Published online Sep 7, 2009. doi: 10.3748/wjg.15.4143
Revised: July 27, 2009
Accepted: August 3, 2009
Published online: September 7, 2009
AIM: To gain molecular insights into the expression and functions of endothelin-1 (ET-1) in pancreatic stellate cells (PSC).
METHODS: PSCs were isolated from rat pancreas tissue, cultured, and stimulated with ET-1 or other extracellular mediators. Cell proliferation was assessed by measuring the incorporation of 5-bromo-2’-deoxyuridine into DNA and cell migration was studied in a transwell chamber assay. Gene expression at the level of mRNA was quantified by real-time polymerase chain reaction. Expression and phosphorylation of proteins were monitored by immunoblotting, applying an infrared imaging technology. ET-1 levels in cell culture supernatants were determined by an enzyme immunometric assay. To study DNA binding of individual transcription factors, electrophoretic mobility shift assays were performed.
RESULTS: Among several mediators tested, transforming growth factor-β1 and tumour necrosis factor-α displayed the strongest stimulatory effects on ET-1 secretion. The cytokines induced binding of Smad3 and NF-κB, respectively, to oligonucleotides derived from the ET-1 promoter, implicating both transcription factors in the induction of ET-1 gene expression. In accordance with previous studies, ET-1 was found to stimulate migration but not proliferation of PSC. Stimulation of ET-1 receptors led to the activation of two distinct mitogen-activated protein kinases, p38 and extracellular signal-regulated kinases (ERK)1/2, as well as the transcription factor activator protein-1. At the mRNA level, enhanced expression of the PSC activation marker, α-smooth muscle actin and two proinflammatory cytokines, interleukin (IL)-1β and IL-6, was observed.
CONCLUSION: This study provides novel lines of evidence for profibrogenic and proinflammatory actions of ET-1 in the pancreas, encouraging further studies with ET-1 inhibitors in chronic pancreatitis.
- Citation: Jonitz A, Fitzner B, Jaster R. Molecular determinants of the profibrogenic effects of endothelin-1 in pancreatic stellate cells. World J Gastroenterol 2009; 15(33): 4143-4149
- URL: https://www.wjgnet.com/1007-9327/full/v15/i33/4143.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.4143
Pancreatic fibrosis represents a key feature of chronic pancreatitis and pancreatic cancer. Fibrosis not only accompanies the tumour, but plays an active role in its progression[1,2]. The cellular and molecular basis of pancreatic fibrogenesis has therefore attracted significant interest in pancreatologists in recent years. In 1998, pancreatic stellate cells (PSC) were identified as the main source of extracellular matrix (ECM) proteins in the diseased pancreas[3,4]. In response to profibrogenic stimuli, PSC undergo phenotypic changes termed activation, which include proliferative activity, expression of myofibroblastic markers [such as α-smooth muscle actin (α-SMA)], and enhanced synthesis of ECM components. The activation process is also triggered when PSC are isolated from healthy pancreas and when cultured in cell culture dishes. Numerous extracellular mediators and intracellular pathways of PSC activation have been described since 1998, which are summarized in recent reviews[2,5]. Thus, platelet-derived growth factor (PDGF) has been identified as a strong PSC mitogen, while transforming growth factor (TGF)-β1 is considered the most relevant stimulator of ECM synthesis[6,7]. In addition, proinflammatory cytokines, including tumour necrosis factor (TNF)-α, and ethanol metabolites, have been implicated in pancreatic fibrogenesis[7-9]. Despite many efforts, however, little progress has been made so far with respect to the development of therapeutic strategies to interrupt fibrogenesis in the context of chronic pancreatitis and pancreatic cancer. One reason is the lack of efficient antifibrotic drugs that are applicable in patients and not in experimental settings only.
Recent studies on fibroproliferative disorders in different organs have indicated an antifibrotic effect of endothelin (ET)-receptor antagonists[10]. Using bosentan, a clinically available dual ET-receptor antagonist targeting the two receptor types ETRA and ETRB, we recently observed inhibition of key functions of activated PSC, including proliferation and collagen synthesis[11]. When bosentan was applied to rats with chronic pancreatitis, at least a tendency towards a diminished disease progression was observed in a subgroup of animals with less severe disease. Based on the results of these experiments, we were further interested in the molecular role of the ET-1/ET receptor axis in the process of stellate cell activation.
In the present study, we have analyzed the biological and molecular effects of ET-1 in PSC and addressed the question of how ET-1 expression in PSC is regulated. The experiments were built on earlier reports by our group and others, which had suggested an autocrine loop of ET-1. Specifically, ET-1 was previously shown to activate extracellular signal-regulated kinases (ERK) 1 and 2, and to enhance PSC contraction and migration[11-13]. Our novel data deciphers ET-1 signalling in greater detail, and identifies, for the first time, target genes of ET-1 in PSC. Interestingly, these genes include not only α-SMA (which is directly linked to the activation process), but also two proinflammatory cytokines, interleukin (IL)-1β and IL-6. Furthermore, our investigations revealed TGF-β1 and TNF-α as potent inducers of ET-1 expression in PSC. Together, the data suggest that ET-1 is an important player in a network of proinflammatory and profibrogenic mediators that fosters interactions between inflammatory cells and PSC in a vicious cycle of inflammation and fibrosis.
