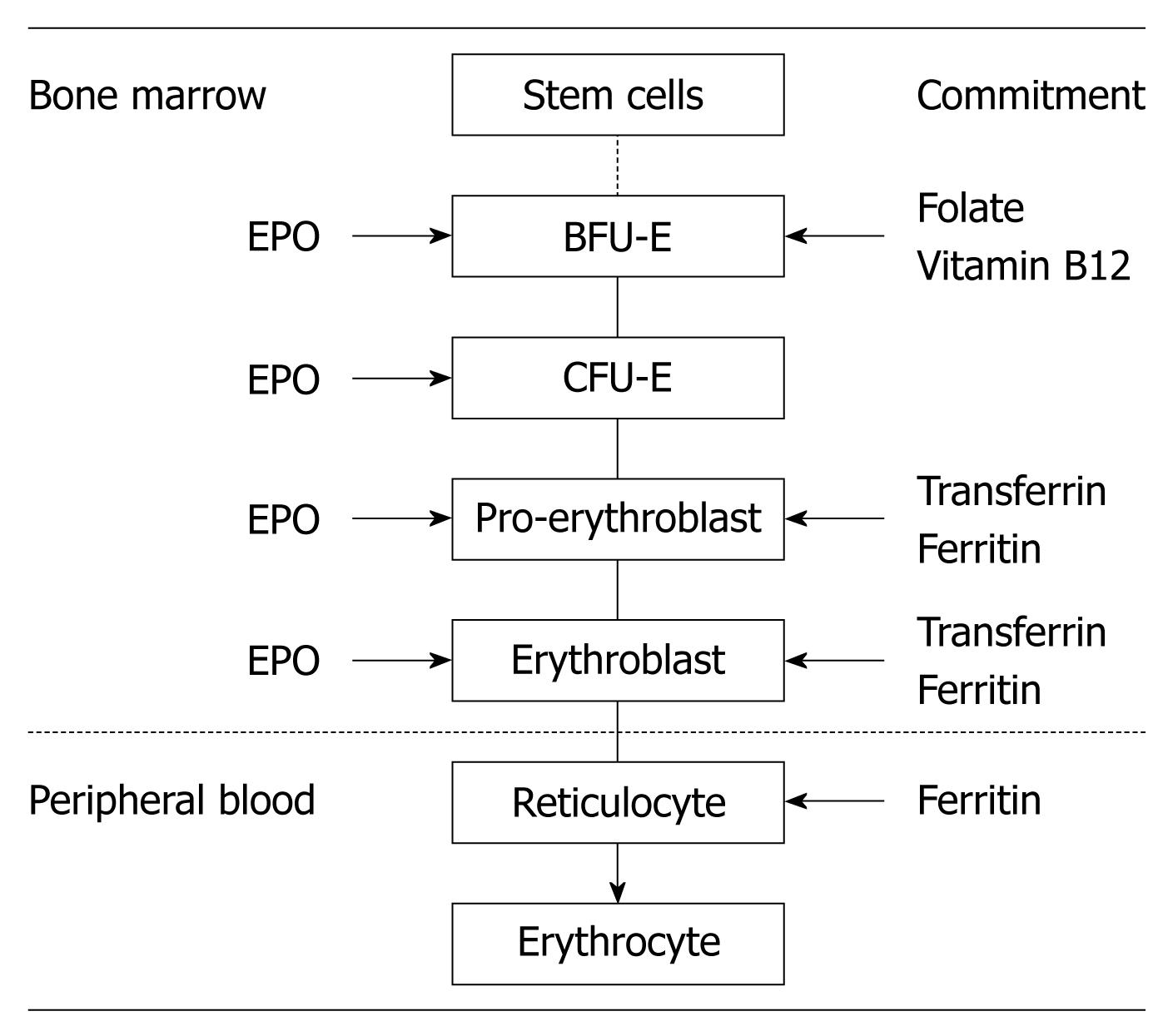
Erythropoiesis is a part of the larger process of hematopoiesis. In the normal adult human, the daily turnover of red blood cells (RBCs) exceeds 1011 cells. In periods of increased RBC loss caused by hemolysis or hemorrhage, the production of RBCs increases rapidly and markedly. However, an overproduction of RBCs (i.e. rebound polycythemia) does not occur even after the most severe loss of RBCs. Thus, erythropoiesis is a finely regulated yet rapidly responsive process that maintains the normal number of circulating RBCs within a narrow range[1].
The major stages of differentiation in human erythropoiesis are depicted in Figure 1. The commitment of multipotent hemopoietic stem cells to erythroid progenitors is driven by several growth factors, such as stem cell factor, thrombopoietin, and interleukin (IL)-3[2]. The most immature stage of committed erythroid progenitors is the burst-forming unit-erythroid, which differentiates into colony-forming unit-erythroid (CFU-E) in approximately 7 d, with declining proliferative potential as the progenitors approach CFU-Es. Each CFU-E develops a single cluster of 8-64 mature erythroblasts within 7 d, after several differentiation stages (pro-erythroblast, basophilic erythroblast, polychromatic erythroblast, and orthochromatic erythroblast). Orthochromatic erythroblasts do not divide but they enucleate, and form nascent RBCs, called reticulocytes, which are release into the bloodstream. After 1 d of circulation in the peripheral blood, reticulocytes mature into RBCs[3]. The normal proliferation and differentiation of erythroid progenitor cells require several essential nutrients, such as iron, folate, and vitamin B12, the interaction with the stromal cells in the bone marrow, and stimulation by erythropoietin (EPO)[3].
For a 70-kg male individual, total body iron is about 3.5 g (50 mg/kg). Most of the iron in the body is distributed within RBC hemoglobin (65%; 2300). Approximately 10% is present in muscle fibers (in myoglobin) and other tissues (in enzymes and cytochromes) (350 mg). The remaining body iron is stored in the liver (200 mg), macrophages of the reticuloendothelial system (RES; 500 mg), and bone marrow (150 mg). In premenopausal women, total body iron (especially the stored fraction, 250-300 mg) is lower than in men. The normal diet contains 15-20 mg of iron, and the body absorbs 1-2 mg/d of dietary iron. This is balanced with losses via sloughed intestinal mucosal cells, menstruation and other blood losses. Therefore, internal turnover of iron is essential to meet the bone marrow requirements for erythropoiesis (20-30 mg/d)[3-5].
On the other hand, the body has no effective means of excreting iron and thus the regulation of absorption of dietary iron from the duodenum plays a critical role in iron homeostasis in the body[5]. This is extremely important as iron is essential for cellular metabolism and aerobic respiration, whilst cellular iron overload leads to toxicity and cell death via free radical formation and lipid peroxidation, thus, iron homeostasis requires tight regulation[3,4,6]. A summary of proteins involved in iron homeostasis, as well as their most frequently used acronyms, is given in Table 1.
