Published online Apr 21, 2009. doi: 10.3748/wjg.15.1816
Revised: March 12, 2009
Accepted: March 17, 2009
Published online: April 21, 2009
AIM: To investigate the inhibitory effect of acetylshikonin on human gastric carcinoma cell line SGC-7901 and its mechanism.
METHODS: MTT assay was used to assess the inhibitory effect of acetylshikonin on proliferation of SGC-7901 cells. Apoptosis-inducing effect was determined by flow cytometry and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end-labeling with Hoechst staining. Expression of mRNA and protein in Bcl-2 and Bax was analyzed by reverse transcription-polymerase chain reaction and Western blot. Antitumor effect of acetylshikonin on a mouse SGC-7901 model was also determined.
RESULTS: Forty-eight hours after treatment with acetylshikonin, MTT assay showed that acetylshikonin inhibited the proliferation of SGC-7901 cells in a dose-dependent manner. The half maximal inhibitory concentration of acetylshikonin to SGC-7901 cells was 0.428 ± 0.07 mg/L. Cell shrinkage, nuclear pyknosis and chromatin condensation, which are the characteristics of cell apoptosis, were observed in treated SGC-7901 cells and the percentage of apoptosis increased in a dose-dependent manner. Acetylshikonin down-regulated the expression of Bcl-2 and up-regulated the expression of Bax in the treated SGC-7901 cells compared with the controls. The experiment in vivo showed that 0.5, 1, and 2 mg/kg of acetylshikonin significantly inhibited the growth of tumor in the mouse SGC-7901 model, with an inhibitory rate of 25.00%-55.76%.
CONCLUSION: Acetylshikonin inhibits the growth of SGC-7901 cells in vitro and in vivo by inducing cell apoptosis.
-
Citation: Zeng Y, Liu G, Zhou LM. Inhibitory effect of acetylshikonin on human gastric carcinoma cell line SGC-7901
in vitro andin vivo . World J Gastroenterol 2009; 15(15): 1816-1820 - URL: https://www.wjgnet.com/1007-9327/full/v15/i15/1816.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1816
Although advances have been made in clinical medicine, no effective treatment modalities are available for patients with advanced or metastatic tumors[1]. Gastric cancer is one of the most common malignant tumors worldwide, and its incidence and mortality rank first in China[23]. At present, gastric cancer patients have certain clinical responses to chemotherapy or radiation therapy, but they cannot tolerate it well[45]. Therefore, it is absolutely necessary to explore drugs capable of preventing and treating gastric cancer and other malignancies.
Chemotherapy is one of the main treatment modalities for advanced cancer[6]. It has been shown that acetylshikonin, one of the shikonin derivatives, is effective against sterilized and infected wounds[78]. It has been demonstrated that acetylshikonin can inhibit tumor-induced cutaneous angiogenesis[9] and has obvious antitumor effects on S180 sarcoma model[10]. However, little is known about the effect of acetylshikonin on gastric cancer in vitro, especially in vivo. This study was to evaluate the ability of acetylshikonin to inhibit cell proliferation and induce apoptosis of human gastric cancer SGC-7901 cells. Our results demonstrate that acetylshikonin could induce apoptosis by down-regulating Bcl-2 and up-regulating Bax in SGC-7901 cells. Significant antitumor effects of acetylshikonin could also be observed in a SGC-7901 tumor xenograft model of nude mice.
Human gastric carcinoma cell line SGC-7901 was obtained from the Institute of Materia Medica, North Sichuan Medical College (Nanchong, China). The cells were cultured in RPMI 1640 (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Minhai Bioengineering Co., Lanzhou, China), and maintained at 37°C in a humidified atmosphere containing 50 mL/L CO2. Acetylshikonin was obtained from Huakang Pharmaceutical Company (Deyang, China). Cyclophosphamide was provided by Hengrui Pharmaceutical Company (Jiangsu, China). MTT was purchased from Sigma Chemical Company (St.louis, MO, USA).
