Published online Jan 14, 2009. doi: 10.3748/wjg.15.248
Revised: October 28, 2008
Accepted: November 4, 2008
Published online: January 14, 2009
We report a case of endocrine cell carcinoma in the sigmoid colon with inferior mesenteric vein (IMV) tumor embolism. A 79-year-old woman was admitted to our hospital with narrowing of the stools. We performed colonoscopy, computed tomography and positron emission tomography, which disclosed sigmoid colon cancer with IMV tumor embolism. She underwent sigmoidectomy and lymph node dissection. The tumor was diagnosed as endocrine cell carcinoma (type 4, pSS, med, INF&agr;, v3, n1, stage IIIb). Immunohistochemically, chromographin A, synaptophysin, cytokeratin 20 and mucicarmine showed partial staining, and CD56 was totally reactive. Three months after operation multiple liver metastases appeared. She was treated with chemotherapy of cisplatin (CDDP) + irinotecan (CPT11). This case highlights the aggressiveness of endocrine cell carcinoma with tumor embolism, and it is essential to establish an accurate diagnosis and effective treatment.
- Citation: Tanoue Y, Tanaka N, Suzuki Y, Hata S, Yokota A. A case report of endocrine cell carcinoma in the sigmoid colon with inferior mesenteric vein tumor embolism. World J Gastroenterol 2009; 15(2): 248-251
- URL: https://www.wjgnet.com/1007-9327/full/v15/i2/248.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.248
Endocrine cell carcinoma of the colon and rectum is uncommon, and accounts for less than 1% of colorectal cancer[1]. It is well known that colorectal carcinoma of common pathology has a relatively good prognosis, whereas endocrine cell carcinoma of the colon and rectum has a very poor prognosis. Many patients with endocrine cell carcinoma have liver and lymph node involvement at the time of diagnosis.
Tumor embolism is an uncommon complication in digestive system cancers. The majority of cases of tumor embolism are related to renal carcinoma and liver carcinoma, not colon and rectum carcinoma. In fact, there has never been a report of endocrine cell carcinoma of the colon and rectum complicated by tumor embolism. Treatment of endocrine cell carcinomas of the colon and rectum are very difficult. We, herein, report the malignant potential, and the treatment of colorectal endocrine cell carcinoma with tumor embolism of the inferior mesenteric vein (IMV).
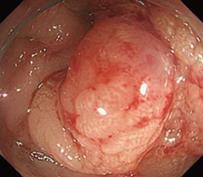
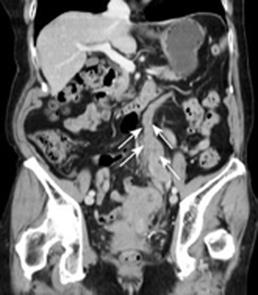
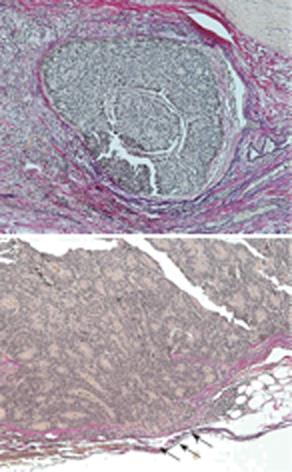
A 79-year-old woman experienced narrowing of the stools that lasted for more than a few days. She had been well since she complained of intermittent abdominal pain. She had no remarkable past medical history. Laboratory findings showed no abnormalities. Colonoscopy disclosed a circumferential type 2 lesion in the sigmoid colon (Figure 1). However, biopsy of this tumor revealed no malignant lesion, only regenerative colon mucosa. Abdominal computed tomography (CT) and positron emission tomography (PET) showed hypertrophy of the sigmoid colon wall with fusiform enlargement of the IMV extending up to the confluence with the splenic vein and a few lymph node metastases in the mesocolon (Figure 2). There were no liver metastases. These findings suggested that she had sigmoid colon cancer (cT3N2M0 Stage IIIB) with IMV tumor embolism. Considering the possibility of progressive cancer obstruction and tumor embolism, we decided to perform radical sigmoidectomy and lymph node dissection without preoperative chemotherapy. Perioperatively, we paid special attention to make an incision in the IMV first before the tumor was isolated. The specimen of tumor was a 11.5 cm × 3.5 cm, type 4 cancer (Figure 3A), pSS, med, INF&agr;, v3, PM0, DM0, RM0, DX with tumor embolism in the IMV, and the clinical stage was stage IIIB pT3N1M0 in the UICC TNM classification. The tumor embolism in the IMV was a solid lesion 14 cm long and 2 cm across in transverse section (Figure 3A and B). Microscopically, it was seen that the tumor had invaded the vein wall and expanded into the extra space (Figure 4).
