Published online Aug 7, 2009. doi: 10.3748/wjg.15.3670
Revised: June 17, 2009
Accepted: June 24, 2009
Published online: August 7, 2009
AIM: To investigate contrast-enhanced ultrasound (CEUS) for early diagnosis of postoperative vascular complications after right-lobe living donor liver transplantation (RLDLT).
METHODS: The ultrasonography results of 172 patients who underwent RLDLT in West China Hospital, Sichuan University from January 2005 to June 2008 were analyzed retrospectively. Among these 172 patients, 16 patients’ hepatic artery flow and two patients’ portal vein flow was not observed by Doppler ultrasound, and 10 patients’ bridging vein flow was not shown by Doppler ultrasound and there was a regional inhomogeneous echo in the liver parenchyma upon 2D ultrasound. Thus, CEUS examination was performed in these 28 patients.
RESULTS: Among the 16 patients without hepatic artery flow at Doppler ultrasound, CEUS showed nine cases of slender hepatic artery, six of hepatic arterial thrombosis that was confirmed by digital subtraction angiography and/or surgery, and one of hepatic arterial occlusion with formation of lateral branches. Among the two patients without portal vein flow at Doppler ultrasound, CEUS showed one case of hematoma compression and one of portal vein thrombosis, and both were confirmed by surgery. Among the 10 patients without bridging vein flow and with liver parenchyma inhomogeneous echo, CEUS showed regionally poor perfusion in the inhomogeneous area, two of which were confirmed by enhanced computed tomography (CT), but no more additional information about bridging vein flow was provided by enhanced CT.
CONCLUSION: CEUS may be a new approach for early diagnosis of postoperative vascular complications after RLDLT, and it can be performed at the bedside.
- Citation: Luo Y, Fan YT, Lu Q, Li B, Wen TF, Zhang ZW. CEUS: A new imaging approach for postoperative vascular complications after right-lobe LDLT. World J Gastroenterol 2009; 15(29): 3670-3675
- URL: https://www.wjgnet.com/1007-9327/full/v15/i29/3670.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.3670
A right-lobe living donor liver transplantation (RLDLT), with or without the middle hepatic vein (MHV), is now used generally for adult-to adult liver transplantation[1]. A right liver graft only involves part of the liver lobes from healthy donors, whose vessels are very slender and the reconstruction of one or more tributaries of the MHV (bridging vein) is sometimes involved. RLDLT can be complicated by a high incidence of postoperative vascular complications, which may cause graft dysfunction and liver failure. Therefore, early diagnosis of postoperative vascular complications is very important after RLDLT. As noninvasive examinations, 2D and Doppler ultrasound play important roles in screening for postoperative complications, but vascular visualization is not always satisfactory[2–4]. Contrast-enhanced ultrasound (CEUS) has been applied gradually in recent years and has provided a great improvement for this defect, because of its fine vascular tracing and perfusion visualization[5–8].
The present study analyzed retrospectively CEUS in patients who underwent RLDLT in West China Hospital, Sichuan University from January 2005 to June 2008, to investigate the diagnostic value of CEUS in postoperative vascular complications.
The study was conducted under the approval of the Ethics Committee of West China Hospital, and written informed consent was obtained from all patients who received CEUS examination. We analyzed retrospectively the ultrasound results of 172 consecutive patients (137 men and 35 women, aged 18-63 years, mean 39.1 years), who underwent RLDLT from January 2005 to June 2008 in West China Hospital. Bypass of MHV tributaries was performed in 83 cases. The primary diseases included 98 with cirrhosis, 58 with malignant liver tumors, 12 with acute liver failure, one with hepatic echinococcosis, one with Budd-Chiari syndrome, and two cases liver re-transplantation.