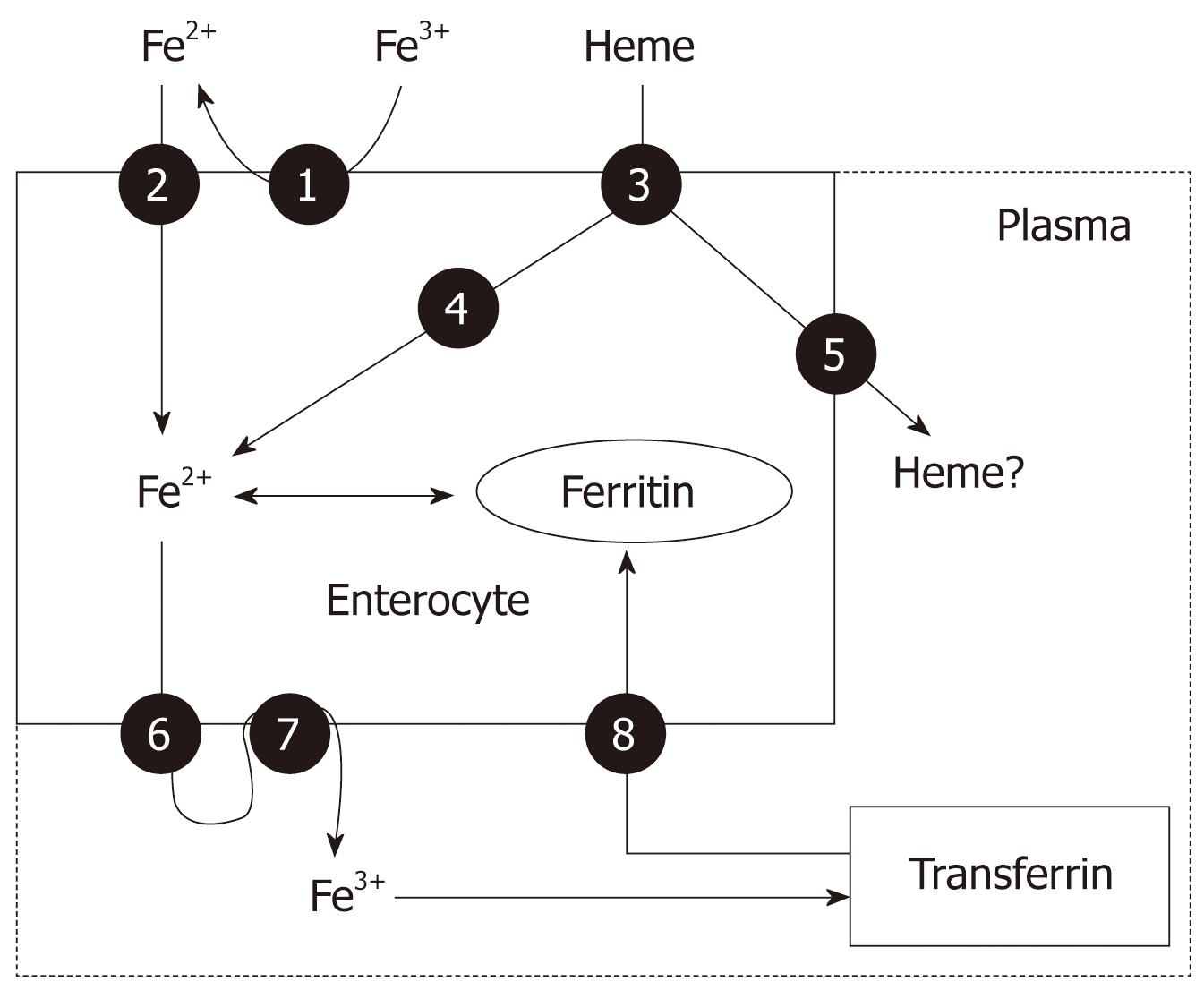
Nearly all absorption of dietary iron occurs in the duodenum. Several steps are involved, including the reduction of iron to a ferrous state, apical uptake, intracellular storage or transcellular trafficking, and basolateral release (Figure 2). Dietary iron is found in heme (10%) and non-heme (ionic, 90%) forms and their absorption occurs at the apical surface of duodenal enterocytes via different mechanisms. Dietary non-heme iron primarily exists in an oxidized (Fe3+) form that is not bioavailable, and must first be reduced to the Fe2+ form by a ferrireductase enzyme, before it is transported across the intestinal epithelium by a transporter called divalent metal transporter 1 (DMT-1), which also transports other metal ions such as zinc, copper and cobalt by a proton-coupled mechanism[3,4]. There is also a siderophore-like iron uptake pathway mediated by lipocalin-2 (that seems to exert an innate immune response to bacterial infection by sequestrating iron) but its physiological role is not fully worked out.
The absorption of non-heme can be diminished by co-administration of tetracyclines, proton pump inhibitors and antacid medication, phytates (high-fiber diets), calcium, and phenolic compounds (coffee and tea). In addition, infection with Helicobacter pylori (H pylori) produces gastric atrophy that, even in the absence of significant bleeding, can lead to profound iron-deficiency anemia (IDA). As expected, this anemia is poorly responsive to oral iron therapy, but can be corrected by eradication of H pylori infection[7].
Heme iron is absorbed into enterocytes by a putative, not totally identified heme carrier protein 1, which is a membrane protein found in the proximal intestine, where heme absorption is greatest[8]. Once internalized in the enterocytes, it is likely that most dietary heme iron is released as ferrous iron by heme oxygenase to enter a common pathway with dietary non-heme iron before it leaves the enterocytes[3,4] (Figure 2). However, it remains uncertain whether some heme might traverse the cells intact, leaving the enterocytes through the action of the recently characterized heme exporters, Bcrp/Abcg2 and feline leukemia virus C receptor (FLVCR)[8]. If this does occur, the subsequent disposition of plasma heme is unknown. In addition, it is not yet known whether heme carrier protein 1 has physiological roles in tissues other than the intestine. The protein is also expressed in the kidneys and liver, which suggests that it may act at those sites. It might, for example, scavenge free heme or mediate cellular uptake of heme from its circulating carrier protein, hemopexin[9].
Once inside the intestinal epithelial cell, iron may either remains in the cell for use or storage (this iron is never absorbed into the body; rather, it is lost when enterocytes senesce and are sloughed into the gut lumen) or exported across the basolateral membrane of the enterocyte into the circulation (absorbed iron). Ferroportin 1 is the only putative iron exporter identified to date. Ferrous iron once exported across the basal membrane by ferroportin 1, is then oxidized by a multi-copper oxidase protein called hephaestin (an enzymatic protein similar to plasma ceruloplasmin) before being bound by plasma transferrin. Ferroportin 1 is also the putative iron exporter in macrophages and hepatocytes (Figure 2)[3,4].
The absorption of iron is dependent on the body’s iron stores, hypoxia and rate of erythropoiesis. Two models have been proposed to explain how the absorption of iron is regulated: the crypt programming model and the hepcidin model.
The crypt programming model: This model proposes that enterocytes in the crypts of the duodenum take up iron from the plasma. The intracellular iron level of the crypt cells corresponds to the body’s iron stores, which in turn determines the amount of iron absorbed from the gut lumen, as these crypt cells migrate upwards to become absorptive cells at the brush border. The crypt cells express both transferrin receptor 1 (TfR1) and TfR2, which mediate the cellular uptake of transferrin-bound iron from plasma[3,5].
TfR1 is expressed ubiquitously and transferrin mediated iron uptake is thought to occur in most cell types. HFE, an MHC-class 1-like molecule that interacts with β2-microglobulin and forms a complex with TfR1, is highly expressed in crypt cells. Its role in the regulation of TfR1-mediated transferrin-bound iron uptake remains unclear, but it seems to enhance transferrin-bound iron uptake from the plasma into crypt cells via TfR1, and may also inhibit the release of iron from the cell via ferroportin 1. In contrast, TfR2 is restricted to hepatocytes, duodenal crypt cells and erythroid cells, which suggests a more specialized role in iron metabolism. The intracellular iron concentration controls the interaction of cytosolic iron regulatory proteins (IRPs) 1 and 2 with iron regulatory elements (IREs; which act as iron sensors in mammalian cells and regulate translation or stability of mRNA-encoding proteins) in the 3’ and 5’ regions of different mRNA molecules. In the absence of iron, IRP1 binds to IREs of TfR1, DMT-1, and ferroportin 1 mRNA, the transcript is stabilized, translation proceeds, and the proteins are synthesized. Thus, a high IRP binding activity reflects low body iron stores and results in upregulation of these proteins in the duodenum and increased dietary iron absorption. When IRPs bind to IRE of ferritin mRNA, translation of the transcript is blocked and synthesis is halted. Thus, ferritin levels are regulated reciprocally - being increased in iron-replete states and decreased in iron-deplete states[3].
