Published online Aug 14, 2009. doi: 10.3748/wjg.15.3748
Revised: June 30, 2009
Accepted: July 7, 2009
Published online: August 14, 2009
AIM: To evaluate in a multicenter study whether the sonographic characterization of focal liver lesions can be improved using SonoVue®-enhancement; and to compare this method with computed tomography (CT) and magnetic resonance imaging (MRI).
METHODS: One hundred and thirty four patients with one focal liver lesion detected in baseline ultrasound (US) were examined with conventional US, contrast-enhanced US (n = 134), contrast-enhanced CT (n = 115) and/or dynamic contrast-enhanced MRI (n = 70). The lesions were classified as malignant, benign or indeterminate and the type of lesion was determined. The final diagnosis based on the combined information of all imaging examinations, clinical information and histology (n = 32) was used. Comparisons were made to see whether the addition of contrast-enhanced US led to the improvement of the characterization of doubtful focal liver lesions.
RESULTS: In comparison with unenhanced US, SonoVue® markedly improves sensitivity and specificity for the characterization (malignant/benign) of focal liver lesions. In comparison with CT and/or dynamic MRI, SonoVue®-enhanced sonography applied for characterization of focal liver lesions was 30.2% more sensitive in the recognition of malignancy and 16.1% more specific in the exclusion of malignancy and overall 22.9% more accurate. In the subgroup with confirmative histology available (n = 30), sensitivity was 95.5% (CEUS), 72.2% (CT) and 81.8% (MRI), and specificity was 75.0% (CEUS), 37.5% (CT) and 42.9% (MRI). The sensitivity and specificity of CEUS for the identification of focal nodular hyperplasia (FNH) and hemangiomas was 100% and 87%, resulting in an accuracy of 94.5%.
CONCLUSION: SonoVue®-enhanced sonography emerges as the most sensitive, most specific and thus most accurate imaging modality for the characterization of focal liver lesions.
- Citation: Trillaud H, Bruel JM, Valette PJ, Vilgrain V, Schmutz G, Oyen R, Jakubowski W, Danes J, Valek V, Greis C. Characterization of focal liver lesions with SonoVue®-enhanced sonography: International multicenter-study in comparison to CT and MRI. World J Gastroenterol 2009; 15(30): 3748-3756
- URL: https://www.wjgnet.com/1007-9327/full/v15/i30/3748.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3748
Whereas un-enhanced ultrasound and color Doppler ultrasonographic examination are widely used to screen for liver lesions, theses techniques have limited performance in the characterization of solid focal tumors[1–3]. Thus contrast enhanced computed tomography (CT) or magnetic resonance imaging (MRI) is required to assess the malignancy of the tumors as basis for therapy decisions. The characterization of lesions with contrast enhanced CT or MRI is based on the vascularity and enhancement pattern within the lesion[4–7]. SonoVue® is a microbubble contrast agent of the 2nd generation, allowing the assessment of vascularity and enhancement pattern of focal lesions with ultrasound in real-time, using low mechanical index scanning technology[8–24]. Low-mechanical index (MI) real-time ultrasound in combination with SonoVue® allows the continuous assessment of tumor vascularity and enhancement during the different vascular phases (arterial, portal and late phase) with better temporal resolution than with CT or MRI[1819]. Safety and effectiveness of this agent have been proved in numerous clinical studies. In this clinical study, we compared the diagnostic performance of this technique in a multicenter study using different systems with conventional unenhanced ultrasound as well as with contrast-enhanced CT and MRI, being the current state-of-the-art methods for characterization of focal liver lesions. Contrast specific US scanning modes, equipments, manufacturers, transducers and range of low mechanical index are extremely varied in clinical practice and validation of SonoVue®-enhanced sonography in a multicenter and multi-equipments study is an essential requirement before introducing this method in routine clinical practice.
Our purpose was to evaluate in a controlled multicenter study whether the characterization of focal liver lesions can be improved by using SonoVue®-enhanced sonography, as compared with unenhanced sonography, contrast-enhanced CT and contrast-enhanced MRI.
The study population consisted of 179 patients [85 men and 94 women, mean age 55.3 years (range 19-93)] with one single focal liver lesion, detected with unenhanced sonography (screening). The lesion had to be clearly identifiable in the different modalities (US, CT, MRI) for matching of results. Exclusion criteria were age < 18 years, pregnant or lactating women, acoustic window insufficient for adequate sonographic examination of the liver, a contra-indication to SonoVue® or any of the diagnostic examinations, and/or inability to give informed consent.
