INTRODUCTION
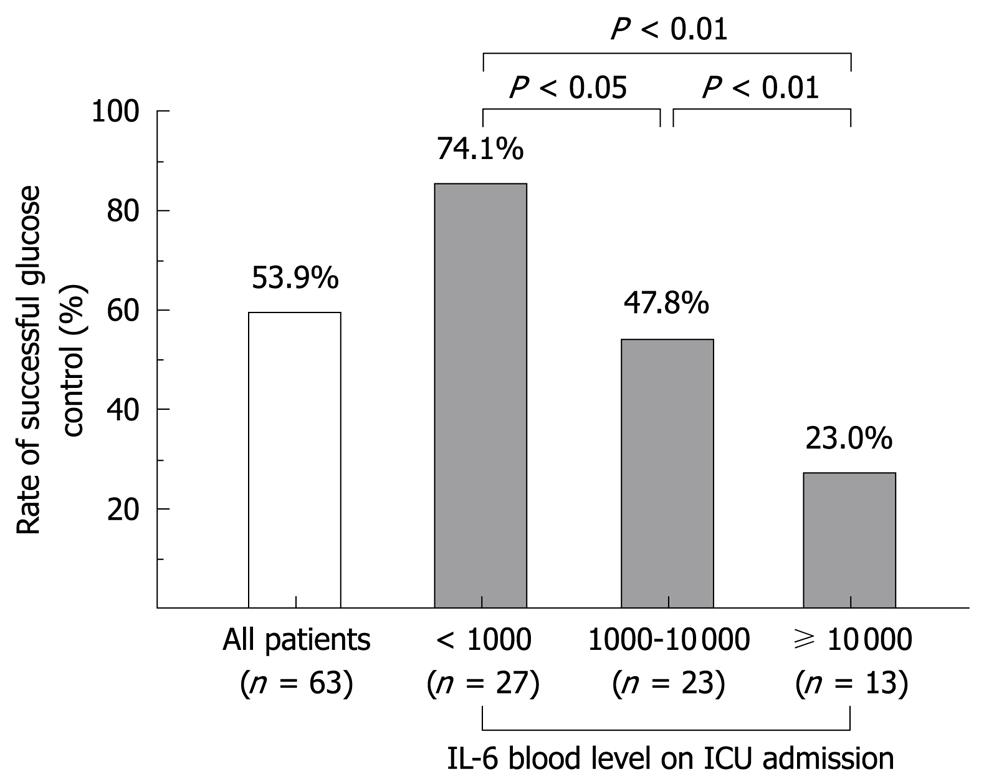
Figure 1 Correlation between IL-6 blood level on ICU admission and success rate of blood glucose control among patients with severe sepsis and septic shock.
The blood glucose level is targeted to be between 110 and 150 mg/dL with intensive insulin therapy.
There are many pathophysiological changes during severe sepsis and septic shock, and one of the most striking is metabolic derangement. Among the metabolic changes, hyperglycemia is the most important[1,2]. Accordingly therapeutic approaches to hyperglycemia in the management of severe sepsis and septic shock have had much attention. Intensive insulin therapy became popular in the intensive care unit (ICU) after Van den Berghe’s research reporting its effectiveness on glycemic control[3,4]. However, a recent large-scale randomized trial indicated that such glycemic control is not effective in reducing ICU mortality and that glycemic control with intensive insulin therapy increases the risk of hypoglycemia, and complications arising from hypoglycemia[5]. Therefore, in this paper we will discuss the effectiveness of intensive insulin therapy in the ICU and the future perspectives on tight glycemic control from the viewpoint of the correlation between inflammatory hypercytokinemia and hyperglycemia.