The hepcidin model: Liver hepcidin is a 25-amino-acid cysteine-rich peptide with antimicrobial properties, which is regulated by a number of factors such as liver iron levels, inflammation, hypoxia and anemia. The hepcidin model proposes that hepcidin is secreted into the blood and interacts with villous enterocytes to regulate the rate of iron absorption, by controlling the expression of ferroportin 1 at their basolateral membranes. The binding of hepcidin to ferroportin 1 results in internalization of ferroportin 1 and loss of its function. Ferroportin 1 molecules present in macrophages and liver are also targets for hepcidin. Thus, it is hypothesized that when hepcidin levels are increased in iron overload (by the uptake of transferrin bound iron via TfR1/HFE and TfR2) or inflammation (via IL-6), iron release from intestinal crypt cells, liver and macrophages is reduced. In contrast, when hepcidin levels are reduced, as in iron deficiency (ID), anemia or hypoxia, it is likely that ferroportin 1 expression and iron release from intestinal cells, liver and cells of reticuloendothelial system is increased[10]. In contrast, a mutation in the ferroportin 1 gene is responsible for type IV hemochromatosis.
There is evidence to support both models and it is possible that both control mechanisms may contribute to the regulation of iron absorption. In this regard, there is emerging evidence that hepcidin may act directly on mature villous enterocytes rather than crypt enterocytes. There are several situations (e.g. acute phase response) when iron absorption can be modulated more rapidly (within hours) than can be accounted for via the mechanism that involves the programming and maturation of crypt enterocytes (lag time of days)[5].
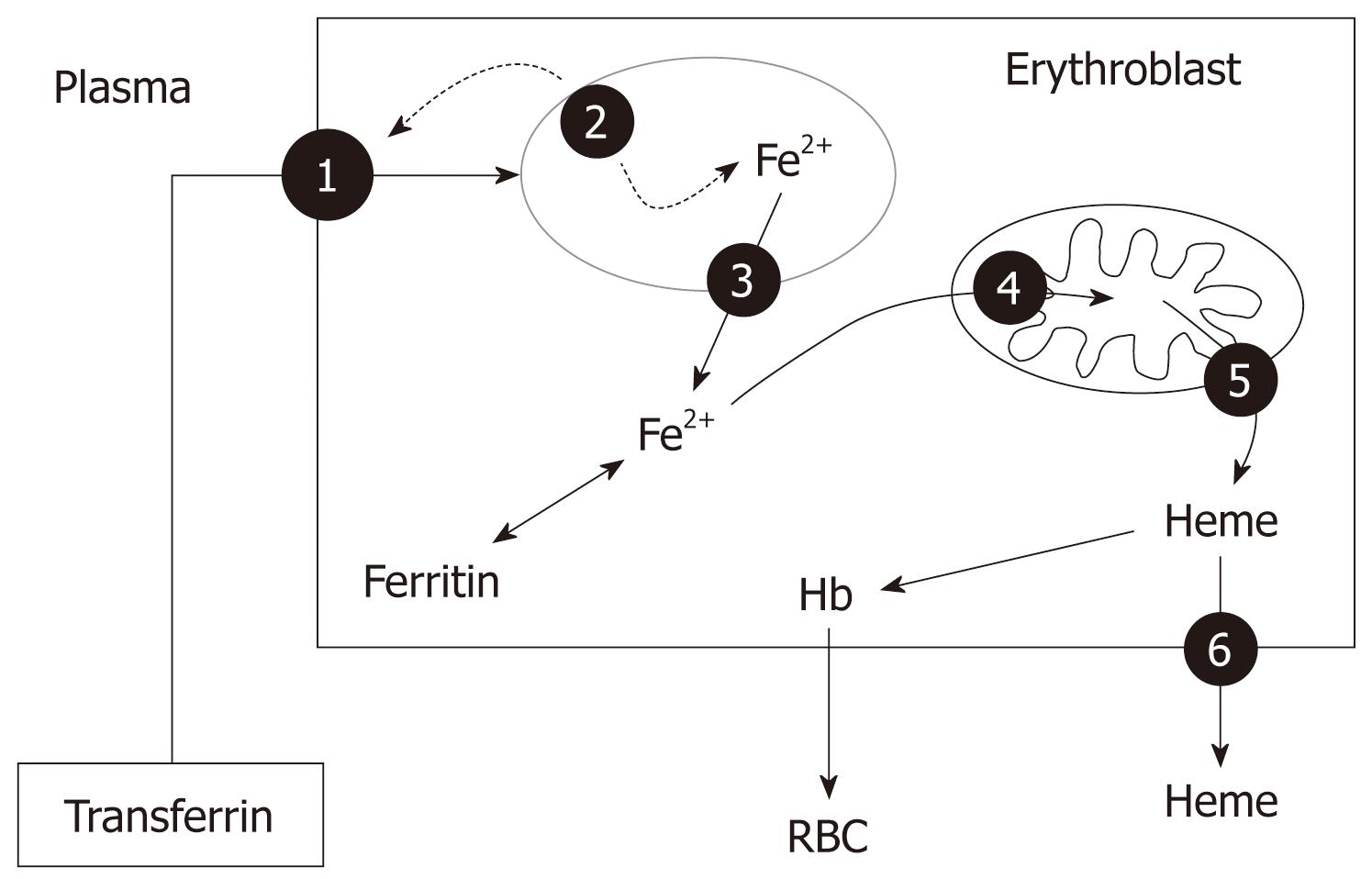
Iron released into the circulation binds to transferrin and is transported to sites of use and storage. Transferrin has two binding sites, binding one iron atom each (thus three forms can be found in plasma: apo-transferrin which contains no iron, monoferric-transferrin and diferric-transferrin). About 30%-40% of these sites are occupied under normal physiological conditions. Thus, transferrin-bound iron is about 4 mg, but this is the most import dynamic iron pool[11]. Transferrin-bound iron enters target cells - mainly erythroid cells, but also immune and hepatic cells- through a process of receptor-mediated endocytosis (Figure 3). As diferric-transferrin has a much higher affinity for TfR than does monoferric-transferrin, it binds to the TfR at the plasma membrane, and patches of cell-surface membrane that carry receptor-ligand complexes invaginate to form clathrin-coated endosomes (siderosomes)[11]. After clathrin is removed, the siderosomes become acidified through an ATP-dependent proton influx, which leads to conformational changes in transferrin and TFR1, and promotes iron release of Fe3+ from transferrin. Fe3+ is then reduced to Fe2+ by a ferrireductase and transported to the cytoplasm through the DMT-1, whereas the TfR is recycled to the cell membrane and transferrin shed back to the circulation[3,12] (Figure 3). Production of hemoglobin by the erythron accounts for most iron use. High-level expression of TfR1 in erythroid precursors ensures the uptake of iron into this compartment. To make heme, iron must again cross an ion-impermeable membrane to enter the mitochondria. The mitochondrial iron importer was recently identified as mitoferrin (also known as SLC25A37), a transmembrane protein that plays a crucial role in supplying iron to ferrochelatase for insertion into protoporphyrin IX to form heme[12] (Figure 3). Recently, different human heme exporters have been identified in erythroblasts, and their activity seems to be essential for erythropoiesis, by transferring heme from the mitochondria to citosol (Abcb6) and removing the excess of heme from the erythroid cells (FLVCR, Bcrp/Abcg2) (Figure 3).