Effect of acetylshikonin on the viability of SGC-7901 cells was detected by MTT assay[11]. Cells were plated in a 96-well plate (5 × 103 cells/well) for 24 h, and then treated with different concentrations of acetylshikonin (0.05, 0.1, 0.2, 0.4, 0.8, 1.6, 3.2, 6.4 mg/L). Tetrazolium dye, 3-(4, 5-dimethylthiazol-2)-2, 5-diphenyl-2H-tetrazolium bromide (MTT, Sigma; 5 mg/mL in PBS) was added to the medium for 4 h, and measured by spectrophotometry after 15 min at room temperature. Optical density (OD) which reflects the viable cell population in each well was determined.
For apoptosis studies, cells were plated on glass cover slips. Samples were assessed 24 h after plated for the detection of TUNEL-positivity (brown cellular nuclei appeared) using an in situ cell death detection kit (Roche Diagnostic Corp., USA). Apoptotic rates of cells were also determined with Hoechst staining and cells showing condensed chromatin were defined as apoptotic[12]. Percentage of apoptotic cells in each sample was counted under microscope and averaged over 10 fields (80-100 cells each).
Cells were harvested and pelleted at 1000 ×g for 5 min, then fixed in 70% ethanol and washed with cold PBS. After suspended in 1 mL propidium, iodide solution (50 &mgr;g/mL), 0.1% (w/v) sodium citrate, and 0.1% Triton X-100. Cells were incubated at 4°C in the dark for at least 15 min and analyzed with a flow cytometer (FACS, Beckman Coulter, USA) to determine the number of cells at each cell cycle and cell apoptosis rate as previously described[11].
Expression of apoptosis-related proteins (Bcl-2 and Bax) was analyzed by Western blotting as previously described[11]. In brief, the cells were lysed in a lysis buffer at 4°C by sonication. Lysates were centrifuged at 15 000 r/min for 15 min at 4°C. Proteins were separated on SDS-PAGE, transferred to nitrocellulose filters and incubated with antibodies against Bcl-2, Bax and actin, respectively. The membrane, were incubated with peroxidase-conjugated secondary antibodies, and detected with an enhanced chemiluminescence reagent.
Expression of Bcl-2 and Bax was semi-quantitatively detected by reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was isolated from the cells using the TRIzol reagent (Gibco) and reverse transcribed with the SuperScript first-strand synthesis system (Invitrogen) according to the manufacturer’s instructions. Sequences of the primers used for RT-PCR are listed in Table 1. PCR for Bcl-2 was performed at 94°C for 3 min, followed by 30 cycles at 94°C for 30 s, at 70°C for 30 s, at 72°C for 45 s and a final extension at 72°C for 7 min. PCR for Bax was performed at 95°C for 3 min, followed by 30 cycles at 95°C for 30 s, at 57°C for 30 s, at 72°C for 1 min, and a final extension at 72°C for 7 min. PCR products were separated on 10 g/L agarose gel stained with ethidium bromide and observed under ultraviolet light.
| Product size | Sense primer | Anti-sense primer | |
| Bax | 311 bp | 5′GCGTCCACCCAAGAAGCTGAG3′ | 5′ACCACCCTGGTCTTGGATCC3′ |
| Bcl-2 | 280 bp | 5′TGTGGCCTTCTTTGAGTTCG3′ | 5′TCACTTGTGGCTCAGATAGG3′ |
| β-actin | 300 bp | 5′TCACCCACACTGTGCCCATCTACGA3′ | 5′CAGCGGAACCGCTCATTGCCAATGG3′ |
Seven-week old male nude mice (supplied by Experimental Animal Center, West China Center of Medical Sciences, Sichuan University, China) were inoculated subcutaneously with transplanted human SGC-7901 gastric carcinoma cell line (5 × 106 cells per mouse). Tumors were allowed to develop to about 75 mm × 75 mm × 75 mm. Thirty mice were randomized into 6 groups (n = 5). Mice in 3 acetylshikonin treatment groups received 0.5, 1 and 2 mg/kg acetylshikonin suspended in castor oil; mice in the castor oil group received an equal volume of castor oil; mice in control group received an equal volume of normal saline (NS), once a day for 15 consecutive days. Mice in the positive control group received cyclophosphamide (60 mg/kg) only on the first day. The animals were sacrificed and their tumors removed and weighed immediately. Tumor inhibitory rate was calculated according to the following formula: tumor inhibitory rate (%) = 1-(Wtreated/Wcontrol) × 100%[11].