Phillips HDI5000 and HD 11 (Phillips Medical Systems, Bothell, WA, USA) with 2-5-MHz probes were used in our study as conventional ultrasound equipment. CEUS examinations were performed with Acuson Sequoia 512 with contrast pulse sequencing (Siemens Medical Solutions, Mountain View, CA, USA) or Phillips IU 22 (Phillips Medical Systems) with the pulse inversion technique. Gray-scale ultrasound was used to observe liver graft parenchyma, and portal and hepatic veins, and Doppler ultrasound was used to measure blood flow and its velocity in the hepatic artery, portal and hepatic veins, and bridging vein. CEUS was performed in patients without hepatic artery and/or portal vein flow and/or patients without bridging vein flow accompanied by liver regional inhomogenous echo. The aim of CEUS was to observe blood flow in the above vessels, as well as perfusion of the liver. Conventional Doppler ultrasound and CEUS were performed by two experienced ultrasound physicians with more than 5 years experience in liver transplantation. Ultrasonography was performed everyday within the first week after operation in the intensive care unit (ICU), and afterwards, the time and interval of ultrasound examination depended on laboratory results and clinical conditions. The contrast agent was SonoVue (Bracco Imaging, Milan, Italy), which consists of an inert gas, sulfur hexafluoride and phospholipid peplos with high flexibility. CEUS was performed using 1.2-2.4 mL SonoVue suspension by rapid bolus injection through the left ulnar vein, followed by 10 mL saline flush and an ultrasonic inner time counter started simultaneously. Dynamic and static images were recorded to observe patency of the hepatic artery, portal and hepatic veins, and bridging vein, as well as perfusion of the liver parenchyma.
Among these 172 patients, 16 patients’ hepatic artery flow and two patients’ portal vein flow was not observed by Doppler ultrasound, and 10 patients’ bridging vein flow was not shown by Doppler ultrasound and there was a regional inhomogeneous echo in the liver parenchyma upon 2D ultrasound. Thus, CEUS examination was performed in these 28 patients.
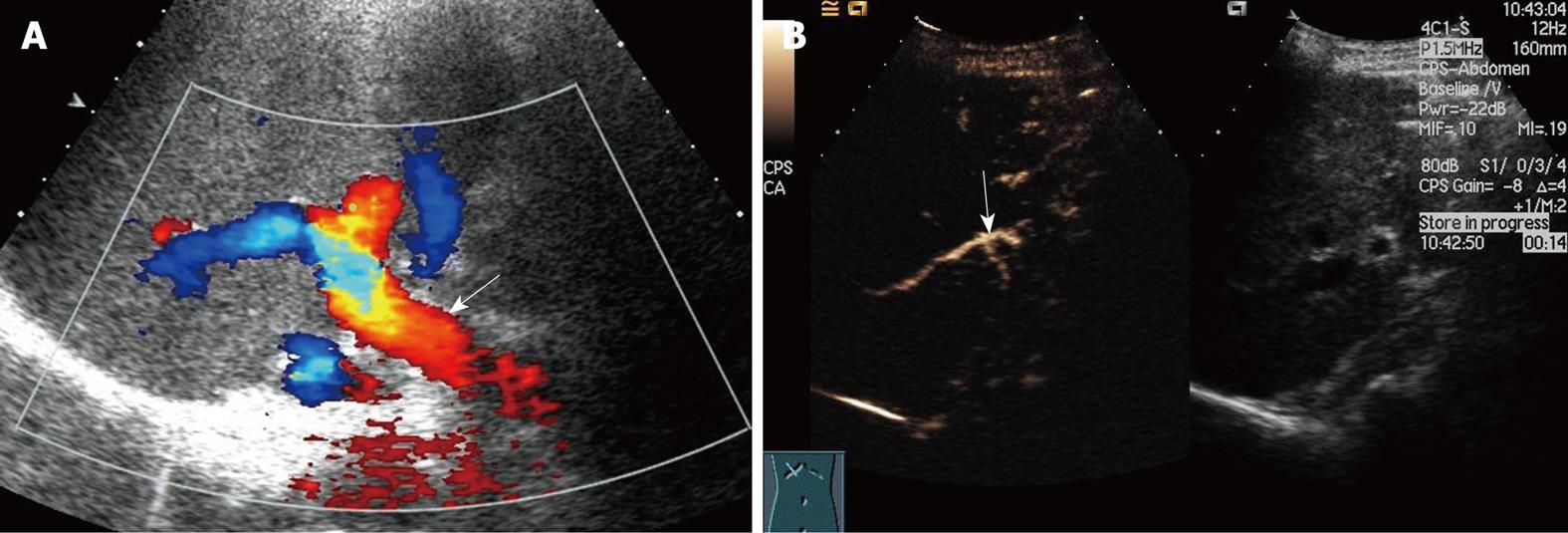
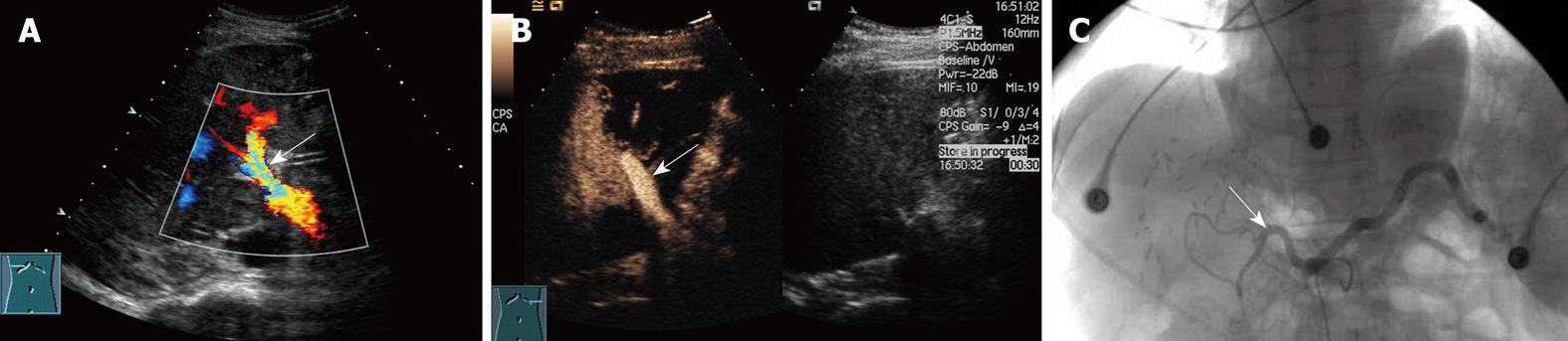
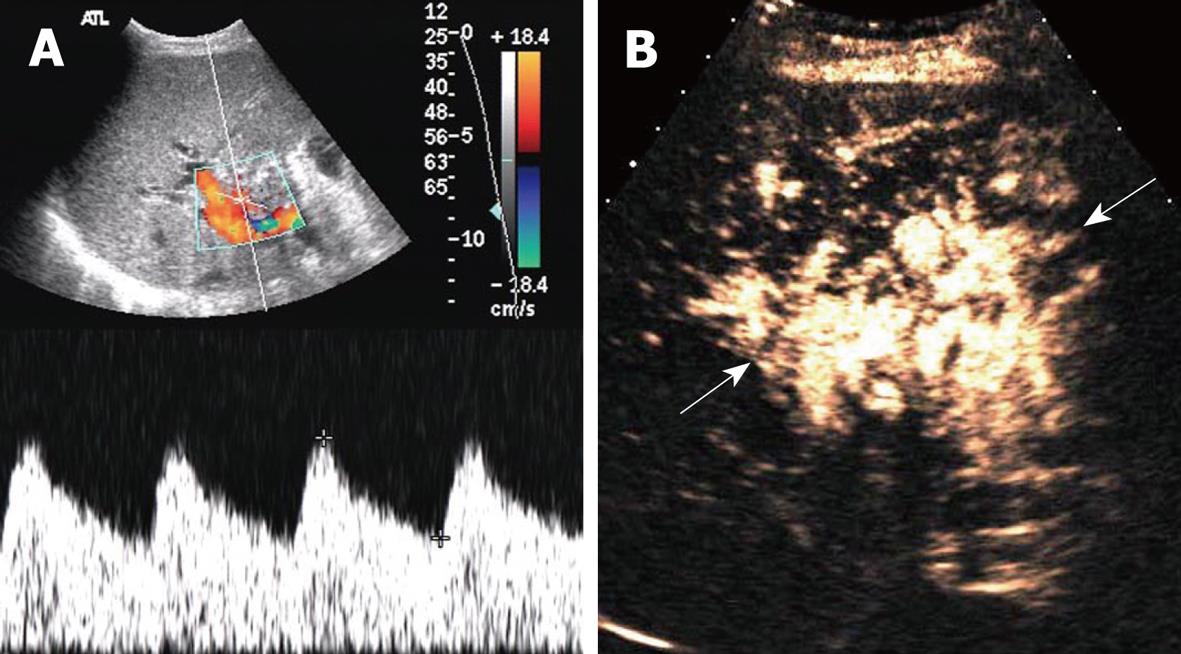