In the erythroid precursors, the expression of TfR1, DMT-1 and ferritin are regulated reciprocally through IRP1 and IRP2, which act on the IRE present in their RNA. Thus, when increased iron uptake is needed, the expression of TfR1 and DMT-1 is increased, whereas the synthesis of ferritin is halted[3]. In addition, there is evidence that EPO activates IRP-1, leading to upregulation of TfR1 expression in the erythroid precursors, which is maintained along with the differentiation process, and DMT-1 and hephaestin gene expression in the duodenum[13]. To date, three patients have been reported with DMT-1 mutations that cause microcytic hypochromic anemia, as a result of decreased erythroid iron utilization, but lead to increased liver iron storage[14].
A truncated form of the TfR can be detected in human serum. The serum concentration of this soluble form of TfR (sTfR; normal median concentration: 1.2-3.0 mg/L, depending on the assessment kit used) is proportional to the total amount of surface TfR. Increased sTfR concentrations indicate ID even during the anemia of chronic disease (ACD), as well as increased erythropoietic activity without ID, whereas lower sTfR concentrations may reflect decreased numbers of erythroid progenitors[3,15].
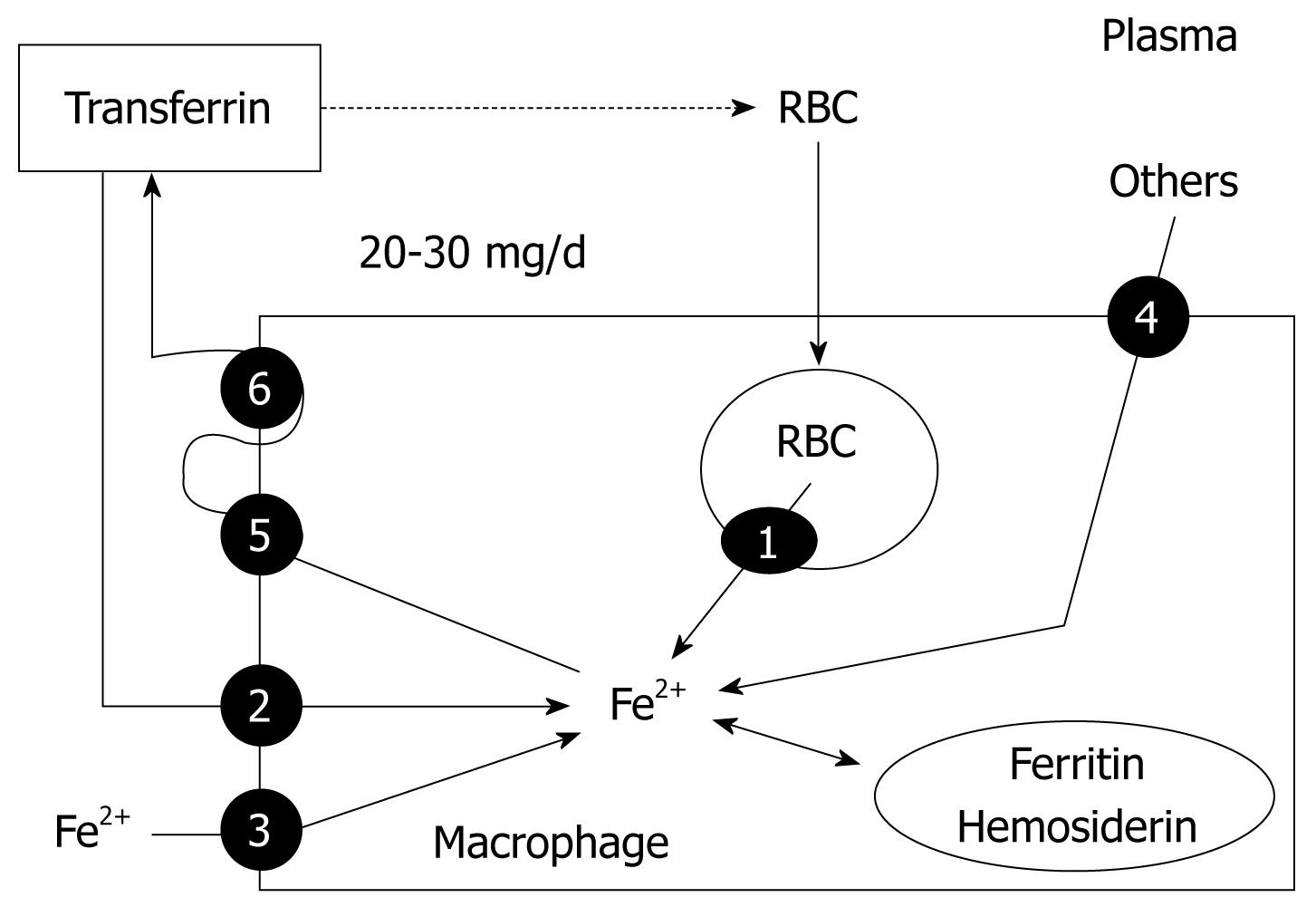
Hemoglobin iron has substantial turnover, as senescent erythrocytes undergo phagocytosis by RES macrophages. Within the phagocytic vesicles, heme is metabolized by heme oxygenase and the released iron is exported to the cytoplasm through the action of natural resistance-associated macrophage protein-1, a transport protein similar to DMT-1 (Figure 4). Macrophages can also obtain iron from bacteria and apoptotic cells, from plasma through the action of DMT-1 and TfR1, and from other sources (Figure 4). Within the cell, iron can be stored in two forms: in the cytosol as ferritin and, after breakdown of ferritin within the lysosomes, as hemosiderin. Hemosiderin represents a very small fraction of normal body iron stores, mostly in macrophages, but increases dramatically in iron overload[11]. Iron export from macrophages to transferrin is accomplished primarily by ferroportin 1, the same iron-export protein expressed in duodenal enterocytes, and hephaestin[3] (Figure 4). The amount of iron required for daily production of 300 billion RBCs (20-30 mg) is provided mostly by recycling iron by macrophages[4]. Importantly, iron storage at the macrophages is safe, as it does not lead to oxidative damage. EPO reduces iron retention in macrophages by decreasing DMT-1 and increasing ferroportin 1 expression[16].