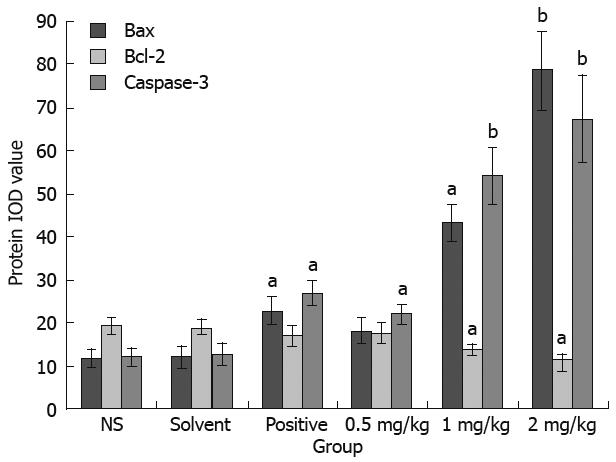
Thirty tumor tissue samples were fixed in 10% buffered formalin and embedded in paraffin. Tumor tissue was cut into 4-&mgr;m thick sections. The sections were stained as previously described[13]. Expressions of Bax, Bcl-2 and Caspase 3 were observed under an inverted phase contrast microscope. Photos were taken and analyzed by Image pro plus, and the integrated optical density (IOD) was calculated.
Data were expressed as mean ± SD. Statistical analysis was performed using Student’s t test and chi-square test. P < 0.05 was considered statistically significant.
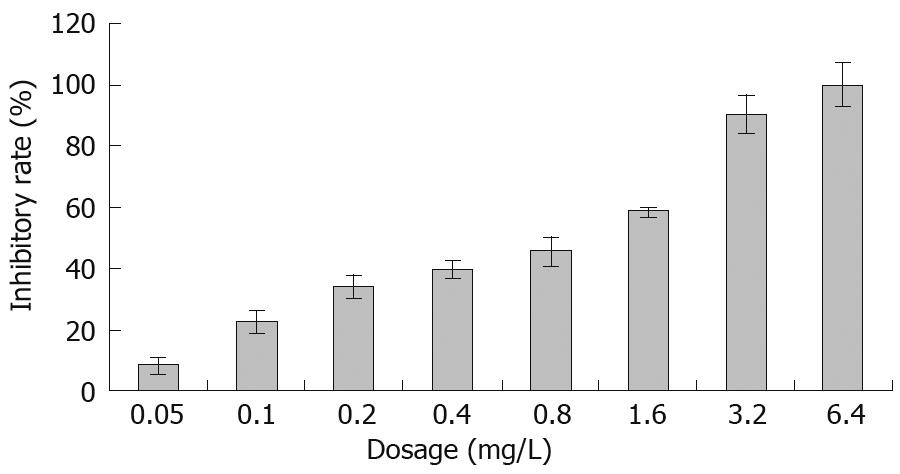
The SGC-7901 cells were treated with various concentrations of acetylshikonin for 48 h and cell viability was determined by MTT assay. Acetylshikonin inhibited the growth of gastric cancer cells in a dose-dependent manner (Figure 1). Cell growth was suppressed by 33.7%, 39.3% and 45.5% 48 h after treatment with 0.2, 0.4 and 0.8 mg/L acetylshikonin, respectively. The inhibitory rate of 3.2 mg/L acetylshikonin for tumor cell growth was over 90%. The half maximal inhibitory concentration (IC50) of acetylshikonin for SGC-7901 cells was 0.428 mg/L.