Among the 16 patients without hepatic artery flow at Doppler ultrasound, CEUS showed a slender right hepatic artery in nine (Figure 1), hepatic artery thrombosis (HAT) in six (Figure 2), all confirmed by digital subtraction angiography and/or surgery, hepatic artery occlusion accompanied by lateral branches in one (Figure 3).
Among the two patients without portal vein flow at Doppler ultrasound, CEUS showed one with hematoma compression and one with portal vein thrombosis, both of which were confirmed by surgery.
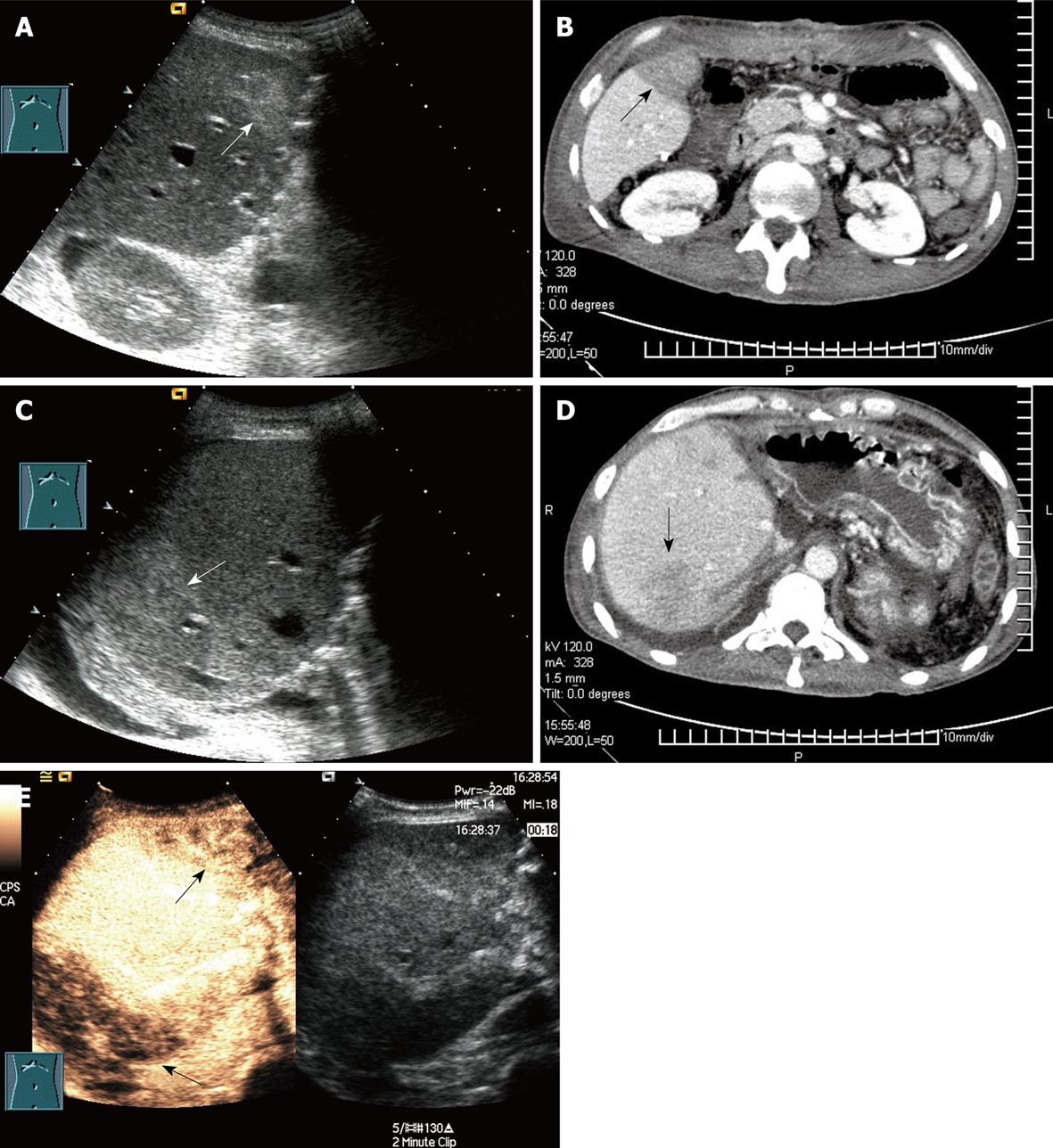
The 10 patients without bridging vein flow and with regional liver inhomogenous echo at Doppler ultrasound were demonstrated by CEUS to have corresponding poor liver graft perfusion. The diagnosis was confirmed by enhanced CT in two cases. However, CT scan did not yield any additional information on bridging vein visualization (Figure 4).
Hepatic artery complications, including HAT, hepatic artery stenosis (HAS) and pseudoaneurysm, are some of the most severe complications after liver transplantation, with an incidence of 5%-15%. HAT often occurs early after surgery, especially within 6 wk. Mortality from HAT is as high as 20%-60% and re-transplantation is usually required[2]. Therefore, early examination and diagnosis after surgery are crucial for graft survival and good prognosis. Ultrasound manifestations of HAT include the disappearance of blood flow at the porta hepatis, disappearance of or abnormal intrahepatic blood flow, such as the tardus-parvus spectrum wave form, possibly followed by secondary changes including biliary complications, hepatic infarction and abscess. HAT is highly suspected if no blood flow signal is present in the hepatic artery at Doppler ultrasound. However, false-positive results must be excluded, and the main reasons include instrumental insensitivity to blood flow, improper adjustment of parameters, incorrect detection approach, early transient artery spasm, and slender graft arteries. HAT sensitivity of Doppler ultrasound has been reported to be 60%-90%[2–49–17]. Color Doppler technology has improved considerably during the past 10 years, and the possibility of non-flow visualization in the hepatic artery has decreased. In the present study, hepatic flow was not demonstrated in 1.3% (16/1231) of scans, similar to the study of Hom et al[5]. Visualization of the hepatic artery is improved significantly by CEUS. The accuracy of CEUS in diagnosing HAT was 100% in our study, similar to previous studies[5–818].