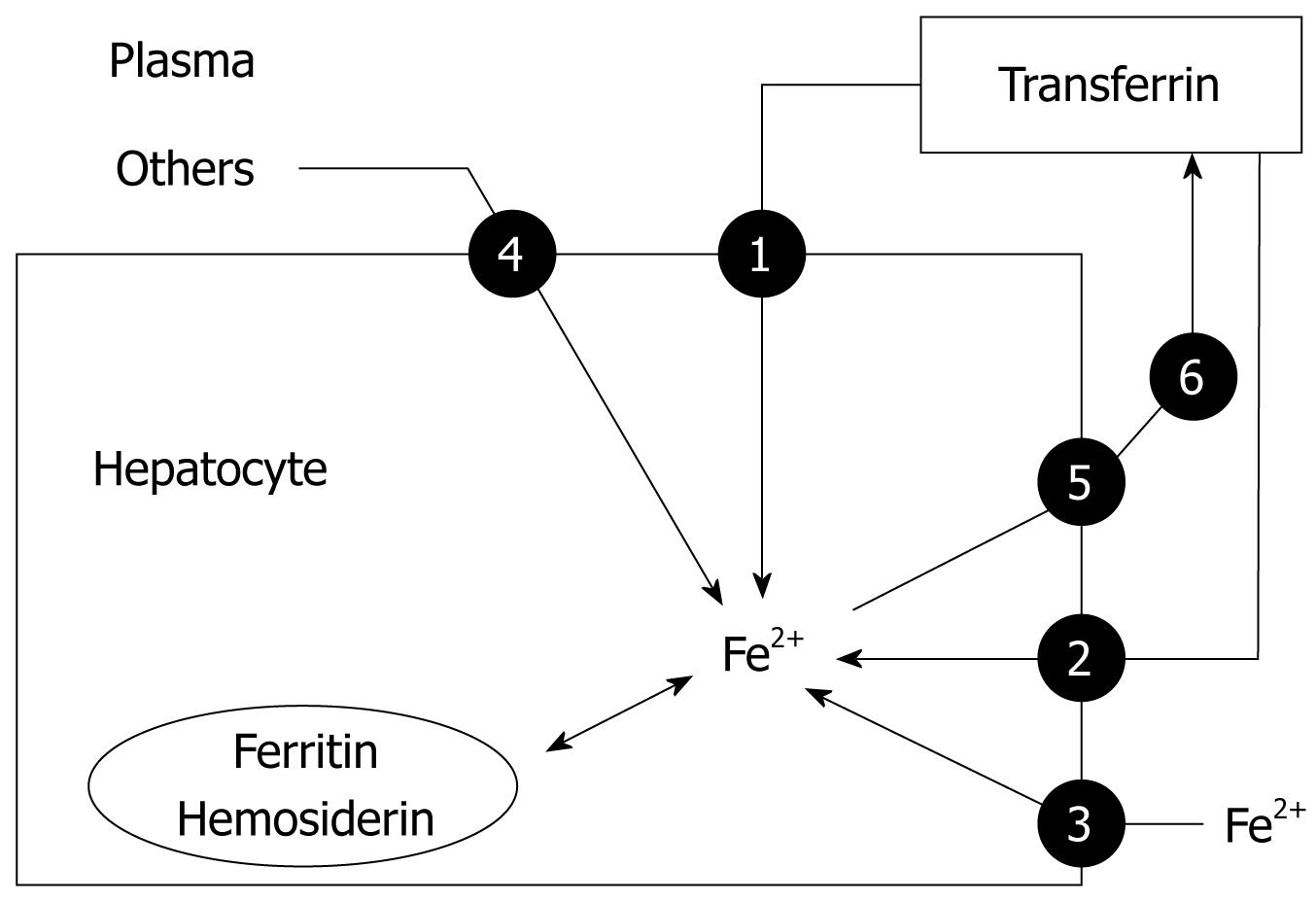
The liver is the other main storage organ for iron. In iron overload, free radical formation and generation of lipid peroxidation products may result in progressive tissue injury and eventually cirrhosis or hepatocellular carcinoma[17]. Iron is sequestrated in hepatocytes predominantly in the form of ferritin or hemosiderin. The uptake of transferrin-bound iron by the liver from plasma is mediated by TfR1 and TfR2 (Figure 5). In iron overload, TfR1 is downregulated in hepatocytes[5]. TfR2 is expressed highly in human liver and is likely to play an important role in liver iron loading in iron overload states. Unlike TfR1, TfR2 lacks an IRE and thus is not regulated reciprocally in response to the level of plasma iron. Instead, TfR2 protein expression is regulated by transferrin saturation (TSAT), and is upregulated in iron overload. In normal and iron-loaded conditions, expression of TfR2 exceeds that of TfR1, which suggests that TfR2 plays an important role in hepatic iron loading in hemochromatosis[5]. In fact, a mutation in TfR2 is responsible for type 3 hemochromatosis[14].
As transferrin becomes saturated in iron overload states, excess iron is also found as non-transferrin-bound iron is transported across the hepatocyte membrane via a carrier-mediated process consistent with DMT-1. The hepatocytes may also store iron from ferritin, hemoglobin-haptoglobin complexes, and heme-hemopexin complexes. In contrast, once again, ferroportin 1 is likely to be the only protein that mediates the transport of iron out of hepatocytes, which is then oxidized by ceruloplasmin and bound to transferrin[3,4] (Figure 5).
Iron storage within cardiomyocytes is also of outstanding interest, as cardiac failure is the leading cause of death among patients with untreated hereditary hemochromatosis or transfusion-associated hemosiderosis[3]. In cardiac cells, excess iron may result in oxidative stress and alteration of myocardial function because of DNA damage by hydrogen peroxide through the Fenton reaction[3].
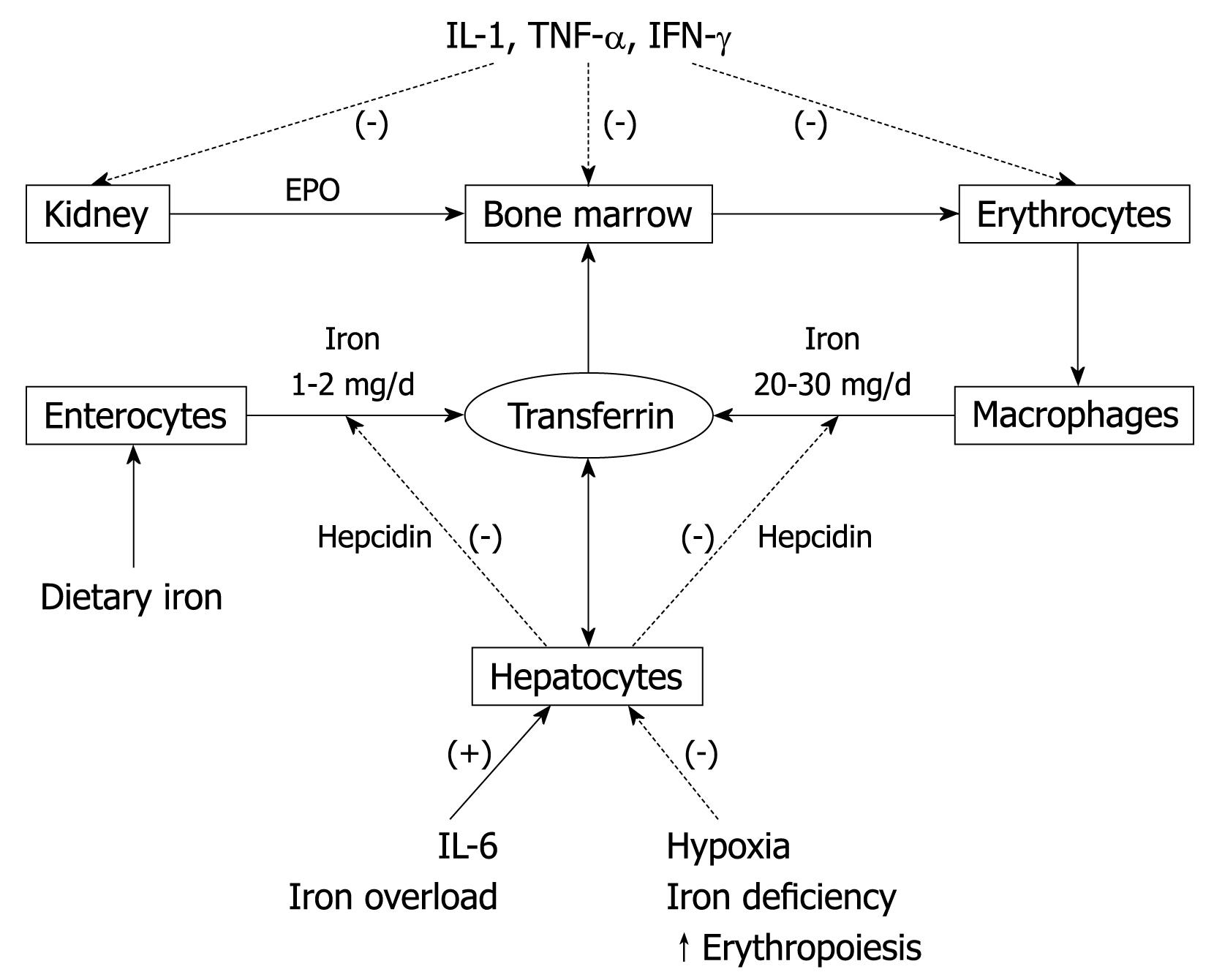
Anemia is a frequent complication of chronic inflammatory diseases (e.g. cancer, rheumatoid arthritis, inflammatory bowel diseases, and congestive heart failure), as well as sepsis and chronic renal failure. In addition to blood loss, hemolysis, hepatic or endocrine disorders, nutritional deficiencies, bone marrow infiltration (cancer cells), or vitamin consumption (bacteria), this anemia may be the result of activation of the immune system by the underlying process, and certain immune and inflammatory cytokines including tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-1, 6, 8 and 10[18,19].