Iscove’s modified Dulbecco’s medium (IMDM) and all supplements for cell culture were obtained from Biochrom (Berlin, Germany), Nycodenz from Nycomed (Oslo, Norway), the rat-specific ET-1 enzyme immunometric assay (EIA) kit from Biotrend (Köln, Germany), and the 5-bromo-2’-deoxyuridine (BrdU) labelling and detection enzyme-linked immunosorbent assay kit as well as the polynucleotide kinase from Roche Diagnostics (Mannheim, Germany). Human connective tissue growth factor (CTGF) was delivered by EMP Genetech (Ingolstadt, Germany), and recombinant cytokines [rat interferon (IFN)-γ and PDGF, human TNF-α and TGF-β1] by R&D Systems (Minneapolis, MN, USA). TRIzol and all reagents used for reverse transcription and Taqman™ real-time polymerase chain reaction (PCR) were from Applied Biosystems (Foster City, CA, USA). PVDF membrane was supplied by Millipore (Schwalbach, Germany), protein- and phospho-protein specific antibodies to ERK1/2 and p38 (all raised in rabbits) by New England BioLabs (Frankfurt, Germany), and Odyssey® blocking buffer, stripping buffer and secondary antibodies for immunoblotting by LI-COR (Bad Homburg, Germany). Immunoglobulins used in gel shift assays were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA), DNA oligonucleotides from BioTeZ (Berlin, Germany), and [γ-32P] ATP from Hartmann Analytic (Braunschweig, Germany). Collagenase, ET-1, tissue culture dishes (Corning plasticware), carboxyfluorescein succinimidyl ester (CFSE), as well as standard laboratory chemicals were from Sigma-Aldrich (St. Louis, MO, USA).
PSC were isolated from the pancreas of male LEW.1W inbred rats by collagenase digestion of the organ and Nycodenz (120 g/L) density gradient centrifugation essentially as previously described[14]. The cells were resuspended in IMDM supplemented with 17% foetal calf serum (FCS), 10 mL/L non-essential amino acids (dilution of a 100 × stock solution), 105 U/L penicillin and 100 mg/L streptomycin, and cultured at 37°C in a 5% CO2 humidified atmosphere. All experiments were performed with cells that were passaged only once. Therefore, PSCs in primary culture were grown to subconfluency, harvested by trypsination (usually, on day seven after isolation), and recultured at equal seeding densities.
Cell proliferation was assessed by measuring incorporation of BrdU into newly synthesized DNA. Therefore, cells growing in 96-well plates were treated under serum-free conditions with ET-1 as indicated. At the time of ET-1 application, BrdU labelling was initiated by adding labelling solution at a final concentration of 10 μmol/L (in the culture medium). Twenty-four hours later, labelling was stopped, and BrdU uptake was measured according to the manufacturer’s instructions.
Serum-starved PSC were labelled with the fluorescence dye CFSE, which was dissolved in dimethyl sulfoxide and applied at a final concentration of 333 μmol/L (chosen based on the results of assay optimization). The cells were incubated with the dye for 20 min in darkness at room temperature. Migration assays were performed under serum-free conditions in 24-transwell plates using inserts with a pore size of 8 μm. Therefore, labelled cells were seeded at a density of 50 000 cells per well into the upper chamber, while ET-1 was added to the medium of the lower chamber as indicated. After an incubation period of 24 h, cells in the transwell inserts were removed by medium aspiration and mechanical detachment from the upper side of the membrane. Cells adhering to the lower side of the filter or the bottom of the lower chamber were harvested by trypsination and transferred into 96-well plates. Fluorescence intensity was then measured using a fluorescence microplate reader (λex = 492 nm, λem = 517 nm).
Protein extracts from equal numbers of PSCs (pretreated as indicated) were prepared and subjected to immunoblot analysis as previously described[14]. After electrophoresis, proteins were blotted onto PVDF membrane. The filters were rinsed in phosphate-buffered saline (PBS) and blocked for 1 h using Odyssey® Blocking Buffer, before primary antibodies were added to the blocking solution. One hour later, the blots were washed four times for 5 min in PBS containing 0.1% Tween 20 (PBS-T), and exposed to secondary antibody (IRDye® 800CW conjugated goat anti-rabbit IgG) for 1 h. Subsequently, the membranes were washed again (PBS-T, three times for 5 min), rinsed in PBS and scanned at a wavelength of 800 nm using an Odyssey® Infrared Imaging System. Signal intensities were quantified by means of the Odyssey® software version 3.0. Prior to reprobing with additional primary antibodies, the blots were treated with stripping buffer according to the instructions of the manufacturer.