This study was a phase IIIb, multicenter, multinational, open label within patient comparison in 9 centers (5 in France, 2 in Czech Republic, 1 in Belgium and 1 in Poland), performed between December 2003 and February 2005. The study was performed according to Good Clinical Practice (GCP) and the ethical principles of the declaration of Helsinki, in particular approval by the responsible Ethical Committees was obtained and all patients gave written informed consent. Due to the fact that a new imaging modality (dynamic low-MI real time sonography) was used, a technical run-in phase was performed to allow establishment of adequate machine settings with 45 patients (5 in each center). The following 134 patients were part of the main phase and constituted the intent-to-treat population. Of these 134 patients 7 patients were excluded due to the following reasons: missing reference examination (CT and MRI) in 3 patients, reference examination outside of acceptable time window (± 3 mo) in 2 patients, ultrasound examination not possible due to technical reasons in 1 patient and final reference diagnosis not supported by valid reference examinations in 1 patient. Therefore, the protocol-correct population used for efficacy analysis consisted of 127 patients [54 men and 73 women, mean age 54.8 ± 19.9 years (range 19-93), mean height 168.7 ± 8.5 cm (range 154-190), mean weight 69.7 ± 13.7 kg (range 43-106)].
All imaging examinations were performed by experienced radiologists. For the contrast-enhanced ultrasound examinations a technical training was performed, including an educational lecture, local setup of contrast-specific software and standard settings in cooperation with contrast ultrasound application specialists and examination of 5 run-in patients under technical supervision. Different US systems were used (Table 1). The sequence of the different imaging procedures (i.e. US, CT and MRI) was not fixed by the study protocol and could be arranged to the organizational situations in the different study center. All examinations should have been performed within 14 d, however in individual cases a time range of up to 3 mo was accepted if there was no treatment or indication of significant changes in between, to avoid repeated radiation exposure.
| Equipment manufacturer | Scanning mode | No. of patients scanned | Transducer | Mechanical index |
| Elegra, Siemens | Ensemble contrast imaging (ECI) | 25 | Convex array | 0.10-0.30 |
| 3.5C40H | ||||
| Aplio, Toshiba | Contrast tissue discrimination (CTD) | 24 | Convex array | 0.1 |
| PVT375AT | ||||
| HDI 5000, Philips | Pulse inversion harmonic contrast imaging (PI) | 18 | Convex array | 0.04-0.12 |
| C5-2 | ||||
| Technos MPX, Esaote | Contrast tuned imaging (CnTI) | 18 | Convex array | 0.09-0.10 |
| CA430 | ||||
| Logic 9, GE | Coded phase inversion (PI) | 16 | Convex array | 0.09-0.17 |
| 3.5C | ||||
| SSD-5500, Aloka | Extended pure harmonic detection (E-PHD) | 16 | Convex array | 0.09-0.20 |
| UST 9126 | ||||
| Sequoia, Siemens | Cadence contrast pulse sequencing (CPS) | 7 | Convex array | 0.15-0.24 |
| 4C1-S | ||||
| IU 22, Philips | Pulse inversion (PI) | 3 | Convex array | 0.06-0.07 |
| C5-2 |
First, an unenhanced sonography was performed to verify the presence of one single focal liver lesion with grey-scale imaging (B-mode). Each focal lesion was measured, localized (Couinaud segments) and the characteristic features (border, shape, echogenicity) were described. The vascularisation was assessed by color Doppler ultrasound. Representative sequences of the examination were stored digitally.
Each patient received at least 2 bolus injections of SonoVue®, a first one for characterization of the detected focal lesion and another one for detection of additional lesions. Contrast-enhanced sequences were obtained using dedicated low-MI contrast-imaging software (MI < 0.2). Standard pre-settings were used, with the possibility to adjust settings to the individual patient. SonoVue® was injected intravenously as a bolus of 2.4 mL, using a 20 gauge cannula placed in the antecubital vein, followed by a flush of 5 mL normal saline solution. Digital cine clips representing the dynamic contrast enhancement within the lesion and the surrounding liver tissue were recorded continuously, starting 5 s before the SonoVue® injection and covering the arterial (i.e. 10-45 sp inject.), portal (i.e. 60-90 sp inject.) and late (i.e. 120-150 sp inject.) phase. In case of a suspected hemangioma, additional 15 s scans were performed after 240 s and 300 s. The injection could be repeated in case of technically insufficient quality, using the same dose (2.4 mL) or double dose (4.8 mL), up to a total dose of 9.6 mL SonoVue®. All sequences were recorded and stored digitally. Intratumoral vascular geometry and enhancement pattern of the lesions were described.