The incidence of HAS is 5%-10%, which occurs mostly at the anastomotic stoma. Severe stenosis may result in similar outcomes to HAT, such as biliary ischemia, graft dysfunction and liver failure. Tardus-parvus changes (RI < 0.5 and accelerating time > 0.08 s) detected at Doppler ultrasound suggest HAS, and it is also suspected when regional blood flow velocity is > 200 m/s at the anastomotic stoma. However, it is difficult to observe directly the stenosis site by ultrasound for intestinal gas or liver artery curvature. The value of CEUS in diagnosing HAS remains controversial. Some studies have revealed that CEUS may be more capable than conventional ultrasound of visualizing the trunk of the hepatic artery, possible sites of stenosis, and peripheral branch circulation[2–4710–1218].
Portal vein complications include portal vein thrombus (PVT), stenosis and phlebangioma. The incidence of PVT and stenosis is about 1%-2%. Monitoring of the portal vein after surgery is also important, because the blood supply from portal vein is essential for graft survival after liver transplantation. Once PVT or severe stenosis in the portal vein of liver grafts occurs after surgery, patients may suffer from primary graft dysfunction, visceral bleeding, bile leakage, ascites, splenectasis, and re-transplantation, re-operation or interventional therapy are required. PVT is not difficult to diagnose using 2D and Doppler ultrasound. CEUS is helpful when the trunk or branches of the portal vein are not well visualized as a result of surgery or other causes[2–57819–21].
Caliber mismatch between the portal vein of the donor and recipient is very common, and is manifested as a hyperechoic ring at the anastomotic stoma and regional blood flow disorder by Doppler ultrasound. Currently, the diagnostic criteria for portal vein stenosis include: regional stenosis with a diameter of 2.5 mm, blood flow aliasing and acceleration at the stenosis site, blood flow velocity > 150 cm/s at the stenosis site, or a prestenotic:stenotic blood flow velocity ratio of > 4:1, accompanied by symptoms of portal hypertension, including splenomegaly, ascites and branched circulation formation. CEUS is useful for diagnosis of portal vein complications, because it can determine clearly the diameter of the portal vein and clarify the severity and range of portal vein stenosis[2–57819–21].
Outflow tract in RLDLT includes the hepatic vein and bridging vein. Hepatic vein complications after surgery include thrombosis (HVT) and stenosis (HVS), with an incidence of about 1%. HVT is not difficult to diagnose by conventional ultrasound, but the diagnosis of HVS by Doppler ultrasound remains controversial. It has been reported that obvious stenosis can be observed by 2D ultrasound, when the ratio of prestenotic/stenotic blood flow velocity is higher than 3-4:1, the distal blood flow spectrum is straight and blood flow is decelerated or reversed, and the negative predictive value of phase loss in HVS is reported to be 98.4%. However, other studies have concluded that ultrasound is inaccurate for the diagnosis of HAS, which should be combined with a pressure gradient for the hepatic vein and inferior vena cava, or depend on clinical manifestations[2–422–25].
As RLDLT without the MHV has been performed increasingly in recent years, reconstruction and bypass of MHV tributaries have received more attention from clinicians, because “small-for-size syndrome” may be caused by early occlusion of bridging veins. However, these vessels are so tiny and deep that postoperative monitoring is difficult. CEUS improve the visualization of these bridging vessels and the perfusion of their drainage area[25–27]. However, there were only 10 patients who underwent bridging vessel monitoring by CEUS in our study, and thorough investigations with a larger sample size are required to clarify its diagnostic value.
Conventional 2D and Doppler ultrasound play important roles in screening and early diagnosis of vascular complications after RLDLT, but their inability to depict flow in some patients remains a substantial problem. Our initial experience shows that CEUS improves visualization of hepatic artery, portal and hepatic vein and bridging vein blood flow, as well as evaluation of perfusion of the liver graft parenchyma. Furthermore, CEUS can be performed at the bedside, and it has no iodine allergy or X-ray exposure. Therefore, CEUS provides a new approach in monitoring postoperative vascular complications after RLDLT, and more studies are needed for further evaluation.