Quantitative reverse transcriptase-PCR using real-time TaqMan™technology
Total RNA from cells pretreated as indicated was isolated with TRIzol reagent according to the manufacturer’s instructions. Next, RNA was reverse transcribed into cDNA by means of TaqMan™ Reverse Transcription Reagents and random hexamer priming. Relative quantification of target cDNA levels by real-time PCR was performed in an ABI Prism 7000 sequence detection system (Applied Biosystems) using TaqMan™ Universal PCR Master Mix and the following Assay-on-Demand™ rat gene-specific fluorescently labelled TaqMan™ MGB probes: Rn00573960_g1 (CTGF), Rn00561129_m1 (ET-1), Rn00580432_m1 (IL-1β), Rn00561420_m1 (IL-6), Rn00572010_m1 (TGF-β1), and Rn01527838_g1 [hypoxanthine phosphoribosyl transferase (HPRT), used as house-keeping control gene]. The Custom TaqMan Gene Expression Assay specific for rat α-SMA (GenBank Accession Number: X06801) has been described previously[11]. Following the guidelines of the manufacturer, PCR was performed under the following conditions: 95°C for 10 min, 50 cycles of 15 s at 95°C, 1 min at 60°C. The reactions were performed in triplicate, and repeated six times with independent samples. Relative expression of each mRNA compared with HPRT was calculated according to the equation ΔCt = Cttarget - CtHPRT. The relative amount of target mRNA in control cells and cells treated as indicated was expressed as 2-(ΔΔCt), where ΔΔCttreatment = ΔCtET-1 - ΔCtcontrol.
ET-1 polypeptide concentrations in PSC culture supernatant were determined by EIA. Therefore, cells were seeded at a density of 50 000 cells per well into 24-well plates. After an overnight incubation, medium was replenished and cells were exposed to different mediators as indicated. 48 h later, supernatants were collected and stored at -80°C until they were subjected to EIA analysis. The assay was performed according to the instructions of the manufacturer. FCS of the culture medium was also analyzed and found to contain no detectable amounts of ET-1 (data not shown).