METABOLIC CHANGE IN SEPSIS
Recent advances in molecular biology have contributed to the tremendous progress in understanding the pathophysiology of sepsis. Now it is widely accepted that the main features of sepsis are the uncontrollable activation of not only pro-inflammatory, but also anti-inflammatory responses, because of overwhelming production of pro-inflammatory and anti-inflammatory mediators[6-8]. Such overwhelming production of mediators causes many pathological changes in vital organs and systems including metabolic changes[9]. One such metabolic change is hyperglycemia arising from muscle glycolysis and lipolysis, and subsequent gluconeogenesis and glycolysis in the liver[9-12]. The other feature of metabolic change in sepsis is hyperlactatemia due to glycolysis in muscle caused by counterregulatory hormones and cytokines, sometimes referred to as the “lactate shuttle”[9,13]. Hyperglycemia in critical illness, such as severe sepsis, is not only a marker of severity of illness and the predictor of poor outcome[1,2], but also has many kinds of adverse effects on vital organs. One such adverse effect on the innate immune system impairs the ability of the host to combat infection, resulting in reduced neutrophil activity such as chemotaxis, formation of reactive oxygen species, and phagocytosis of bacteria despite accelerated diapedesis of leukocytes into peripheral tissue, as well as specific alterations in cytokine patterns, with increased concentrations of the early proinflammatory cytokines, tumor necrosis factor-α and interleukin (IL)-6, and a reduction of endothelial nitric oxide formation[14]. Recently it has also been reported that the variability of the glucose level in blood is independently associated with hospital mortality in septic patients[15,16] and that severity of sepsis has a strong effect on glycemic variability in blood[13].
Without question, treatment of severe sepsis and septic shock starts with control of the infection source. Antibiotics, drainage of abscesses, and operations to control the source, when indicated, are essential in the initial treatment[17]. Furthermore, hemodynamic stabilization is also very important for the initial treatment of such patients[18].
On the other hand, an epoch-making paper by Van den Berghe in 2001 reported that tight glycemic control with intensive insulin therapy in the surgical ICU, to control the blood glucose level between 80 and 110 mg/dL, resulted in improvement in survival and a shortened length of hospital stay[3]. The same authors reported later that intensive insulin therapy reduced morbidity but not mortality in the medical ICU[4]. These studies[3,4] led the influential guidelines for the management of severe sepsis and septic shock to recommend tight glycemic control as one of the most important therapeutic approaches[19]. Since the publication of the guidelines, tight glycemic control in ICU patients has become popular and it is now one of the standard clinical practices in the ICU. This recommendation remained the same in the revised version of the guidelines published in 2008[20].
CLINICAL EFFECT OF GLYCEMIC CONTROL IN SEPTIC PATIENTS
Since the publication of the papers by Van den Berge[3,4], indicating that tight glycemic control between 80 and 110 mg/dL with intensive insulin therapy reduces morbidity and mortality among critically ill patients in the surgical ICU, and that the intensive insulin therapy significantly reduces morbidity but not mortality among all patients in the medical ICU, many secondary clinical trials on tight glycemic control have been carried out. A meta-analysis and systematic reviews on tight glycemic control were also published. In a review, the Van den Berghe group reconfirmed that maintaining strict normoglycemia with the use of intensive insulin improves the outcome of critically ill patients[21]. They also published a paper in which they concluded that intensive insulin therapy reduced mortality of all medical/surgical ICU patients, except those with a prior history of diabetes, that intensive insulin therapy did not cause harm, and that a blood glucose target < 110 mg/dL was most effective, but also carried the highest risk of hypoglycemia[22].
On the other hand, Brunkhorst and the SepNet group from Germany published a paper which found that use of intensive insulin therapy placed critically ill patients with sepsis at increased risk for serious adverse events related to hypoglycemia, without showing any benefit, and they stopped the trial for safety reasons[23]. Treggiari and colleagues also showed that a policy of intensive insulin therapy in a group of ICU patients was not associated with a decrease in hospital mortality, and they concluded that further study was needed prior to widespread implementation of intensive insulin therapy in critically ill patients[24]. The meta-analysis by Wiener and colleagues published in JAMA concluded that, in critically ill adult patients, tight glucose control is not associated with significantly reduced hospital mortality, but that it is associated with an increase risk of hypoglycemia[25]. Finally, the long-awaited result of the NICE-SUGAR (Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulator) trial was published recently. This concluded that intensive glucose control increased mortality among adults in the ICU and that a blood glucose target of 180 mg/dL or less resulted in lower mortality than did a target of 81-108 mg/dL[5]. Furthermore it was also pointed out that intensive insulin therapy increased the labor requirement of nursing staff in the ICU[26]. Now the statement by Merz and Finfer, that each ICU should define a blood glucose range which can be achieved without causing a significant increase in severe hypoglycemia, and which fits within the constraints of their nursing and economic resources sounds fair and acceptable[26]. They also concluded that the upper limit of glucose control should currently be 140-180 mg/dL[26]. Preiser also suggested that a blood glucose range of 80-110 mg/dL may not be normal or desirable and that lowering of blood glucose by intensive insulin therapy can induce a shortage in the provision of glucose, the predominant source of energy useable by the myocardium during ischemia, and neuroglycopenia in cases of cerebral injury[27].