Right-lobe living donor liver transplantation (RLDLT) is considered to be complicated liver surgery, with a high incidence of postoperative vascular complications that may cause graft dysfunction and liver failure. Noninvasive 2D and Doppler ultrasound play important roles in screening for postoperative complications, but vascular visualization is not always satisfactory. Contrast-enhanced ultrasound (CEUS) provides a great improvement for this defect because of its fine vascular tracing and perfusion visualization. Therefore, the authors investigated if CEUS improved the diagnosis of postoperative vascular complications after RLDLT.
CEUS has been applied gradually in recent years and focuses mainly on characterization of focal liver lesion and ablation monitoring. CEUS has the ability to show macro- as well as microvasculature, but there have been more studies based microvasculature.
CEUS can improve flow visualization of the hepatic artery, portal and hepatic veins and bridging vein, as well as evaluation of perfusion of liver graft parenchyma after RLDLT.
RLDLT is now generally used for adult-to adult liver transplantation worldwide. CEUS provides a new approach in monitoring postoperative vascular complications after RLDLT.
This is a good descriptive study in which the authors analyzed the value of CEUS in vascular complications after ALDLT. The results suggest that CEUS can be performed at the bedside; it has no iodine allergy, no X-ray exposure, and can be repeated with little interference to the vessels. Thus, CEUS will be a new advanced and noninvasive technique for vascular complications in RLDLT.
| 1. | Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074-1082. |
| 2. | Vaidya S, Dighe M, Kolokythas O, Dubinsky T. Liver transplantation: vascular complications. Ultrasound Q. 2007;23:239-253. |
| 3. | Crossin JD, Muradali D, Wilson SR. US of liver transplants: normal and abnormal. Radiographics. 2003;23:1093-1114. |
| 4. | Asakura T, Ohkohchi N, Katoh H, Orii T, Kikuchi H, Sekiguchi S, Kawagishi N, Takayama J, Oikawa K, Satomi S. Doppler ultrasonography in living-related liver transplantation. Transplant Proc. 1998;30:3190-3194. |
| 5. | Hom BK, Shrestha R, Palmer SL, Katz MD, Selby RR, Asatryan Z, Wells JK, Grant EG. Prospective evaluation of vascular complications after liver transplantation: comparison of conventional and microbubble contrast-enhanced US. Radiology. 2006;241:267-274. |
| 6. | Caiado AH, Blasbalg R, Marcelino AS, da Cunha Pinho M, Chammas MC, da Costa Leite C, Cerri GG, de Oliveira AC, Bacchella T, Machado MC. Complications of liver transplantation: multimodality imaging approach. Radiographics. 2007;27:1401-1417. |
| 7. | Sidhu PS, Marshall MM, Ryan SM, Ellis SM. Clinical use of Levovist, an ultrasound contrast agent, in the imaging of liver transplantation: assessment of the pre- and post-transplant patient. Eur Radiol. 2000;10:1114-1126. |
| 8. | Herold C, Reck T, Ott R, Schneider HT, Becker D, Schuppan D, Hahn EG. Contrast-enhanced ultrasound improves hepatic vessel visualization after orthotopic liver transplantation. Abdom Imaging. 2001;26:597-600. |
| 9. | Vivarelli M, Cucchetti A, La Barba G, Bellusci R, De Vivo A, Nardo B, Cavallari A, Pinna AD. Ischemic arterial complications after liver transplantation in the adult: multivariate analysis of risk factors. Arch Surg. 2004;139:1069-1074. |