PSC growing in six-well plates were incubated for 16 h in FCS-free culture medium, before they were treated as indicated. Nuclear extracts were prepared as previously described[14,15]. For EMSA experiments, nuclear proteins of 105 cells were incubated with double-stranded oligonucleotides which were end-labelled with [γ-32P] ATP by polynucleotide kinase. The sequence of the activator protein (AP)-1 probe was 5'-CGCTTGATGACTCAGCCGATC-3' (consensus binding motif underlined). To study inducible protein binding to the ET-1 promoter, the following probes derived from the rat promoter sequence[16] (accession number: S76970) were applied: 5'-GATTGTCAGACGGCGGGCGTCTGCCTCTGAAG-3' (corresponding to bases -196 to -165), and 5'-AGCCGTGATTTCCTCTAGAGC-3' (-163 to -144). The first oligonucleotide contains two consensus motifs for Smad transcription factors (underlined), which in the homologous region of the human ET-1 promoter have been identified as core sequences within a TGF-β response element[17]. The underlined bases of the second oligonucleotide correspond to a putative, although imperfect NF-κB/c-Rel binding site. Binding reaction and supershift analysis (initiated by adding 0.5 μg antibody) were performed as described[14,15]. Protein-DNA complexes were analyzed by electrophoretic separation on a 6% non-denaturating polyacrylamide gel. Dried gels were exposed to X-ray film.
Results are expressed as mean ± SE for the indicated number of separate cultures per experimental protocol. Statistical significance was analyzed using Wilcoxon’s rank sum test. P < 0.05 was considered to be statistically significant.
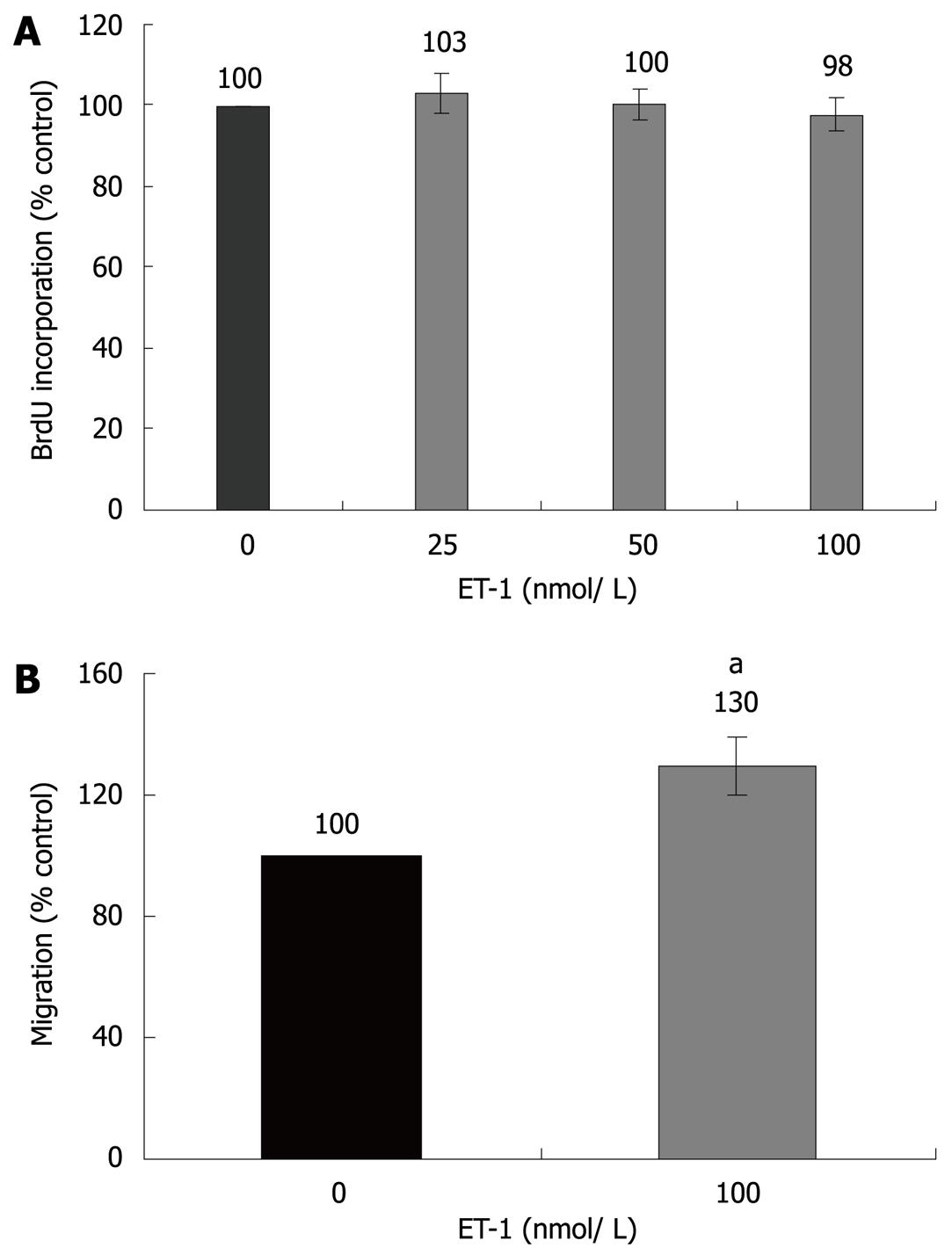
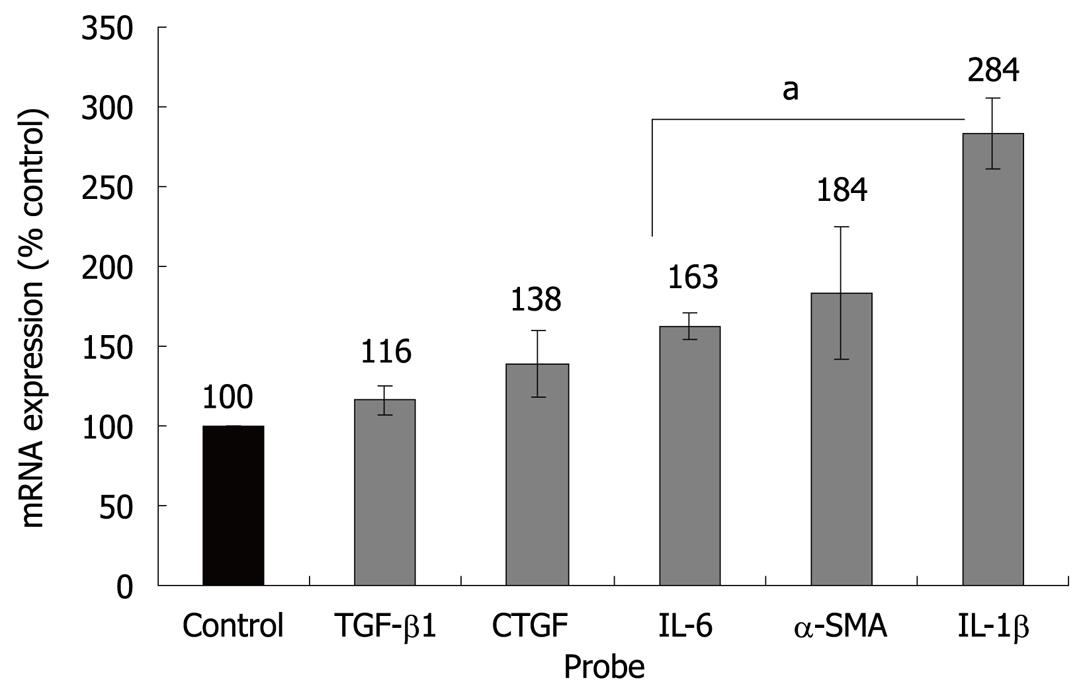
Initial studies dealt with the biological effects of ET-1 in PSC. In accordance with previous reports[12,13], ET-1 stimulated migration but had no effect on PSC proliferation (Figure 1). Next, we analyzed regulation of gene expression by ET-1, using real-time PCR. Interestingly, ET-1 significantly enhanced expression of α-SMA, suggesting direct stimulation of myofibroblastic differentiation (Figure 2). Furthermore, we focussed on cytokines and growth factors that have previously been implicated in autocrine or paracrine maintenance and enhancement of PSC activation[6-8,18,19]. ET-1 significantly increased expression of two proinflammatory mediators, IL-1β and IL-6, but not of TGF-β1 and CTGF, two potent inducers of collagen synthesis.