Twenty-four hours after exposure to acetylshikonin, SGC-7901 cells began to show morphologic features of apoptosis and some cells rounded up off the plate, exhibiting a smaller and circular shape. Cell cycle analysis revealed that the number of cells was different at the G2/M phase after exposure to 0.2 and 0.4 mg/L acetylshikonin, but significantly decreased at the G2/M phase after exposure to 0.8 mg/L acetylshikonin. Hoechst assay showed nuclear condensation, DNA fragmentation and perinuclear apoptotic bodies after treatment with 0.2 mg/L acetylshikonin, indicating that acetylshikonin induces apoptosis in a dose-dependent manner (Table 2).
| Concentration (mg/L) | Cell cycle phase distribution (%) | Apoptotic index (%) | ||||
| G0/G1 | S | G2/M | Hoechst | TUNEL | FACScan | |
| 0 | 53.73 ± 4.61 | 36.29 ± 7.32 | 9.98 ± 2.35 | 5.76 ± 0.58 | 5.34 ± 0.64 | 6.15 ± 0.75 |
| 0.2 | 54.82 ± 4.12 | 33.45 ± 6.05 | 11.73 ± 3.90 | 11.05 ± 2.10a | 11.56 ± 1.62a | 10.13 ± 1.69a |
| 0.4 | 56.92 ± 5.04 | 30.15 ± 5.84 | 12.93 ± 5.32 | 28.76 ± 3.55b | 30.89 ± 2.35b | 25.40 ± 2.46b |
| 0.8 | 57.12 ± 2.94 | 28.58 ± 5.21 | 14.30 ± 4.79a | 52.25 ± 6.16b | 56.33 ± 5.78b | 48.73 ± 6.55b |
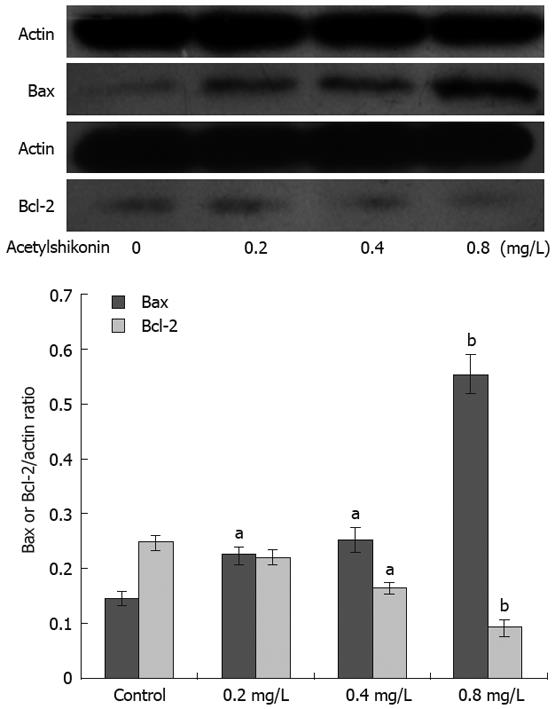
Western blot assay showed that acetylshikonin could induce or inhibit the expression of Bcl-2 and Bax. Twenty-four hours after treatment with indicated concentrations of acetylshikonin, the expression of Bax was markedly increased, while that of Bcl-2 had a trend to decrease. The Bax/Bcl-2 protein ratio was also elevated remarkably. The expression of actin remained unchanged (Figure 2).