The lesion was classified separately for unenhanced and SonoVue®-enhanced sonography as benign, indeterminate or malignant. In addition, the particular type of lesion was determined according to pre-defined criteria (Table 2).
| Lesion type | Arterial phase | Portal venous phase | Sinusoidal phase |
| Malignant lesions | |||
| Hepatocellular carcinoma (HCC) | Hyperenhanced, often with prominent delineation of feeding vessels around and inside of the lesion, however in well differentiated tumors sometimes only weak arterial enhancement | Iso- or hypoenhanced, usually rapid contrast wash-out | Mostly hypoenhanced, however in well differentiated tumors some portal venous enhancement may be present |
| Cholangiocarcinoma (CCC) | Moderately hyperenhanced | Iso- or hypoenhanced | Mostly hypoenhanced |
| Hypervascular metastases (MET) | Hyperenhanced, often restricted to the margin of the lesion | Iso- or hypoenhanced | Always hypoenhanced (black spots) |
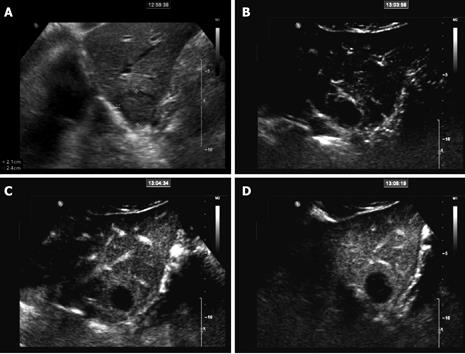
| Hypovascular metastases (MET) | Not enhanced or only few isolated spots inside of the lesion | Hypoenhanced | Always hypoenhanced (black spots) |
| Other malignant | |||
| Benign lesions | |||
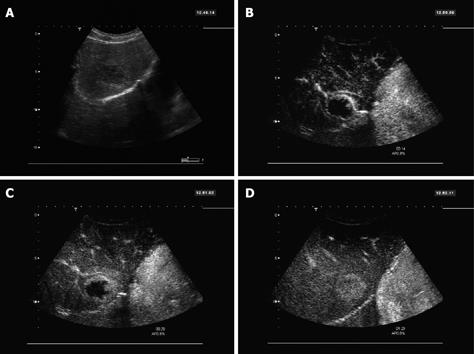
| Hemangioma (typical) | Peripheral nodular enhancement, sharp margin in high-flow hemangiomas: complete filling of the lesion during arterial phase | Slow centripetal progression of the enhancement (‘iris diaphragm sign’), leading to an iso- or hyperenhancedappearance; fill-in can be very slowly (lasting minutes) or rather fast (lasting less than a minute) | More or less complete enhancement, prolonged compared to surrounding liver tissue and therefore hyperenhanced at later time points; enhancement may be incomplete in case of (partial) thrombosis |
| Hemangioma (atypical) | Peripheral nodular enhancement, sharp margin or no enhancement (complete thrombosis) or complete enhancement (high flow hemangioma) | Slow centripetal progression of the enhancement (‘iris diaphragm sign’), leading to an iso- or hyperenhanced appearance; fill-in can be very slowly (lasting minutes) or rather fast (lasting less than a minute) | More or less complete enhancement, prolonged compared to surrounding liver tissue and therefore hyperenhanced at later time points; enhancement may be incomplete in case of (partial) thrombosis |
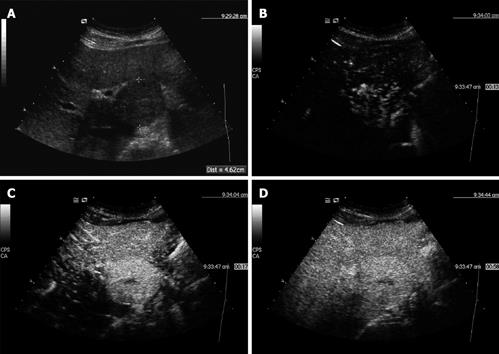
| Focal nodular hyperplasia (FNH) | Hyperenhanced, with fast centrifugal filling of the lesion; usually a central vessel and radial vascular branches can be delineated, especially in larger lesions (‘spoke and wheel sign’) | Iso- or hyperenhanced | Iso- or hyperenhanced, central scar may become visible |
| Hepatic adenoma | Hyperenhanced, frequently with fast centrifugal filling and rapid contrast wash-out; no radial vascular structures visible | Iso- or hyperenhanced; hypoenhanced areas in case of central bleeding or scar | Iso- or hyperenhanced; hypoenhanced areas in case of central bleeding or scar; no central scar or radial intralesional structures |
| Large regenerating or dysplastic nodules | Isoenhanced | Isoenhanced | Isoenhanced |
| Focal fatty accumulation | Isoenhanced | Isoenhanced | Isoenhanced |
| Focal fatty sparing | Isoenhanced | Isoenhanced | Isoenhanced |
| Cyst | No enhancement | No enhancement | No enhancement |
| Other benign |
With unenhanced sonography the classification was based on echogenicity, morphological criteria, and color Doppler signals reflecting abnormal vascularity, using the following criteria: lesion size, lesion depth, border definition (clear or blurred), shape (round, oval or irregular) and echogenicity (hypoechoic, isoechoic or hyperechoic).