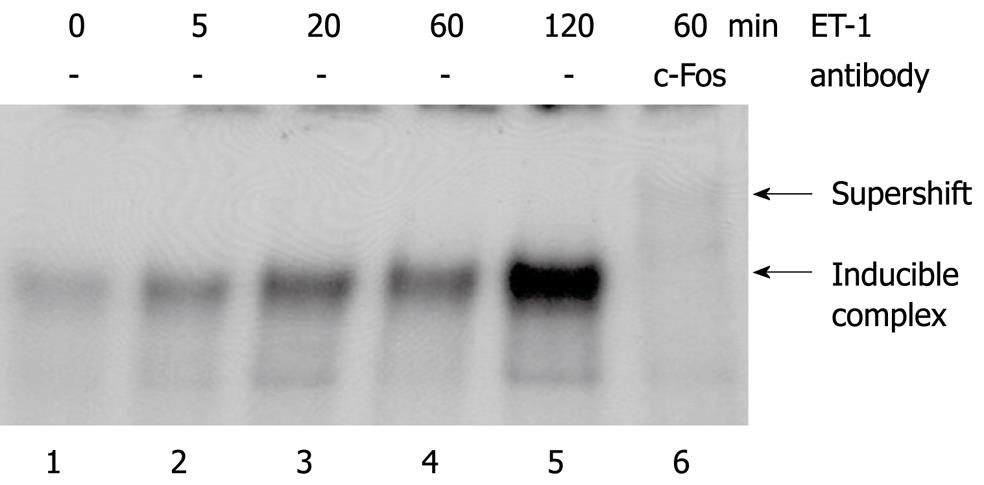
Extending our previous pilot study[11], we observed that ET-1 induced a rapid and transient phosphorylation of two distinct types of mitogen-activated protein (MAP) kinases, ERK1/2 and p38 (Figure 3). EMSA experiments revealed that ET-1 stimulation strongly increased DNA binding of the transcription factor complex AP-1 (Figure 4). As indicated by the results of a supershift analysis, the DNA/protein complex contained the AP-1 subunit c-Fos (Figure 4, lane 6). ET-1 stimulation of PSC was also associated with some enhancement of the DNA binding of NF-κB. The relevance of this finding, however, remained uncertain since activation of NF-κB was quite weak (data not shown).
In the course of the studies, four mediators involved in PSC activation (PDGF, CTGF, TNF-α and TGF-β1)[2] as well as one antagonist, the antiproliferative and antifibrotic cytokine IFN-γ[20], were tested regarding their effects on ET-1 synthesis in PSC (Figure 5). TGF-β1 and TNF-α strongly stimulated secretion of ET-1 by the cells, whereas PDGF displayed the opposite effect. After application of CTGF or IFN-γ, statistically significant but nevertheless small increases of ET-1 levels in culture supernatants were observed.
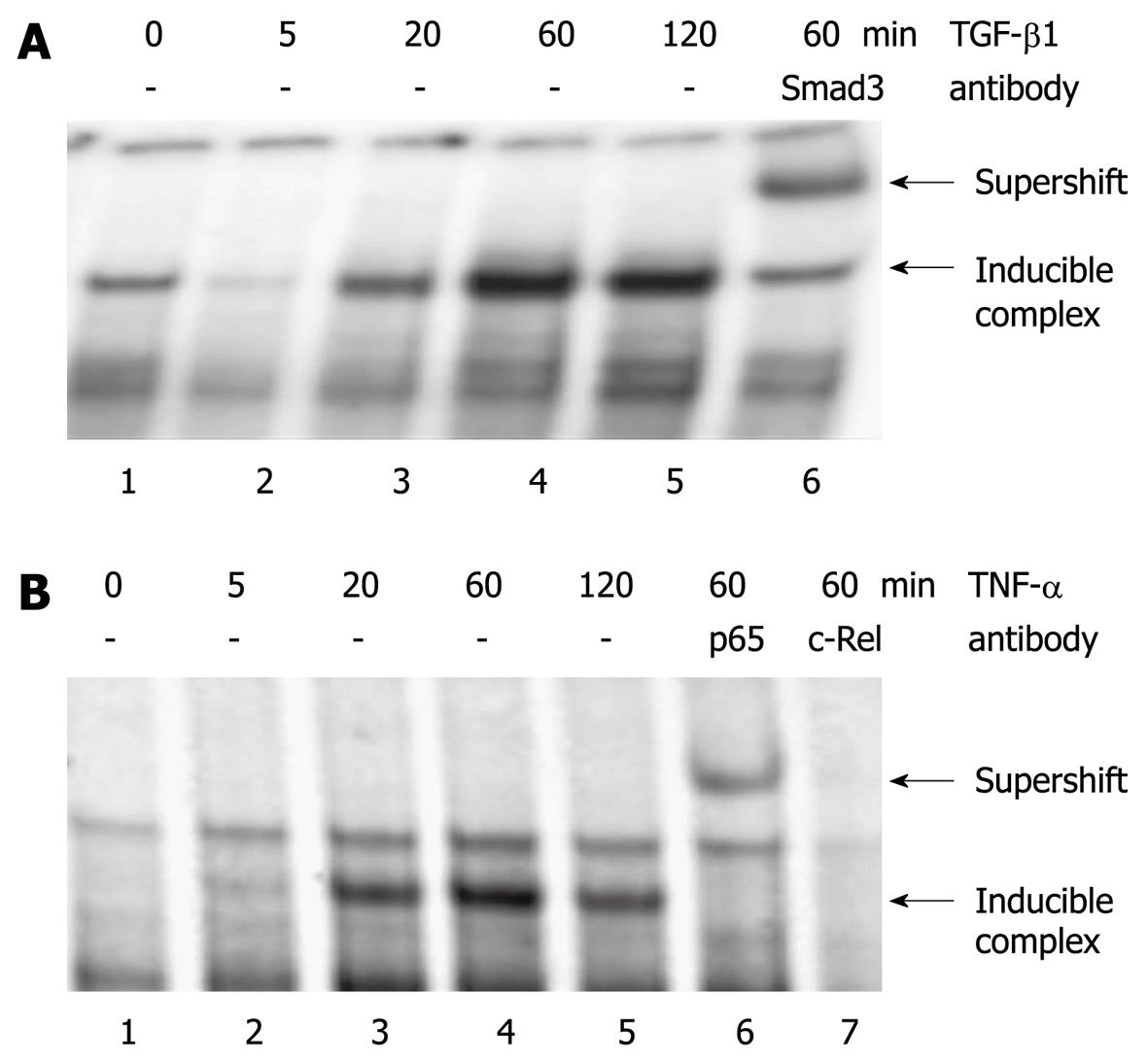
To study the molecular mechanisms underlying the effects of TGF-β1 and TNF-α, EMSA experiments using oligonucleotides derived from the rat ET-1 promoter were performed. TGF-β1 induced the binding of a transcription factor complex containing Smad3 to a DNA sequence that corresponds to the TGF-β response element of the human ET-1 gene[17] (Figure 6A). TNF-α stimulation of PSC was associated with enhanced protein binding to a putative NF-κB site in the ET-1 promoter. Antibodies to the NF-κB subunits p65RelA and c-Rel shifted the protein/DNA complex or weakened DNA/protein interaction, suggesting the presence of both proteins (Figure 6B).
PSC are the principal effector cells in pancreatic fibrosis[2,21]. Progressive replacement of pancreatic parenchyma by connective tissue promotes the development of exocrine and endocrine organ failure in the course of chronic pancreatitis. Furthermore, paracrine interactions between stroma cells and pancreatic cancer cells have been implicated in accelerated tumour growth and resistance to chemotherapeutics[22-24]. PSC are therefore considered attractive targets for the adjuvant treatment of pancreatic cancer based on inhibition of fibrogenesis. One important prerequisite for such an antifibrotic therapy is to understand the molecular processes underlying PSC activation in pancreatitis and cancer.