As for chronic inflammatory diseases (and sepsis), these inflammatory mediators lead to anemia through several of the following pathophysiological mechanisms[19] (Figure 6): (1) decreased RBC half-life because of dyserythropoiesis, RBC damage and increased erythrophagocytosis (TNF-α); (2) EPO responses are inadequate for the degree of anemia in most, but not all (e.g. systemic-onset of juvenile chronic arthritis) (IL-1 and TNF-α)[20]; (3) impaired responsiveness of erythroid cells to EPO (IFN-γ, IL-1, and TNF-α); (4) inhibited proliferation and differentiation of erythroid cells (IFN-γ, IL-1, TNF-α, and α-1-antitrypsin); and (5) pathological iron homeostasis caused by increased DMT-1 (IFN-γ) and TfR (IL-10) expression in macrophages, reduced ferroportin 1 expression (IFN-γ and IL-6-induced high hepcidin levels) in enterocytes (inhibition of iron absorption) and macrophages (inhibition of iron recirculation), and increased ferritin synthesis (TNF-α, IL-1, IL-6, IL-10) (increased iron storage). All these lead to hypoferremia through iron diversion to the RES [functional iron deficiency (FID) that is characterized by low serum iron and decreased TSAT], iron-restricted erythropoiesis, and mild-to-moderate anemia.
Thus, the immunological pathophysiology of ACD includes disturbances of iron homeostasis, impaired proliferation of erythroid progenitor cells, and a blunted EPO response to anemia[19]. However, the pathophysiology of acute inflammation-related anemia (e.g. trauma or surgery) is somewhat different. In this setting, inflammatory responses are mediated mainly by IL-6 and IL-8 (with transient contribution of TNF-α and IL-1 in some visceral surgery, such as gastrointestinal or cardiac procedures), whereas IFN-γ plasma levels are undetectable or within the normal range[21-23]. Therefore, in most of these conditions, the two major mechanisms that lead to anemia are perioperative or traumatic blood loss and blunted erythropoiesis caused by decreased iron availability (caused by IL-6-induced high hepcidin levels), whereas EPO levels are normal or near-to-normal[24]. Finally, with persisting decreased iron absorption and/or chronic blood loss, ACD may evolve to ACD with true ID (ACD + ID).
On the other hand, it must be borne in mind that iron is not only required for erythropoiesis and oxidative metabolism. Cellular immune responses are also dependent on the presence of iron, and specific defects in cell-mediated immunity have been described in detail, even in mild ID, including the impaired proliferation and function of lymphocytes and natural killer cells, and a depressed neutrophil respiratory burst[25,26]. Thus, ID or FID may lead not only to a blunted erythropoiesis and chronic fatigue, but also to an inappropriate immune response. For this reason, systemic inflammatory response episodes last longer in critically ill patients with FID, and result in prolonged stay in the intensive care unit and increased morbidity[27]. On the other hand, the effectiveness of the administration of iron sucrose, alone or in combination with EPO, has been assessed in a population of anemic, critically ill patients[28]. Compared to those in the control group who only received folic acid, patients treated with iron sucrose experienced an amelioration of systemic inflammatory response [decreased C-reactive protein (CRP) levels]. These beneficial effects were not as evident in patients who received iron sucrose plus recombinant human EPO (rHuEPO), probably because of the persistence of FID caused by rHuEPO-enhanced erythropoietic activity.
Under physiological conditions, there is a balance between iron absorption, iron transport and iron storage in the human body. However, ID and IDA are common conditions among medical, surgical and critically ill patients, and result from the interplay of three distinct risk factors: increased iron requirements [e.g. growth or use of erythropoiesis-stimulating agents (ESAs), pregnancy and post-bleeding recovery], limited external supply (e.g. malnutrition, malabsorption caused by inflammatory bowel disease, use of gastric antiacid agents, infection with H pylori, even in the absence of significant bleeding), and increased blood loss (e.g. chronic gastrointestinal bleeding)[28]. ID can be either absolute or functional. In absolute ID, iron stores are depleted; in FID, iron stores, although replete, cannot be mobilized as fast as necessary from the macrophages of the RES to the bone marrow. As stated above, FID occurs in anemia of inflammatory diseases because iron is trapped in the RES[3,29].
Thus, laboratory tests for investigating ID fall into two categories: measurements providing evidence of iron depletion in the body, and measurements reflecting iron-deficient RBC production[30] (Table 2). The appropriate combination of these laboratory tests will help to establish a correct diagnosis of anemia and ID status[19].
Normal hemoglobin level does not exclude ID, because individuals with normal body iron stores must lose a large amount of body iron before the hemoglobin falls below the laboratory definition of anemia. According to WHO criteria, laboratory definition of anemia is Hb < 12 g/dL for female and < 13 g/dL for male. However, although we will refer to these values in the following paragraph, it is worth noting that WHO criteria have been challenged recently. Analysis of the large NHANES-III (the third US National Health and Nutrition Examination Survey) and Scripps-Kaiser databases has indicated that a hemoglobin concentration < 13.7 g/dL in a white man aged between 20 and 60 years would have only an approximately 5% chance of being a normal value. The corresponding value for female of all ages would be 12.2 g/dL[31]. Should these new definitions of anemia be applied to our inflammatory bowel disease patient population, the prevalence of anemia would be higher than that reported in several previous studies.
In non-anemic patients, the most important clinical clue of ID is the symptom of chronic fatigue (iron is required for the enzymes involved in oxidative metabolism). However, it is of little screening value because clinicians rarely consider the presence of ID in patients who are not anemic, and therefore ID is invariably diagnosed in the laboratory[30]. A normal hemoglobin level with a mean corpuscular hemoglobin (MCH) in the lower limit of normality (normal range: 28-35 pg), or an increased RBC distribution width (RDW, normal range: 11-15) point to mild ID without anemia, but the main laboratory finding is low ferritin level. Although ferritin is an intracellular iron storage protein, small amounts of ferritin are secreted into the circulation and can be measured in the laboratory, and 1 ng/mL serum ferritin corresponds to approximately 8 mg of stored iron. Thus, measurement of ferritin provides the aID can be defined by a ferritin level < 30 ng/mL in the absence of inflammation (normal serum concentrations of CRP: < 0.5 mg/dL) (true ID). In the presence of inflammation, a normal ferritin level (acute phase reactant) does not exclude ID, and TSAT also should be measured. As transferrin is the only iron-binding protein involved in iron transport, TSAT reflects iron availability for the bone marrow. Thus, in the presence of inflammation, ID should be better defined by normal ferritin concentrations and low TSAT (FID). FID may also occur in response to the therapeutic use of ESAs, such as epoetin or darbopoetin, which place a significant demand on iron stores that may surpass the iron-release capacity of the RES[32].