| 10. | Uzochukwu LN, Bluth EI, Smetherman DH, Troxclair LA, Loss GE Jr, Cohen A, Eason JD. Early postoperative hepatic sonography as a predictor of vascular and biliary complications in adult orthotopic liver transplant patients. AJR Am J Roentgenol. 2005;185:1558-1570. |
| 11. | Vit A, De Candia A, Como G, Del Frate C, Marzio A, Bazzocchi M. Doppler evaluation of arterial complications of adult orthotopic liver transplantation. J Clin Ultrasound. 2003;31:339-345. |
| 12. | De Gaetano AM, Cotroneo AR, Maresca G, Di Stasi C, Evangelisti R, Gui B, Agnes S. Color Doppler sonography in the diagnosis and monitoring of arterial complications after liver transplantation. J Clin Ultrasound. 2000;28:373-380. |
| 13. | Chen W, Facciuto ME, Rocca JP, Marvin MR, Sheiner PA, Rachlin S, Rodriguez MI. Doppler ultrasonographic findings on hepatic arterial vasospasm early after liver transplantation. J Ultrasound Med. 2006;25:631-638. |
| 14. | Nolten A, Sproat IA. Hepatic artery thrombosis after liver transplantation: temporal accuracy of diagnosis with duplex US and the syndrome of impending thrombosis. Radiology. 1996;198:553-559. |
| 15. | Dodd GD 3rd, Memel DS, Zajko AB, Baron RL, Santaguida LA. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology. 1994;192:657-661. |
| 16. | Fistouris J, Herlenius G, Bäckman L, Olausson M, Rizell M, Mjörnstedt L, Friman S. Pseudoaneurysm of the hepatic artery following liver transplantation. Transplant Proc. 2006;38:2679-2682. |
| 17. | Kim HJ, Kim KW, Kim AY, Kim TK, Byun JH, Won HJ, Shin YM, Kim PN, Ha HK, Lee SG. Hepatic artery pseudoaneurysms in adult living-donor liver transplantation: efficacy of CT and Doppler sonography. AJR Am J Roentgenol. 2005;184:1549-1555. |
| 18. | Sidhu PS, Ellis SM, Karani JB, Ryan SM. Hepatic artery stenosis following liver transplantation: significance of the tardus parvus waveform and the role of microbubble contrast media in the detection of a focal stenosis. Clin Radiol. 2002;57:789-799. |
| 19. | Settmacher U, Nüssler NC, Glanemann M, Haase R, Heise M, Bechstein WO, Neuhaus P. Venous complications after orthotopic liver transplantation. Clin Transplant. 2000;14:235-241. |
| 20. | Woo DH, Laberge JM, Gordon RL, Wilson MW, Kerlan RK Jr. Management of portal venous complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:233-239. |
| 21. | Jia YP, Lu Q, Gong S, Ma BY, Wen XR, Peng YL, Lin L, Chen HY, Qiu L, Luo Y. Postoperative complications in patients with portal vein thrombosis after liver transplantation: evaluation with Doppler ultrasonography. World J Gastroenterol. 2007;13:4636-4640. |
| 22. | Darcy MD. Management of venous outflow complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:240-245. |
| 23. | Kim KW, Kim TK, Kim SY, Kim MJ, Park MS, Lee MG, Lee SG. Doppler sonographic abnormalities suggestive of venous congestion in the right lobe graft of living donor liver transplant recipients. AJR Am J Roentgenol. 2007;188:W239-W245. |
| 24. | Ko EY, Kim TK, Kim PN, Kim AY, Ha HK, Lee MG. Hepatic vein stenosis after living donor liver transplantation: evaluation with Doppler US. Radiology. 2003;229:806-810. |
| 25. | Kim SY, Kim KW, Lee SS, Song GW, Hwang S, Kim PN, Lee SG. Doppler sonography to diagnose venous congestion in a modified right lobe graft after living donor liver transplantation. AJR Am J Roentgenol. 2008;190:1010-1017. |
| 26. | Yan L, Wu H, Chen Z, Luo Y, Lu Q, Zhang Z, Zhao J, Wang W, Ma Y, Wen T. Intrahepatic venous collaterals formation following outflow block in adult-to-adult living donor liver transplantation. J Surg Res. 2008;146:172-176. |