We and others have recently shown that ET-1 acts on PSCs in an autocrine fashion[11-13]. In agreement with a previous study[13], we have now found that application of ET-1 to PSC cultures promotes migration. Furthermore, our novel data indicate that ET-1 directly fosters exhibition of an activated, myofibroblastic phenotype, since α-SMA expression in PSC was enhanced. As previously suggested by Masamune et al[13], exogenous ET-1 did not stimulate cell proliferation. Nevertheless, the dual-specific ET receptor antagonist bosentan significantly inhibits PSC growth[11], suggesting a contribution of endogenous ET-1 to the induction of mitogenesis. Together, these findings clearly point to a profibrogenic role of the ET-1/ET receptor axis in PSC. Additional support for this conclusion comes from our studies at the molecular level: ET-1 induced an activation of the MAP kinases ERK1/2, p38 and the transcription factor AP-1. All these signalling molecules have previously been identified as key components of the intracellular network triggering PSC activation[14,15,25]. Furthermore, ET-1 significantly stimulated expression of two cytokines that have previously been suggested as autocrine enhancers of PSC activation, IL-1β and IL-6[18,19]. Since both cytokines are well-established pro-inflammatory mediators, our data also implicate, for the first time, ET-1 in the enhancement of local inflammatory reactions in the pancreas.
In the present study, experiments aimed at elucidating regulation of ET-1 gene expression in PSC revealed that TGF-β1 and TNF-α strongly enhanced the release of ET-1 by culture-activated PSC. Based on our molecular studies, Smad transcription factors and NF-κB are likely to be involved in the induction of ET-1 expression by TGF-β1 and TNF-α, respectively. Although both cytokines have previously been shown to stimulate ET-1 synthesis in other types of cells[17,26], this finding is interesting since it suggests the existence of a regulatory network in which TGF-β1 and TNF-α exert their biological effects on stellate cells in part through the ET-1/ET receptor axis. Given that ET-1 induces expression of IL-1β/IL-6, and TGF-β1 is secreted by PSC[27], a vicious cycle may develop which leads to enhanced inflammation and progressive fibrosis.