With contrast-enhanced sonography, the classification was based on the dynamic enhancement pattern reflecting vascularity and perfusion pattern of the lesion, using the following criteria: vascularity (no intratumoral vessel, straight feeding vessels, irregular feeding vessels, radial intratumoral vessels/spoke and wheel sign or basket-like vessels around the lesion), enhancement pattern in arterial and portal-venous phase (hypoenhancing, isoenhancing or hyperenhancing) and homogeneity of enhancement (homogeneous or non-homogeneous). The characteristic enhancement pattern used for classification were in accordance with pattern described earlier for CT, MRI and contrast-enhanced US[17].
All patients received at least one triple phase contrast-enhanced reference examination with CT or MRI. Single-slice CT was available in 37/127 patients, multi-slice CT in 78/127 patients and dynamic MRI in 70/127 patients. In all (except one) patients having just a single-slice CT, an additional dynamic MRI examination was performed to be sure to have a proper representation of the different enhancement phases in the reference examination. In the one patient with just a single slice CT without additional MRI, all imaging examinations resulted in the same diagnosis (HCC), which was confirmed by histology, so that the reference examination was considered to be acceptable. In patients having a CT and MRI examination available, a combined tomographic diagnosis (consensus) was made.
In 32 patients, a histological examination of the lesion was available. Samples for histology could be obtained by biopsy or surgical resection, according to the clinical situation and requirements. Due to ethical reasons, no tissue samples were taken for study reasons only without clinical indication[25]. From 32 histology specimen 2 were classified as indeterminate, so that in 30 patients a histological diagnosis could be obtained.
After all examinations were completed, a final diagnostic assessment was performed, taking into consideration all-available imaging examinations and clinical information (i.e. US, CT, MRI, clinical data, biochemical markers and histology, if available) of the respective patient. This was considered to reflect the true-disease-state of the patient and used as gold standard. For the final reference diagnosis, malignancy and lesion type was determined using the same classification as described above.
All continuous variables were presented with its summary statistics (n, mean, standard deviation and range). Categorical data were given by frequency distribution tables.
Sensitivity, specificity and accuracy for the assessment of malignancy were calculated for unenhanced ultrasound, SonoVue®-enhanced ultrasound and combined CT/MRI, using the final reference diagnosis as gold standard. Indeterminate classifications were rated as incorrect classifications. Patients for whom the final reference diagnosis was indeterminate (n = 4) were excluded from these analyses. Differences between unenhanced ultrasound, SonoVue®-enhanced ultrasound and combined CT/MRI were analysed by using McNemar test two-sided test. P < 0.05 was considered as statistically significant.
Complete data for unenhanced and SonoVue®-enhanced ultrasound were obtained in 127 patients. Four patients were excluded because no decisive final reference diagnosis could be obtained (indeterminate character of the lesion), so that in 123 patients the accuracy of the ultrasound examinations versus the final reference diagnosis could be calculated.
According to the final reference diagnosis, 68 focal liver lesions were benign and 55 were malignant. The detailed lesion characters of target lesions are presented in Table 3. The number of correctly classified lesions was significantly higher with SonoVue®-enhanced ultrasound compared to unenhanced ultrasound (benign lesions: 60/68 vs 25/68, malignant lesions: 54/55 vs 22/55). This results in a sensitivity, specificity and accuracy of 98.2%, 88.2% and 92.7% for CEUS compared to 40.0%, 36.8% and 38.2% for unenhanced ultrasound, respectively. In comparison unenhanced US was significantly (P < 0.0001) less sensitive, specific and accurate than SonoVue®-enhanced ultrasound.