MECHANISM OF BENEFICIAL OR HARMFUL EFFECTS OF TIGHT GLYCEMIC CONTROL
There are many papers which illustrate the mechanism of the beneficial effect of tight glycemic control. Wade tell us that hyperglycemia can alter cytokine production and phagocytosis both by means of hyperosmotic stress and by mechanisms other than hyperosmolality[28]. Egi from Bellomo’s group suggested that variability of glucose concentration is a significant independent predictor of ICU and hospital mortality, and that decreasing the variability of blood glucose concentration might be an important aspect of glucose management[29]. The question whether intensive insulin therapy per se or a lowered glucose level by intensive insulin therapy is the main mechanism of the beneficial effect of tight glycemic control with intensive insulin therapy has not yet been answered.
On the other hand, Jeschke et al[30] reported that insulin therapy improves the systemic inflammatory reaction to severe trauma. Vanhorebeek from Van den Berghe’s group reported that protection of hepatocyte mitochondrial ultrastructure and function is one of the mechanisms of the beneficial effect of strict blood glucose control with insulin in critically ill patients[31]. Another researcher from Van den Berghe’s group also reported that intensive insulin therapy prevented critical polyneuropathy/myopathy and the necessity for treatment with prolonged mechanical ventilation[32,33]. Dugo and colleagues showed, in an experimental study, that the inhibitory effect of insulin on the activity of glycogen synthase kinase-3β, contributed to the protective effect of insulin against organ injury/dysfunction caused by excessive systemic inflammation, independently of any effects on blood glucose[34]. Another possible mechanism of the beneficial effect of intensive insulin therapy or tight glycemic control is through RAGE (receptor of advanced glycation end product). Now RAGE is considered to play an important role in the pathophysiology of severe sepsis and septic shock. It is possible that tight glycemic control can reduce the production of AGE (advanced glycation end product), and that tight glycemic control can thereby reduce the inflammatory response mediated through AGE and RAGE interaction[35]. This could be another mechanism of the beneficial effect of tight glycemic control in severe sepsis.
There are many papers reporting that hypoglycemia during intensive insulin therapy is the main reason why intensive insulin therapy cannot show a beneficial effect on mortality in severe sepsis and septic shock. Waeschle et al[16] showed that the risk of hypoglycemia with intensive insulin therapy is very high among patients with severe sepsis and septic shock. The meta-analysis mentioned above on the benefit and risks of tight glucose control in critically ill adults by Wiener[24], indicated that intensive insulin therapy increased the risk of hypoglycemia among critically ill patients including those with severe sepsis and septic shock. Krinsley and Grover indicated that even a single episode of severe hypoglycemia was independently associated with increased risk of mortality and therefore that safe implementation of tight glycemic control requires appropriate monitoring to reduce the risk of this complication[36]. They proposed to move beyond tight glucose control to safe effective glucose control avoiding hypoglycemia[37]. The mechanism by which hypoglycemia increased mortality in severe sepsis and septic shock has not yet been fully elucidated. However, brain damage because of an energy deficit in the brain through hypoglycemia is possibly one mechanism[38].
FUTURE PERSPECTIVES ON TIGHT GLYCEMIC CONTROL
It is now clear that to benefit from tight glycemic control, we should avoid hypoglycemia. On the other hand it has also become clear that it is not so easy to keep the blood glucose level within the targeted range even though the targeted range is not very tight such as between 150 and 180 mg/dL in patients with severe sepsis and septic shock.