Unexpectedly, IFN-γ displayed a small stimulatory effect on ET-1 release by PSC, although it inhibited expression of the ET-1 gene in a cell line of immortalized PSC[28]. Here and in the case of PDGF, which diminished ET-1 synthesis, the underlying molecular mechanisms warrant further investigation.
As indicated by the results of our previous study[11], interruption of autocrine and paracrine loops at the level of ET-1 action represents a suitable approach to target activated PSC. However, the antifibrotic efficiency of the ET receptor antagonist bosentan in our rat model of severe chronic pancreatitis[29] was quite limited. Based on our previous and novel data, we therefore suggest that in future preclinical studies ET-1 receptor antagonists should be combined with drugs interfering with a different critical step of fibrogenesis and inflammation, such as transduction of profibrogenic signals in PSC.
Fibrosis is a key feature of chronic pancreatitis and pancreatic cancer. The extensive deposition of extracellular matrix (ECM) proteins fosters the development of an exocrine and endocrine organ insufficiency, and accelerates progression of the tumour. Pancreatic stellate cells (PSC) are the principal effector cells in pancreatic fibrosis. They are activated by profibrogenic stimuli, which include, for example, cytokines and ethanol metabolites. So far, there are no specific therapies available to interfere with dysregulated fibrogenesis in the diseased pancreas.
The molecular mechanisms underlying induction and maintenance of PSC activation are incompletely understood. This deficit hampers to some degree the development of therapeutic approaches aimed at the inhibition of pancreatic fibrosis in pancreatitis and cancer.
The results of this study provide molecular insights into endothelin-1 (ET-1) action in PSC. The data indicate that ET-1 stimulates exhibition of an activated stellate cell phenotype. The intracellular signalling molecules found to be activated by ET-1, ERK1/2, p38 and AP-1, have previously been linked to the process of stellate cell activation. With IL-1β and IL-6, two proinflammatory cytokines were identified as target genes of ET-1, suggesting for the first time a direct link between inflammation and fibrosis at the level of ET-1 action. In addition, studies on the regulation of ET-1 expression revealed that transforming growth factor-β1 and tumour necrosis factor-α strongly stimulate ET-1 secretion by PSC. Together, these data suggest that ET-1 is part of a network of proinflammatory and profibrogenic mediators that fosters interactions between inflammatory cells and PSC, ultimately enhancing inflammation and fibrosis.
The results encourage further studies aimed at the inhibition of fibrogenesis by targeting the ET-1/ET receptor axis. The authors suggest that combinations of ET receptor antagonists with drugs interfering with independent steps of stellate cell activation should be tested in animal models of pancreatic fibrosis.
Pancreatic fibrosis refers to the process of progressive replacement of pancreatic tissue by connective tissue. PSC are fibroblast-like cells that produce most of the ECM in the diseased organ. ET-1 is a polypeptide originally identified as an endothelial cell-derived hormone with vasoconstrictive activity. Additional cellular origins and functions of ET-1 have been described.
The authors revealed alteration of several factors involved in inflammation and fibrogenesis focusing on ET-1 action in isolated PSC. The study is well designed and provided some interesting results of scientific value, implying future therapeutic strategy using ET-1 receptor antagonists against chronic pancreatitis.
| 1. | Talukdar R, Saikia N, Singal DK, Tandon R. Chronic pancreatitis: evolving paradigms. Pancreatology. 2006;6:440-449. [Cited in This Article: ] |
| 2. | Jaster R, Emmrich J. Crucial role of fibrogenesis in pancreatic diseases. Best Pract Res Clin Gastroenterol. 2008;22:17-29. [Cited in This Article: ] |
| 3. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grunert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [Cited in This Article: ] |
| 4. | Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut. 1998;43:128-133. [Cited in This Article: ] |
| 5. | Omary MB, Lugea A, Lowe AW, Pandol SJ. The pancreatic stellate cell: a star on the rise in pancreatic diseases. J Clin Invest. 2007;117:50-59. [Cited in This Article: ] |
| 6. | Luttenberger T, Schmid-Kotsas A, Menke A, Siech M, Beger H, Adler G, Grunert A, Bachem MG. Platelet-derived growth factors stimulate proliferation and extracellular matrix synthesis of pancreatic stellate cells: implications in pathogenesis of pancreas fibrosis. Lab Invest. 2000;80:47-55. [Cited in This Article: ] |
| 7. | Apte MV, Haber PS, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Wilson JS. Pancreatic stellate cells are activated by proinflammatory cytokines: implications for pancreatic fibrogenesis. Gut. 1999;44:534-541. [Cited in This Article: ] |