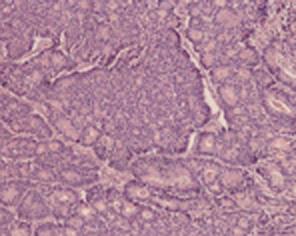
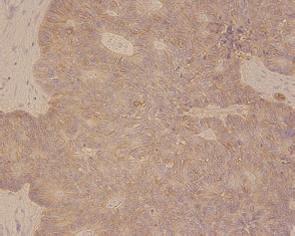
Histological features included an irregular pattern of the nuclei in size and mitotis (Figure 5). The tumor was diagnosed pathologically as an endocrine cell carcinoma histochemically, using CD56, chromographin A, synaptophysin, cytokeratin 7, cytokeratin 20 and mucicarmine. Chromographin A, synaptophysin, cytokeratin 20 and mucicarmine were partly stained and CD56 was totally reactive (Figure 6).
The postoperative course was mostly uneventful. However, three months after operation, multiple liver metastasis appeared. Now, she is being treated with chemotherapy of cisplatin (CDDP) + irinotecan (CPT11) and still alive after 14 mo.
This case is the first report of a sigmoid colon endocrine cell carcinoma invading into IMV as a tumor embolism. Endocrine cell carcinomas of the colon and rectum are uncommon, comprising less than 1% of colon and rectal cancer[1]. Endocrine cell carcinomas do not include carcinoid tumors that have a benign course compared to adenocarcinoma and endocrine cell carcinoma. Iwafuchi et al have proposed four classifications of endocrine cell carcinoma derived from: (1) preexisting general-histological adenocarcinomas; (2) preexisting carcinoids; (3) nonneoplastic multipotential stem cells and (4) nonplastic immature endocrine cells[2]. Recently, it has been thought that endocrine cell carcinomas predominantly arise from endocrine precursor cell clones occurring in preexisting adenocarcinoma components, transforming into endocrine cell carcinomas during rapid clonal expansion under the influence of p53 gene alteration[3].
From 1986 to 2007, a total of 87 cases of endocrine cell carcinomas of the colon and rectum were reported in Japan (Table 1). The average patient age was 60.8 years (range, 34-89 years). There were 44 males and 43 females. Tumors were located as follows: 2 in appendix, 9 in cecum, 17 in ascending colon, 9 in transverse colon, 2 in descending colon, 2 in sigmoid colon and 47 in rectum. It suggests that endocrine cell carcinomas arise in any part of the colon, but particularly in rectum. The lesions invaded into subserosa or pericolic tissue (51/66; 77%). The rate of vascular invasion (32/44; 72%) was also very high. Endocrine cell carcinomas are aggressive neoplasms easily invading and expanding, which are reported to occur frequently with metastasis and carry a poor prognosis. It was reported that the liver metastasis rate of 52.1% was higher compared to general colon and rectal carcinoma which had a rate of 10%[4]. Actual 1-year survival rates were 39%[5]. Therefore, one would predict the presence of vascular invasion to be associated with an increased incidence of liver metastasis and cause poor prognosis.
| Clinical features of 87 patients with endocrine cell carcinoma | |
| Characteristic | |
| Sex M/F (n = 87) | 44/43 |
| Average age (Yr, n = 87) | 60.4 (34-89) |
| Location ( n = 87) | Appendix: 2 |
| Cecum: 9 | |
| Ascending colon: 17 | |
| Transverse colon: 9 | |
| Descending colon: 2 | |
| Sigmoid colon: 1 | |
| Rectum: 47 | |
| Depth (n = 66) | m-sm: 5 |
| Mp: 10 | |
| a1/ss: 20 | |
| a2/se: 23 | |
| ai/si: 8 | |
| Lym (n = 44) | Positive 38 |
| Negative 6 | |
| V (n = 44) | Positive 32 |
| Negative 12 | |
| N (n = 59) | Positive 46 |
| Negative 13 | |
| H (n = 56) | Positive 11 |
| Negative 45 | |
| Prognosis (death n = 56) | > 6 mo: 22 |
| > 1 year: 19 | |
| >= 1 year: 15 | |
The phenomenon of tumor embolism of colon and rectal carcinoma is rare. As regards our research on tumor embolism of colon and rectum cancers in the mesenteric vein, we could find only 14 case reports including our case in Japanese papers (Table 2). In these, it was considered that histologically immature carcinomas were more likely to be complicated by a tumor embolism; but, there have been no reports of endocrine cell carcinoma showing mesenteric vein tumor embolism.