In our ICU, we routinely check IL-6 blood levels in every patient every day. The reason why we specifically measure IL-6 is not that we think IL-6 is the most important proinflammatory cytokine, but rather that IL-6 is the most easily measurable cytokine because of its relatively high blood level and relatively long half-time in the blood[39]. Since blood levels of many kinds of cytokines change synergistically in sepsis, we do not need to measure blood levels of various cytokines to make a diagnosis of hypercytokinemia but we can measure only one of the most easily measurable cytokines to make the diagnosis of hypercytokinemia in sepsis. It is reported that if a patient has an IL-6 level of more than 1000 pg/mL, this patient can be diagnosed with systemic inflammatory response syndrome or hypercytokinemia[40].
We applied tight glycemic control with a target blood glucose level of 110-150 mg/dL in patients with severe sepsis and septic shock. The overall success rate for tight glycemic control was only 53.9%. However when we subgrouped the patients according to the IL-6 blood level on ICU admission, we found that the success rate of tight glycemic control was relatively high in subgroups whose IL-6 blood level on ICU admission was lower than 1000 pg/mL. On the other hand, the success rate of tight glycemic control was very low among the patients whose initial IL-6 blood level in the ICU was higher than 10 000 pg/mL as shown in Figure 1. These data indicate that hypercytokinemia correlates with hyperglycemia in sepsis and that countermeasures to hypercytokinemia in sepsis would be one of the key factors for successful glycemic control. Recently, we published a paper showing the efficacy of continuous hemodiafiltration (CHDF) with a cytokine-adsorbing hemofilter made from polymethyl methacrylate (PMMA) membrane in patients with septic shock[41]. We found that, in septic patients with severe hypercytokinemia, blood glucose control became easier once we lowered the blood level of cytokines with PMMA-CHDF.
Since one of the reasons why tight glycemic control could not show a beneficial effect on critically ill patients is hypoglycemia during intensive insulin therapy, avoidance of hypoglycemia should always be considered[36,37]. For this purpose an artificial pancreas has promising potential[42]. On the other hand, a pharmacological dose of a steroid is recommended in the guidelines for the management of severe sepsis and septic shock[18,19]. However, steroid administration may create some difficulties in glycemic control in septic patients[43]. An artificial pancreas may be most effective on such patients with severe sepsis and septic shock receiving steroid therapy.
Another important issue of tight glycemic control in critically ill patients, including those with severe sepsis and septic shock, is whether tight glycemic control would also be effective in critically ill diabetics. It is proposed that precisely defined target glucose levels, treatment intervention and the avoidance of hypoglycemic episodes during insulin therapy should be studied before the widespread application of tight glycemic control in critically ill diabetic patients[44]. Egi and colleagues reported that, unlike nondiabetic patients, diabetic patients showed no clear association between hyperglycemia during the ICU stay and mortality, and there were markedly lower odds ratios of death at all levels of hyperglycemia suggesting that hyperglycemia may have different biological and/or clinical implications in critically patients with diabetes mellitus[45].
As mentioned above, it has not yet been fully elucidated whether intensive insulin therapy or normoglycemia with intensive insulin therapy really has the beneficial effect on severely septic patients. However, if normoglycemia is the key mechanism of tight glycemic control with intensive insulin therapy, modulation of glucose use and gluconeogenesis in sepsis with adrenergic β receptor blockade is one of the future approaches in this area[46].
CONCLUSION
It is now suggested that tight glycemic control with a target blood glucose level of 90-110 mg/dL does not improve clinical outcome and that less strict glycemic control with a target blood glucose level of 140-180 mg/dL is more effective. Also specific targeting of glycemic control in diabetic patients should be considered. Since there is a significant correlation between success rate of glycemic control and the degree of hypercytokinemia in septic patients, some countermeasures to hypercytokinemia may be an important aspect of successful glycemic control. Thus, in future, use of an artificial pancreas to avoid hypoglycemia during insulin therapy, special consideration of septic diabetic patients, and control of hypercytokinemia should be considered for more effective glycemic control in patients with severe sepsis and septic shock.
Peer reviewer: Raymund R Razonable, MD, Division of Infectious Diseases, Mayo Clinic, 200 First Street SW, Rochester, Minnesota 55905, United States
S- Editor Li LF L- Editor Cant MR E- Editor Zheng XMb