| 8. | Mews P, Phillips P, Fahmy R, Korsten M, Pirola R, Wilson J, Apte M. Pancreatic stellate cells respond to inflammatory cytokines: potential role in chronic pancreatitis. Gut. 2002;50:535-541. [Cited in This Article: ] |
| 9. | Apte MV, Phillips PA, Fahmy RG, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Naidoo D, Wilson JS. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780-794. [Cited in This Article: ] |
| 10. | Clozel M, Salloukh H. Role of endothelin in fibrosis and anti-fibrotic potential of bosentan. Ann Med. 2005;37:2-12. [Cited in This Article: ] |
| 11. | Fitzner B, Brock P, Holzhuter SA, Nizze H, Sparmann G, Emmrich J, Liebe S, Jaster R. Synergistic growth inhibitory effects of the dual endothelin-1 receptor antagonist bosentan on pancreatic stellate and cancer cells. Dig Dis Sci. 2009;54:309-320. [Cited in This Article: ] |
| 12. | Klonowski-Stumpe H, Reinehr R, Fischer R, Warskulat U, Luthen R, Haussinger D. Production and effects of endothelin-1 in rat pancreatic stellate cells. Pancreas. 2003;27:67-74. [Cited in This Article: ] |
| 13. | Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Endothelin-1 stimulates contraction and migration of rat pancreatic stellate cells. World J Gastroenterol. 2005;11:6144-6151. [Cited in This Article: ] |
| 14. | Jaster R, Sparmann G, Emmrich J, Liebe S. Extracellular signal regulated kinases are key mediators of mitogenic signals in rat pancreatic stellate cells. Gut. 2002;51:579-584. [Cited in This Article: ] |
| 15. | Fitzner B, Sparmann G, Emmrich J, Liebe S, Jaster R. Involvement of AP-1 proteins in pancreatic stellate cell activation in vitro. Int J Colorectal Dis. 2004;19:414-420. [Cited in This Article: ] |
| 16. | Paul M, Zintz M, Bocker W, Dyer M. Characterization and functional analysis of the rat endothelin-1 promoter. Hypertension. 1995;25:683-693. [Cited in This Article: ] |
| 17. | Rodriguez-Pascual F, Redondo-Horcajo M, Lamas S. Functional cooperation between Smad proteins and activator protein-1 regulates transforming growth factor-beta-mediated induction of endothelin-1 expression. Circ Res. 2003;92:1288-1295. [Cited in This Article: ] |
| 18. | Aoki H, Ohnishi H, Hama K, Ishijima T, Satoh Y, Hanatsuka K, Ohashi A, Wada S, Miyata T, Kita H. Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2006;290:C1100-C1108. [Cited in This Article: ] |
| 19. | Aoki H, Ohnishi H, Hama K, Shinozaki S, Kita H, Yamamoto H, Osawa H, Sato K, Tamada K, Sugano K. Existence of autocrine loop between interleukin-6 and transforming growth factor-beta1 in activated rat pancreatic stellate cells. J Cell Biochem. 2006;99:221-228. [Cited in This Article: ] |
| 20. | Baumert JT, Sparmann G, Emmrich J, Liebe S, Jaster R. Inhibitory effects of interferons on pancreatic stellate cell activation. World J Gastroenterol. 2006;12:896-901. [Cited in This Article: ] |
| 21. | Haber PS, Keogh GW, Apte MV, Moran CS, Stewart NL, Crawford DH, Pirola RC, McCaughan GW, Ramm GA, Wilson JS. Activation of pancreatic stellate cells in human and experimental pancreatic fibrosis. Am J Pathol. 1999;155:1087-1095. [Cited in This Article: ] |
| 22. | Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907-921. [Cited in This Article: ] |
| 23. | Vonlaufen A, Joshi S, Qu C, Phillips PA, Xu Z, Parker NR, Toi CS, Pirola RC, Wilson JS, Goldstein D. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Res. 2008;68:2085-2093. [Cited in This Article: ] |
| 24. | Muerkoster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML, Sebens T, Kloppel G, Kalthoff H, Folsch UR. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and interleukin-1beta. Cancer Res. 2004;64:1331-1337. [Cited in This Article: ] |
| 25. | Masamune A, Satoh M, Kikuta K, Sakai Y, Satoh A, Shimosegawa T. Inhibition of p38 mitogen-activated protein kinase blocks activation of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2003;304:8-14. [Cited in This Article: ] |
| 26. | Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-alpha. Am J Physiol. 1992;262:C854-C861. [Cited in This Article: ] |
| 27. | Shek FW, Benyon RC, Walker FM, McCrudden PR, Pender SL, Williams EJ, Johnson PA, Johnson CD, Bateman AC, Fine DR. Expression of transforming growth factor-beta 1 by pancreatic stellate cells and its implications for matrix secretion and turnover in chronic pancreatitis. Am J Pathol. 2002;160:1787-1798. [Cited in This Article: ] |
| 28. | Fitzner B, Brock P, Nechutova H, Glass A, Karopka T, Koczan D, Thiesen HJ, Sparmann G, Emmrich J, Liebe S. Inhibitory effects of interferon-gamma on activation of rat pancreatic stellate cells are mediated by STAT1 and involve down-regulation of CTGF expression. Cell Signal. 2007;19:782-790. [Cited in This Article: ] |
| 29. | Sparmann G, Merkord J, Jaschke A, Nizze H, Jonas L, Lohr M, Liebe S, Emmrich J. Pancreatic fibrosis in experimental pancreatitis induced by dibutyltin dichloride. Gastroenterology. 1997;112:1664-1672. [Cited in This Article: ] |