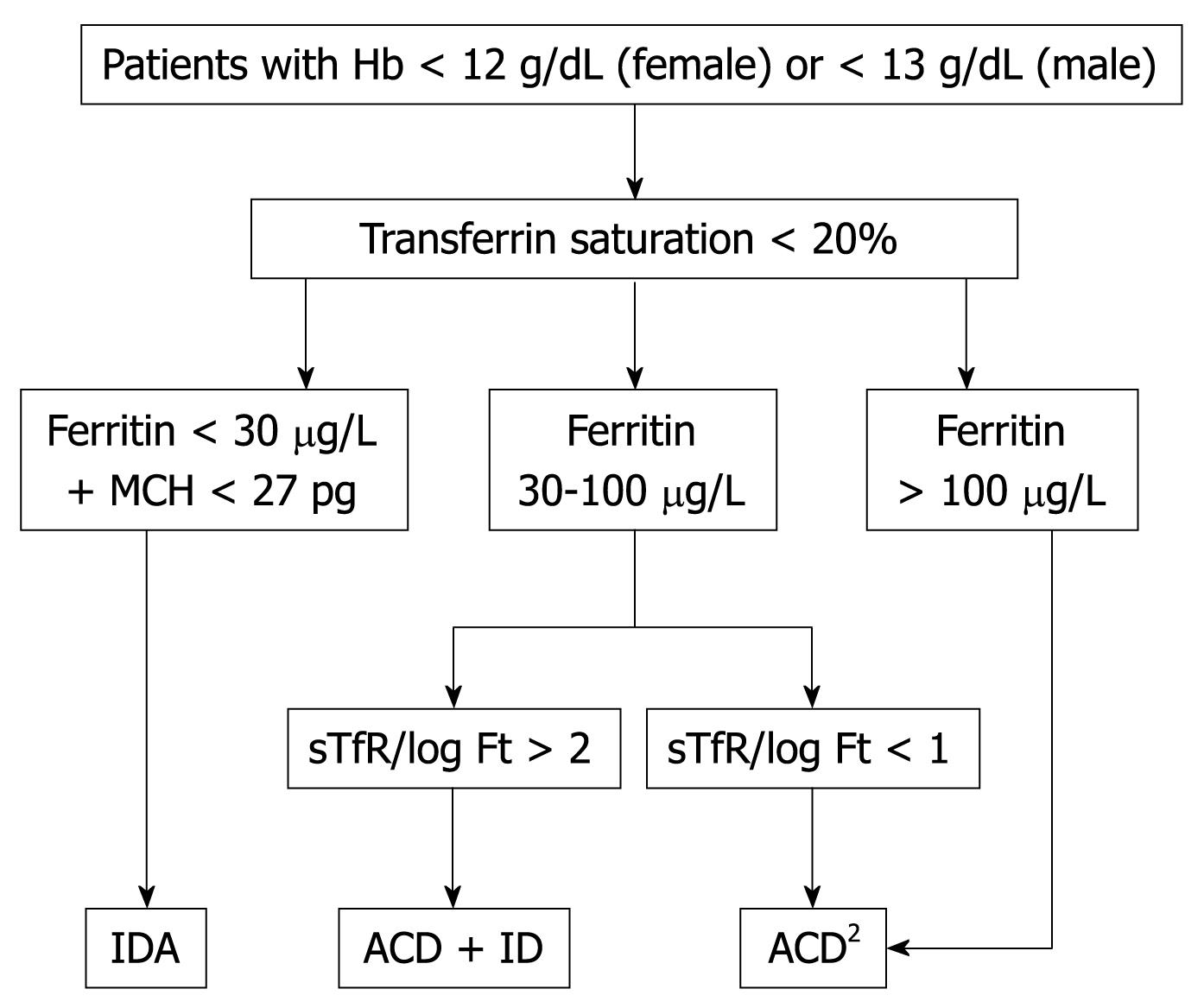
Patients should be considered to suffer from IDA when they presented with low Hb (male < 13 g/dL and female < 12 g/dL), TSAT (< 20%) and ferritin concentrations (< 30 ng/mL) but no signs of inflammation[30]. The MCH rather than mean corpuscular volume (MCV) has become the most important RBC marker for detecting ID in circulating RBCs (Figure 7). MCV is a reliable and widely available measurement but is a relatively late indicator in patients who are not actively bleeding. In addition, patients may present with IDA and without microcytosis, with coexisting vitamin B12 or folate deficiency. sTfR levels are usually high or very high, but they are not usually required for the diagnosis of uncomplicated IDA.
Patients should be considered to suffer from ACD when they have: (1) evidence of chronic inflammation (high CRP level); (2) hemoglobin concentration < 13 g/dL for male and < 12 g/dL for female; and (3) low TSAT < 20%, but normal or increased serum ferritin concentration (> 100 ng/mL) or low serum ferritin concentration (30-100 ng/mL), and an sTfR/log ferritin ratio < 1[19,33-35] (Figure 7). ACD, as well as FID, are frequent among patients with inflammatory disease without apparent blood loss (e.g. rheumatoid arthritis, renal failure or chronic hepatitis).
On the other hand, although ACD is typically mild to moderate, and RBCs may not show any stigmata of ID (normochromic, normocytic anemia), the underlying iron etiology is evident: macrophages that normally recycle iron are found to sequester it, intestinal iron absorption is interrupted, and erythroid precursors respond very rapidly when iron-transferrin is made available, especially by the administration of iv iron preparations. Thus, it can be speculated that the normocytic RBCs result from the combination of iron insufficiency and an as-yet-unexplained tendency to macrocytosis (e.g. alterations in folate or B12 metabolism in response to inflammation)[12].
Patients should be considered to have ACD + ID when they have: (1) chronic inflammation (high CRP level); (2) hemoglobin concentration < 13 g/dL for male and < 12 g/dL for female; and (3) low TSAT < 20%, serum ferritin concentration > 30 and < 100 ng/mL, and an sTfR/log ferritin ratio > 2[19,33-35] (Figure 7). This type of anemia is more frequent in patients with inflammatory diseases and chronic blood losses (e.g. inflammatory bowel disease). There are two important hematological indices that may also help in the diagnosis of ACD + ID: reticulocyte hemoglobin content (CHr) and hypochromic red blood cells (HYPO). In non-ferropenic patients, the 2.5 percentile values were 28 pg for CHr and 5% for HYPO[15]. However, these hematological indices are only available in specific hematological analyzers. The Advia 120 hematology analyzer determines CHr and RBC hemoglobin content (≥ 27 pg), whereas the Sysmex XE-2100 hematology analyzer determines RET-Y, which can be considered as the reticulocyte hemoglobin equivalent, as well as RBC-Y, which can be considered as the RBC hemoglobin equivalent[36,37]. These hematological indices (CHr and HYPO) are direct indicators of FID, in contrast to the majority of biochemical markers, which measure FID indirectly via iron-deficient erythropoiesis and demonstrate weaknesses in the diagnosis of FID as defined by hematological indices[15]. New hematological indices are being developed for other hematological analyzers (e.g. Beckman-Coulter LH 750) and their clinical utility in the diagnosis of ID will be evaluated in the near future.