| Unenhanced sonography | SonoVue®-enhanced sonography | CT and/or MRI | Histology | Final reference diagnosis | |
| n | 127 | 127 | 115 | 31 | 127 |
| Benign | 25 (19.7) | 61 (48.0) | 41 (35.7) | 8 (25.8) | 68 (53.5) |
| Hemangioma (typical) | 10 (40.0) | 11 (18.0) | 12 (29.3) | 1 (12.5) | 10 (14.7) |
| Hemangioma (atypical) | 5 (20.0) | 16 (26.2) | 12 (29.3) | 1 (12.5) | 19 (27.9) |
| Focal nodular hyperplasia | 6 (24.0) | 20 (32.8) | 9 (22.0) | 3 (37.5) | 24 (35.3) |
| Hepatic adenoma | 0 | 2 (3.3) | 2 (4.9) | 2 (25.0) | 3 (4.4) |
| Regenerating or dysplastic nodules | 1 (4.0) | 2 (3.3) | 1 (2.4) | 0 | 2 (2.9) |
| Focal fatty accumulation | 0 | 2 (3.3) | 1 (2.4) | 0 | 2 (2.9) |
| Focal fatty sparing | 2 (8.0) | 3 (5.0) | 1 (2.4) | 0 | 3 (4.4) |
| Cyst | 0 | 0 | 0 | 0 | 0 |
| Other benign | 1 (4.0) | 5 (8.2) | 3 (7.3) | 1 (12.5) | 5 (7.4) |
| Malignant | 25 (19.7) | 57 (44.9) | 38 (33.0) | 21 (67.7) | 55 (43.3) |
| Hepatocellular carcinoma | 14 (56.0) | 34 (59.7) | 21 (55.3) | 13 (61.9) | 33 (60.0) |
| Cholangiocarcinoma | 0 | 0 | 0 | 1 (4.8) | 1 (1.8) |
| Hypervascular metastasis | 2 (8.0) | 9 (15.8) | 4 (10.5) | 2 (9.5) | 7 (12.7) |
| Hypovascular metastasis | 9 (36.0) | 10 (17.5) | 10 (26.3) | 3 (14.3) | 10 (18.2) |
| Other malignant lesion | 0 | 4 (7.0) | 3 (7.9) | 2 (9.5) | 4 (7.3) |
| Indeterminate | 77 (60.6) | 9 (7.1) | 34 (29.6) | 2 (6.5) | 4 (3.2) |
In 2 patients, no CT/MRI evaluation of the target lesion could be obtained. Therefore, a comparison of the accuracy of SonoVue®-enhanced ultrasound and combined CT/MRI examination could be performed in 121 patients.
Correct classifications of benign lesions could be obtained with CEUS in 59/67 lesions and with CT/MRI in 50/67 lesions. Correct classifications of malignant lesions could be obtained with CEUS in 53/54 lesions and with CT/MRI 37/54 lesions. This results in a sensitivity, specificity and accuracy of 98.2%, 88.1% and 92.6% for CEUS compared to 68.5%, 74.6% and 71.9% for CT/MRI. In comparison CT/MRI was significantly less sensitive (P < 0.0001), less specific (P < 0.029) and less accurate (P < 0.0001) than SonoVue®-enhanced ultrasound. In the subgroup of patients with histological diagnosis as part of the final reference standard (n = 30) the excellent performance of SonoVue®-enhanced ultrasound could be confirmed. Sensitivity, specificity and accuracy of the 3 modalities were 95.5%, 75.0% and 90.0% for CEUS, 72.7%, 37.5% and 63.3% for CT and 81.8%, 42.9% and 66.6% for MRI, respectively.
Looking on the false positive classifications (malignant or indeterminate instead of benign) of the different methods CEUS was indeterminate in 5 patients and wrongly classified two FNH (as HCC and hypervascular metastasis) and one adenoma (as hypovascular metastasis). CT was indeterminate in 17 patients and missed the lesion in 2 patients. MRI was indeterminate in 11 patients. With regard to false negative findings (benign or indeterminate instead of malignant), CEUS was indeterminate in 1 patient with an HCC. CT was indeterminate in 17 patients and misdiagnosed 1 hypovascular metastasis as hemangioma. MRI was indeterminate in 9 patients, missed one hypervascular metastasis and misdiagnosed one hypovascular metastasis as hemangioma.
Safety assessment was performed in all patients receiving SonoVue® (n = 179). Four adverse events were reported, 3 with mild and 1 with moderate intensity. Three of these adverse events were local reactions at the injection site (2 pain and 1 pruritic rash), from which 2 (1 pain and 1 rash; 1.1% of the patients population) were considered to be possibly related to the contrast agent. One adverse event was systemic (nausea), but this was considered related to a recent cholecystectomy and not to the study agent. The related adverse events were of mild intensity and both patients recovered spontaneously without sequelae.
Characterization of a focal liver lesion requires the assessment of morphological characteristics as well as vascularity and enhancement patterns within the lesion. Therefore, the administration of a contrast agent, demonstrating the intratumoral vascularity and blood flow, gives essential information for the characterization of focal liver lesions. In CT and MRI, the acquisition of contrast-enhanced images in different phases is a well-established standard procedure[4–6]. In ultrasound, the standard approach for focal lesion characterization includes only the use of color-coded Doppler imaging, which is not able to demonstrate microvascular flow and dynamic enhancement patterns[1–3]. With the introduction of second generation ultrasound contrast agents like SonoVue®, in combination with appropriate scanner technology (low-MI real-time contrast imaging), the assessment of intratumoral vascularity and dynamic enhancement pattern became possible, comparable to the information obtained by CT and dynamic MRI[1726–28].