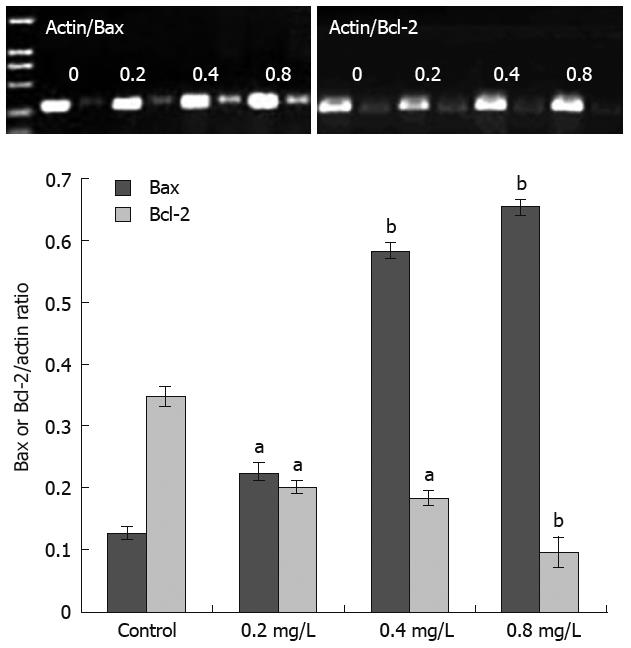
The expression of Bcl-2 and Bax was semi-quantitatively detected with β-actin as an internal standard. Acetylshikonin inhibited the expression of Bcl-2 in SGC-7901 cells in a dose-dependent manner. However, the expression of Bax mRNA increased with the concentration of acetylshikonin used (Figure 3).
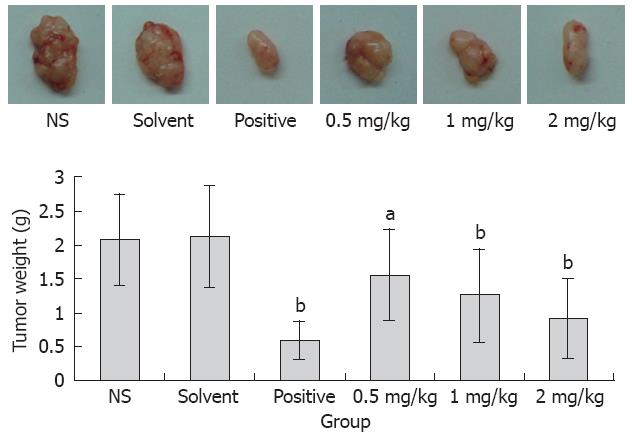
To determine whether acetylshikonin inhibits tumor growth in vivo, an equal number of SGC-7901 cells were injected into the right flanks of nude mice. Tumor growth was notably inhibited in mice after treatment with acetylshikonin (Figure 4). The inhibitory rate of tumor growth in 3 acetylshikonin groups and cyclophosphamide group was 55.76%, 39.42%, 25.00% and 58.65%, respectively. In addition, no toxicity judged by parallel monitoring of body weight was observed in acetylshikonin-treated mice. Immunohistochemistry data showed that the IOD value for Bcl-2 was 19.26 ± 2.15 in the negative control group and 11.59 ± 1.04 in the 2 mg/kg acetylshikonin group (Figure 5). However, the expression of Bax and Caspase 3 was markedly higher in 3 acetylshikonin groups than in negative control group.
Shikonin, isolated from roots of the medicinal herb Lithospermum erythrorhizon Siebold Zucc[14], is a Chinese herbal medicine with various biological activities, including anti-microbial, anti-fungal, anti-HIV, anti-inflammatory, anti-tumor, and wound-healing activities[15–17]. However, its toxicity has limited it as a therapeutic agent[1417]. Acetylshikonin, one of the shikonin derivatives, has been shown to possess anti-cancer activity[910] with less toxicity[18], which has made acetylshikonin a promising anti-tumor agent. However, its effects on gastric cancer cells have not been reported.