Although hepcidin affects iron traffic in ACD and ACD + ID, individuals suffering from ACD + ID have significantly lower hepcidin levels than ACD subjects, and ACD + ID individuals, in contrast to ACD subjects, are able to absorb some dietary iron from the gut and to mobilize some iron from macrophages. Thus, hepcidin determination may also aid differentiation between ACD and ACD + ID and in selecting appropriate therapy for these patients[33].
Iron is an essential micronutrient, as it is required for an adequate erythropoietic function, oxidative metabolism and cellular immune response. Although the absorption of dietary iron (1-2 mg/d) is regulated tightly, it is just balanced with losses. Therefore, internal turnover of iron is essential to meet the requirements for erythropoiesis (20-30 mg/d). Hepcidin, which is primarily made in hepatocytes in response to liver iron levels, inflammation, hypoxia and anemia, is the main iron regulatory hormone for iron absorption and recirculation. Increased iron requirements, limited external supply, and increased blood loss may lead to ID and IDA. During inflammation, the excess of hepcidin decreases iron absorption and prevents iron recycling, which results in hypoferremia and iron-restricted erythropoiesis, despite normal iron stores, and finally, in ACD, which can later evolve to ACD + ID. An appropriate combination of laboratory tests that provides evidence of iron depletion or reflects iron-deficient RBC production will help to establish a correct diagnosis of ID status and anemia[38].
Peer reviewer: Emiko Mizoguchi, MD, PhD, Department of Medicine, Gastrointestinal Unit, GRJ 702, Massachusetts General Hospital, Boston, MA 02114, United States
S- Editor Tian L L- Editor Kerr C E- Editor Zheng XM
| 1. | Koury MJ. Progress in understanding erythropoiesis. rhErythropoietin in cancer supportive treatment. New York: Marel Dekker 1996; 1-12. |
| 2. | Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med. 2006;354:2034-2045. |
| 3. | Muñoz Gómez M, Campos Garríguez A, García Erce JA, Ramírez Ramírez G. [Fisiopathology of iron metabolism: diagnostic and therapeutic implications]. Nefrologia. 2005;25:9-19. |
| 4. | Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986-1995. |
| 5. | Siah CW, Ombiga J, Adams LA, Trinder D, Olynyk JK. Normal iron metabolism and the pathophysiology of iron overload disorders. Clin Biochem Rev. 2006;27:5-16. |
| 6. | Fleming RE, Bacon BR. Orchestration of iron homeostasis. N Engl J Med. 2005;352:1741-1744. |
| 7. | Marignani M, Angeletti S, Bordi C, Malagnino F, Mancino C, Delle Fave G, Annibale B. Reversal of long-standing iron deficiency anaemia after eradication of Helicobacter pylori infection. Scand J Gastroenterol. 1997;32:617-622. |
| 8. | Krishnamurthy P, Xie T, Schuetz JD. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol Ther. 2007;114:345-358. |
| 9. | Andrews NC. Understanding heme transport. N Engl J Med. 2005;353:2508-2509. |
| 10. | Nemeth E, Ganz T. Hepcidin and iron-loading anemias. Haematologica. 2006;91:727-732. |
| 11. | Crichton RR, Danielsson BG, Geisser P. Iron metabolism: biologic and molecular aspects. Iron therapy with special emphasis on intravenous administration. 4th ed. Bremen: UNI-Med Verlag AG 2008; 14-24. |
| 13. | Weiss G, Houston T, Kastner S, Jöhrer K, Grünewald K, Brock JH. Regulation of cellular iron metabolism by erythropoietin: activation of iron-regulatory protein and upregulation of transferrin receptor expression in erythroid cells. Blood. 1997;89:680-687. |
| 14. | Iolascon A, De Falco L, Beaumont C. Molecular basis of inherited microcytic anemia due to defects in iron acquisition or heme synthesis. Haematologica. 2009;94:395-408. |
| 15. | Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem. 2002;48:1066-1076. |
| 16. | Kong WN, Zhao SE, Duan XL, Yang Z, Qian ZM, Chang YZ. Decreased DMT1 and increased ferroportin 1 expression is the mechanisms of reduced iron retention in macrophages by erythropoietin in rats. J Cell Biochem. 2008;104:629-641. |
| 17. | Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640-642. |
| 18. | Nowrousian MR, Kasper C, Oberhoff C, Essers U, Voigtmann R, Gallasch W, Quarder O. Pathophysiology of cancer-related anemia. rhErythropoietin in cancer supportive treatment. New York: Marcel Dekker 1996; 13-34. |
| 19. | Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011-1023. |
| 20. | Cazzola M, Ponchio L, de Benedetti F, Ravelli A, Rosti V, Beguin Y, Invernizzi R, Barosi G, Martini A. Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood. 1996;87:4824-4830. |
| 21. | Jansson K, Redler B, Truedsson L, Magnuson A, Matthiessen P, Andersson M, Norgren L. Intraperitoneal cytokine response after major surgery: higher postoperative intraperitoneal versus systemic cytokine levels suggest the gastrointestinal tract as the major source of the postoperative inflammatory reaction. Am J Surg. 2004;187:372-377. |
| 22. | Franke A, Lante W, Fackeldey V, Becker HP, Kurig E, Zöller LG, Weinhold C, Markewitz A. Pro-inflammatory cytokines after different kinds of cardio-thoracic surgical procedures: is what we see what we know? Eur J Cardiothorac Surg. 2005;28:569-575. |
| 23. | Muñoz M, García-Vallejo JJ, Sempere JM, Romero R, Olalla E, Sebastián C. Acute phase response in patients undergoing lumbar spinal surgery: modulation by perioperative treatment with naproxen and famotidine. Eur Spine J. 2004;13:367-373. |
| 24. | van Iperen CE, Kraaijenhagen RJ, Biesma DH, Beguin Y, Marx JJ, van de Wiel A. Iron metabolism and erythropoiesis after surgery. Br J Surg. 1998;85:41-45. |
| 25. | van Iperen CE, Gaillard CA, Kraaijenhagen RJ, Braam BG, Marx JJ, van de Wiel A. Response of erythropoiesis and iron metabolism to recombinant human erythropoietin in intensive care unit patients. Crit Care Med. 2000;28:2773-2778. |
| 27. | Oppenheimer SJ. Iron and its relation to immunity and infectious disease. J Nutr. 2001;131:616S-633S; discussion 633S-635S. |
| 28. | Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. Am J Clin Nutr. 1997;66:464S-477S. |
| 29. | Hersko C. Prevalence and causes of iron deficiency anaemia. Disorders of iron homeostasis, erythrocytes, erythropoiesis. Paris: European School of Haematology 2006; 409-419. |
| 30. | Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319-332. |
| 31. | Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747-1750. |
| 32. | Goodnough LT. The Relevance of Iron in Erythropoietin-Stimulated Erythropoiesis. Semin Hematol. 2006;43:S3-S8. |
| 33. | Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, Schroll A, Sonnweber T, Eberwein L, Witcher DR, Murphy AT. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277-5286. |
| 34. | Punnonen K, Irjala K, Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052-1057. |
| 35. | Beguin Y, Clemons GK, Pootrakul P, Fillet G. Quantitative assessment of erythropoiesis and functional classification of anemia based on measurements of serum transferrin receptor and erythropoietin. Blood. 1993;81:1067-1076. |
| 36. | Thomas L, Franck S, Messinger M, Linssen J, Thomé M, Thomas C. Reticulocyte hemoglobin measurement--comparison of two methods in the diagnosis of iron-restricted erythropoiesis. Clin Chem Lab Med. 2005;43:1193-1202. |
| 37. | Brugnara C, Schiller B, Moran J. Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states. Clin Lab Haematol. 2006;28:303-308. |