Contrast-enhanced imaging with ultrasound has two major advantages: (1) the microbubble contrast agents are real blood-pool agents not leaving the intravascular space and (2) a continuous imaging over the whole enhancement period with high temporal resolution is possible, not limited to distinct, pre-defined time points[1819]. Furthermore contrast-enhanced ultrasound has many other advantages, such no exposure to radiation, and absence of nephrotoxic contrast agents and large availability of machines.
There are already several studies published, demonstrating the safety and efficacy of this method for the diagnosis of focal liver lesions, however most of these studies were single-center studies performed with just one type of machine[91026]. This clinical study investigated the diagnostic performance of SonoVue®-enhanced ultrasound in the characterization of focal liver lesion in an international multicenter and multi-equipment setting in comparison to established CT and MRI examinations, using a final reference diagnosis based on all available imaging, histology and clinical information as gold standard.
The results of this study confirm the excellent performance of real-time contrast-enhanced ultrasound for focal liver lesion characterization which is clearly superior to that of unenhanced ultrasound, as shown already in other studies with SonoVue® and other microbubble contrast agents[10–1726–29]. Furthermore, this study demonstrates, that the diagnostic accuracy of SonoVue®-enhanced ultrasound is even better than that of contrast-enhanced CT and MRI. The markedly improved accuracy of SonoVue®-enhanced compared to unenhanced sonography (+ 54.5%) is statistically significant (P < 0.0001, McNemar’s test) and of high clinical relevance. Usually, sonography is the first-line examination for the assessment of focal liver lesions and the high number of ‘indeterminate’ evaluations found with unenhanced ultrasound causes the high number of follow-up examinations found in today’s clinical practice, along with all the costs and discomfort for the patient. The comparable low accuracy for unenhanced sonography in our study could be explained by the high number of patients with malignant lesions (43.3%) in our study population, which was intended to obtain a balanced study population for characterization but does not reflect the distribution in an unselected study population. In our study population, benign lesions with typical B-Mode appearance (cysts, typical hemangiomas, typical FNH), which are easy to characterize based on morphological criteria, were less frequent, making the characterization more difficult compared to an unselected patient population. However, even in an unselected patient population unenhanced sonography is known to be clearly less accurate than the 92.7% accuracy obtained in our study with SonoVue®-enhanced Sonography[1–3].
For the diagnosis of benign lesions (especially FNH and hemangioma), correct diagnosis is the only issue since usually no further activities are required. Therefore, incorrect classification of a malignant lesion is the main concern. In our study, there was only one false-negative characterization with SonoVue®-enhanced sonography (1 out of 55 patients with malignancies), a lesion which was diagnosed as indeterminate instead of malignant. The tumor was an HCC with hypoenhancement in arterial and portal venous phase. The final diagnosis could only be obtained by histology, since also with CT just an indeterminate diagnosis could be made due to the non-characteristic enhancement pattern. For exclusion of malignancy we observed 5 indeterminate and 3 false malignant classifications out of 68 patients with benign lesion. All of these lesions corresponded to atypical enhancement patterns (4 FNH, 1 adenoma, 2 hemangioma, and 1 hepatic fibrosis).
SonoVue®-enhanced ultrasound turned out to be even more sensitive, specific, and accurate for the characterization of doubtful focal liver lesions compared to CT/MRI. Percentage point differences were 29.7% for sensitivity (recognition of malignancy), 13.6% for specificity (exclusion of malignancy), and 20.8% for accuracy. Sensitivity was nearly perfect for the SonoVue®-enhanced sonography (98.2%) in comparison to the much lower sensitivity of CT/ MRI (68.5%). The higher accuracy of SonoVue®-enhanced sonography may be explained by the real-time capabilities of dynamic low-MI contrast sonography, allowing a continuous assessment of the whole enhancement period, whereas in CT and MRI spiral scans at distinct time points (usually early arterial, late arterial, portal-venous and - in MRI - late phase)[4–6]. Especially the very early contrast period (8-12 s post injection) is usually missed with CT/MRI, but may provide crucial diagnostic information especially in highly arterialized lesions. For example, this early arterial phase was particularly useful for the FNH demonstrating the centrifugal hyper-enhancement with a central vessel and radial vascular branches (Figure 1), for haemangioma showing the very early nodular enhancement (Figure 2) or for hypovascular metastasis to demonstrate few isolated spots in the lesions with marked wash-out in portal and late phase (Figure 3). Furthermore, the blood-pool characteristics of SonoVue® prevent the extravasation of the contrast agent (present in conventional CT and MRI contrast agents), which may obscure vascular flow information especially at later time points (equilibrium phase in CT/MRI)[26]. The data collected in this study suggest that contrast-enhanced sonography is the best imaging modality for the characterization (malignant/benign) of focal liver lesions.