| Age | Sex | Location | Histology | Depth | Size (mm) | H | Recurrent | Prognosis |
| 50 | F | A/SMV | Poor | - | 10 × 50 | - | Liver, lung | 4 mo |
| 53 | M | S/IMV | Mod | - | - | + | Liver, local | - |
| 77 | F | T/SMV | Poor | - | 15 × 40 | - | - | - |
| 62 | M | A/SMV | Poor | se | 8 | - | None | - |
| 65 | F | A/SMV | Mod | si | - | - | None | 7 mo alive |
| 68 | M | S/IMV | Mod | a2 | 5 × 50 | - | None | 24 mo alive |
| 75 | M | A/SMV | - | - | 40 | - | None | - |
| 82 | F | A/SMV | Mod | - | - | - | - | - |
| 67 | M | A/SMV | Mod | se | - | + | Liver | - |
| 56 | F | A/SMV | Muc | si | - | - | - | - |
| 78 | F | R/IMV | Well | se | 10 × 185 | - | None | - |
| 47 | F | T/SMV | Mod | - | - | - | - | 5 mo alive |
| 78 | F | A/SMV | Poor | ss | - | - | - | 5 mo |
| 79 | F | S/IMV | End | se | - | - | Liver | 9 mo alive |
Little is known about the causes of tumor embolism of colon and rectum carcinomas; but, we consider that they involve vessel invasion. The biological and pathological processes of tumor emboli formation consis of several phases: (1) tumor growth in the primary lesion; (2) invasion of the primary tumor into the surrounding vessels; (3) detachment of tumor cells from the primary lesion; (4) dissemination of tumor cells by blood flow; (5) adhesion of tumor cells to endothelial cells or tumor embolism formation. In these processes, the metastatic potential of tumor cells depends in part on the ability to undergo cell aggregation, leading to the embolization of tumor cells in the microcapillaries, and additionally in macro-vessels. A strong correlation has been demonstrated between in vitro aggregation and in vivo metastatic potential[6]. Poorly differentiated cell carcinomas including endocrine cell carcinomas which are well known to have strong aggregation potential related to adhesion factors, for example integrin or CD56, are thought to form a tumor embolism easily.
Because of this invasion propensity, endocrine cell carcinomas develop distant metastasis, particularly in the liver. It is not clearly known whether such metastasis can be prevented or not; but, it has been suggested that early ligation of the tumor invading the vein in the operation is useful.
The treatment strategy for patients with endocrine cell carcinomas is to detect the origin and metastases at an early stage, and to monitor carefully after the operation because of its malignant potential. Surgical resection remains the mainstay of treatment, with modest impact on survival. In cases of metastasis or cases of adjuvant therapy, the literature suggests that treatment be initiated using CDDP + CPT11 based on lung small cell carcinoma[78]. A response rate of 41.5%[9] and median survival range of 10.4 mo have been reported[10]. In another report, four of 11 patients with endocrine cell carcinomas who were treated with S-1 survived for over 2 years after surgery[1112].
In conclusion, this case highlights the aggressiveness of endocrine cell carcinomas of the colon and rectum. We showed that endocrine cell carcinomas are clinically more aggressive than colorectal adenocarcinomas, and they are capable of rapid distant spread; so, the prognosis is generally worse. So far, many studies have emphasized prognostic features, immunohistochemical characteristics, and pitfalls in diagnosis and treatment of endocrine cell carcinoma[13]. Practically, there are uncharted territories left. Further improvement in diagnosis and treatment is awaited.
| 1. | Yaziji H, Broghamer WL Jr. Primary small cell undifferentiated carcinoma of the rectum associated with ulcerative colitis. South Med J. 1996;89:921-924. |
| 2. | Iwafuchi M, Watanabe H, Ishihara N, Noda Y, Ajioka Y. Histopathology of carcinoid tumor and endocrine cell carcinoma in gastrointestinal tract (in Japanese). Clin Gastroenterol. 1990;5:1669-1681. |
| 3. | Nishikura K, Watanabe H, Iwafuchi M, Fujiwara T, Kojima K, Ajioka Y. Carcinogenesis of gastric endocrine cell carcinoma: analysis of histopathology and p53 gene alteration. Gastric Cancer. 2003;6:203-209. |
| 4. | Yasunori I, Shigeru S, Yousuke U, Hiroyuki I, Motomichi U, Hiroyuki K, Tsuguo Y, Fujihiko S. A case of endocrine cell carcinoma of the ascending colon. Prog Digest Endosc. 2003;62:122-123. |
| 5. | Lotan R, Raz A. Low colony formation in vivo and in culture as exhibited by metastatic melanoma cells selected for reduced homotypic aggregation. Cancer Res. 1983;43:2088-2093. |
| 6. | Moertel CG, Kvols LK, O’Connell MJ, Rubin J. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68:227-232. |
| 7. | Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346:85-91. |
| 8. | Mitry E, Baudin A, Ducreux M, Sabourin JC, Rufie P, Aparicio1 T, Lasser P, Elias D, Duvillard P, Schlumberger M. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer. 1999;81:1351-1355. |
| 9. | Bernick PE, Klimstra DS, Shia J, Minsky B, Saltz L, Shi W, Thaler H, Guillem J, Paty P, Cohen AM. Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum. 2004;47:163-169. |
| 10. | Vilor M, Tsutsumi Y, Osamura RY, Tokunaga N, Soeda J, Ohta M, Nakazaki H, Shibayama Y, Ueno F. Small cell neuroendocrine carcinoma of the rectum. Pathol Int. 1995;45:605-609. |
| 11. | Burke AB, Shekitka KM, Sobin LH. Small cell carcinomas of the large intestine. Am J Clin Pathol. 1991;95:315-321. |
| 12. | Koide N, Suzuki A, Saito H, Sato T, Murakami M, Ota H, Miyagawa S. Gastric small cell carcinoma successfully treated by surgery and postoperative chemotherapy consisting of cisplatin and S-1: report of a case. Surg Today. 2007;37:989-994. |