A limitation of this study is the lack of an off-site assessment with blinded and separated unenhanced and combined unenhanced/contrast-enhanced sequences, to evaluate the accuracy of the pure image sequences without any clinical patient information and with validated blinding with regard to reference examinations. Information about patient’s characteristics (e.g. laboratory values) may influence the pre-test probability for malignancy and thus facilitate the classification by imaging. However, this reflects the situation in routine clinical practice. The improvement of the sonographic characterization with SonoVue® can be explained by the additional information on vascularity and perfusion obtained with the analysis of the dynamic enhancement pattern of the lesions, which is standard in CT and MRI examinations (generally using contrast enhanced sequences for characterization) but not in conventional sonography (just assessing morphological criteria and Doppler signals in large vessels). An unenhanced examination should be considered as inadequate for the characterization of focal liver lesions, which can’t be clearly classified as benign due to unequivocal morphological criteria. Contrast-enhanced ultrasound should be the preferred first-line examination for the characterization of such lesions, followed by supplementary CT/MRI examinations or histological confirmation, if required.
The characterization of liver lesions with imaging is based on the examination of the vascularity and enhancement pattern within the lesion. SonoVue® is an Ultrasonographic contrast agent allowing the assessment of vascularity and enhancement pattern of focal lesions with ultrasound in real-time.
The authors demonstrate the higher accuracy of contrast-enhanced sonography related to the real-time capabilities of dynamic contrast sonography, allowing a continuous assessment of the whole enhancement period, whereas in computed tomography (CT) and in magnetic resonance imaging (MRI) spiral scans at distinct time points (usually early arterial, late arterial, portal-venous and - MRI) -late phase).
There are already several studies published, demonstrating the safety and efficacy of contrast-enhanced ultrasonography for the diagnosis of focal liver lesions. However most of these studies were single-center studies performed with just one type of machine. This clinical study investigated the diagnostic performance of contrast-enhanced ultrasound in the characterization of focal liver lesion in an international multicenter and multi-equipment setting in comparison to established CT and MRI examinations.
Contrast-enhanced ultrasound should be the preferred first-line examination for the characterization of liver lesions, followed by supplementary CT/MRI examinations or histological confirmation, if required.
SonoVue® is an ultrasonographic contrast agent composed by micro bubbles. It is used with specific contrast-enhanced sequences obtained using low-mechanical index (MI) imaging software (MI < 0.2). Low-mechanical index or low acoustic pressure is used to do not destroy the microbubbles too fast to allow multiphasic imaging. The real-time capability of sonography in comparison with CT or MRI is related to a better temporal resolution of this technique.
The authors evaluated in a controlled multicenter study whether the characterization of focal liver lesions can be improved by using SonoVue®-enhanced sonography, as compared with unenhanced sonography, contrast-enhanced CT and contrast-enhanced MRI. Contrast-enhanced sonography emerges as the most sensitive, most specific and thus most accurate imaging modality for the characterization of focal liver lesions.
| 1. | Reinhold C, Hammers L, Taylor CR, Quedens-Case CL, Holland CK, Taylor KJ. Characterization of focal hepatic lesions with duplex sonography: findings in 198 patients. AJR Am J Roentgenol. 1995;164:1131-1135. |
| 2. | Lee MG, Auh YH, Cho KS, Chung YH, Lee IC, Kang EM. Color Doppler flow imaging of hepatocellular carcinomas. Comparison with metastatic tumors and hemangiomas by three-step grading for color hues. Clin Imaging. 1996;20:199-203. |
| 3. | Wernecke K, Rummeny E, Bongartz G, Vassallo P, Kivelitz D, Wiesmann W, Peters PE, Reers B, Reiser M, Pircher W. Detection of hepatic masses in patients with carcinoma: comparative sensitivities of sonography, CT, and MR imaging. AJR Am J Roentgenol. 1991;157:731-739. |
| 4. | Freeny PC, Marks WM. Patterns of contrast enhancement of benign and malignant hepatic neoplasms during bolus dynamic and delayed CT. Radiology. 1986;160:613-618. |
| 5. | Petersein J, Spinazzi A, Giovagnoni A, Soyer P, Terrier F, Lencioni R, Bartolozzi C, Grazioli L, Chiesa A, Manfredi R. Focal liver lesions: evaluation of the efficacy of gadobenate dimeglumine in MR imaging--a multicenter phase III clinical study. Radiology. 2000;215:727-736. |
| 6. | Seltzer SE, Getty DJ, Pickett RM, Swets JA, Sica G, Brown J, Saini S, Mattrey RF, Harmon B, Francis IR. Multimodality diagnosis of liver tumors: feature analysis with CT, liver-specific and contrast-enhanced MR, and a computer model. Acad Radiol. 2002;9:256-269. |
| 7. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. |
| 8. | Schneider M. Characteristics of SonoVuetrade mark. Echocardiography. 1999;16:743-746. |
| 9. | Leen E. The role of contrast-enhanced ultrasound in the characterisation of focal liver lesions. Eur Radiol. 2001;11 Suppl 3:E27-E34. |
| 10. | Spinazzi A, Llull JB. Diagnostic performance of SonoVue-enhanced color duplex sonography of vascular structures. Acad Radiol. 2002;9 Suppl 1:S246-S250. |
| 11. | Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del Maschio A, Summaria V, Maresca G, Pezzoli C, Llull JB. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol. 2002;41:200-206. |
| 12. | Leen E, Ceccotti P, Kalogeropoulou C, Angerson WJ, Moug SJ, Horgan PG. Prospective multicenter trial evaluating a novel method of characterizing focal liver lesions using contrast-enhanced sonography. AJR Am J Roentgenol. 2006;186:1551-1559. |
| 13. | Catala V, Nicolau C, Vilana R, Pages M, Bianchi L, Sanchez M, Bru C. Characterization of focal liver lesions: comparative study of contrast-enhanced ultrasound versus spiral computed tomography. Eur Radiol. 2007;17:1066-1073. |
| 14. | Dietrich CF, Kratzer W, Strobe D, Danse E, Fessl R, Bunk A, Vossas U, Hauenstein K, Koch W, Blank W. Assessment of metastatic liver disease in patients with primary extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J Gastroenterol. 2006;12:1699-1705. |
| 15. | Ricci P, Laghi A, Cantisani V, Paolantonio P, Pacella S, Pagliara E, Arduini F, Pasqualini V, Trippa F, Filpo M. Contrast-enhanced sonography with SonoVue: enhancement patterns of benign focal liver lesions and correlation with dynamic gadobenate dimeglumine-enhanced MRI. AJR Am J Roentgenol. 2005;184:821-827. |
| 16. | Bleuzen A, Tranquart F. Incidental liver lesions: diagnostic value of cadence contrast pulse sequencing (CPS) and SonoVue. Eur Radiol. 2004;14 Suppl 8:P53-P62. |
| 17. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. |
| 18. | Nicolau C, Vilana R, Catalá V, Bianchi L, Gilabert R, García A, Brú C. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol. 2006;186:158-167. |
| 19. | Wilson SR, Burns PN. An algorithm for the diagnosis of focal liver masses using microbubble contrast-enhanced pulse-inversion sonography. AJR Am J Roentgenol. 2006;186:1401-1412. |
| 20. | Liu GJ, Xu HX, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Enhancement pattern of hepatocellular carcinoma: comparison of real-time contrast-enhanced ultrasound and contrast-enhanced computed tomography. Clin Imaging. 2006;30:315-321. |
| 21. | Celli N, Gaiani S, Piscaglia F, Zironi G, Camaggi V, Leoni S, Righini R, Bolondi L. Characterization of liver lesions by real-time contrast-enhanced ultrasonography. Eur J Gastroenterol Hepatol. 2007;19:3-14. |
| 22. | Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol. 2008;48:848-857. |
| 23. | Kim SH, Lee JM, Lee JY, Han JK, An SK, Han CJ, Lee KH, Hwang SS, Choi BI. Value of contrast-enhanced sonography for the characterization of focal hepatic lesions in patients with diffuse liver disease: receiver operating characteristic analysis. AJR Am J Roentgenol. 2005;184:1077-1084. |
| 24. | Wilson SR, Jang HJ, Kim TK, Iijima H, Kamiyama N, Burns PN. Real-time temporal maximum-intensity-projection imaging of hepatic lesions with contrast-enhanced sonography. AJR Am J Roentgenol. 2008;190:691-695. |
| 25. | Kim SH, Lim HK, Lee WJ, Cho JM, Jang HJ. Needle-tract implantation in hepatocellular carcinoma: frequency and CT findings after biopsy with a 19.5-gauge automated biopsy gun. Abdom Imaging. 2000;25:246-250. |
| 26. | Burns PN, Wilson SR. Focal liver masses: enhancement patterns on contrast-enhanced images--concordance of US scans with CT scans and MR images. Radiology. 2007;242:162-174. |
| 27. | Wilson SR, Burns PN. Liver mass evaluation with ultrasound: the impact of microbubble contrast agents and pulse inversion imaging. Semin Liver Dis. 2001;21:147-159. |
| 28. | Solbiati L, Martegani A, Leen E, Correas JM, Burns PN, Becker D. Contrast-enhanced ultrasound of liver diseases. Milan: Springer-Verlag 2003; . |
| 29. | Fan ZH, Chen MH, Dai Y, Wang YB, Yan K, Wu W, Yang W, Yin SS. Evaluation of primary malignancies of the liver using contrast-enhanced sonography: correlation with pathology. AJR Am J Roentgenol. 2006;186:1512-1519. |