In the present study, acetylshikonin suppressed the proliferation of SGC-7901 cells in vitro, and inhibited tumor growth in a SGC-7901 tumor xenograft model of nude mice. Generally, tumorigenesis and tumor progression are strongly associated with abnormal apoptosis. It has been shown that shikonin and its derivatives induce apoptosis of tumor cells[161920]. Our data demonstrate that acetylshikonin could also induce apoptosis of SGC-7901 cells with typical apoptotic alterations, including morphological changes and positive TUNEL assay, DNA fragmentation and apoptotic sub-G1 peak.
Apoptosis is a complex process regulated by a variety of factors[2122]. Members of the Bcl-2 family are the important regulators in the apoptotic pathway with individual members that promote or suppress apoptosis. As an anti-apoptotic protein, Bcl-2 confers a negative control in the pathway of cellular suicide machinery, whereas Bax, a Bcl-2-homologous protein, promotes cell death by competing with Bcl-2[2324]. To explore whether apoptosis-related genes contribute to the inhibitory effect of acetylshikonin on SGC-7901 cells, the relative Bcl-2 and Bax expression levels induced by acetylshikonin were determined in this study. We found that acetylshikonin decreased the Bcl-2 expression while increased the Bax expression in a dose-dependent manner. Moreover, acetylshikonin up-regulated the expression level of Caspase 3 in vivo. Taken together, the reduced Bcl-2/Bax ratio, activating Caspases and cell apoptosis ultimately[24], might serve as a mechanism of acetylshikonin underlying apoptosis of SGC-7901 cells.
In conclusion, acetylshikonin has significant antitumor effects both in vitro and in vivo by inducing apoptosis of SGC-7901 cells involved in down-regulation of Bcl-2 expression and up-regulation of Bax expression. Acetylshikonin can be used as a potent and selective agent in the treatment of human gastric adenocarcinoma.
Patients with gastric cancer have certain clinical responses to chemotherapy or radiation therapy, but they cannot tolerate it. Therefore, it is absolutely necessary to explore drugs capable of preventing and treating gastric cancer and other malignancies.
Previous studies on shikonin, a chemical derived from a Chinese medicinal herb, have shown that shikonin has anti-tumor effects although it is toxic. Acetylshikonin, an acetyl derivative, has less toxicity and suppresses the growth of sarcomas. However, little is known about its effect on gastric cancer in vitro, especially in vivo.
The study demonstrated that acetylshikonin could exert significant effects on SGC-7901 cells both in vitro and in vivo by inducing cell apoptosis.
Acetylshikonin can be used as a potent and selective agent in treatment of human gastric adenocarcinoma.
Apoptosis: A type of cell death, in which cells use a specialized cellular machinery to kill themselves, as well as a cell suicide mechanism enabling metazoans to control the cell number and eliminate them.
The study analyzed the effect of acetylshikonin, derived from a Chinese medicinal herb, on gastric cancer cell line SGC-7901 in vivo, which is an interesting finding.
| 1. | Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat Rev Cancer. 2006;6:546-558. |
| 2. | Sun X, Mu R, Zhou Y, Dai X, Qiao Y, Zhang S, Huang X, Sun J, Li L, Lu F. 1990-1992 mortality of stomach cancer in China. Zhonghua Zhongliu Zazhi. 2002;24:4-8. |
| 3. | Lu JB, Sun XB, Dai DX, Zhu SK, Chang QL, Liu SZ, Duan WJ. Epidemiology of gastroenterologic cancer in Henan Province, China. World J Gastroenterol. 2003;9:2400-2403. |
| 4. | Macdonald JS. Clinical overview: adjuvant therapy of gastrointestinal cancer. Cancer Chemother Pharmacol. 2004;54 Suppl 1:S4-11. |
| 5. | Yu W, Kim HS, Choi GS, Suh IS. Perigastric lymph nodes with metastasis in gastric cancer. Hepatogastroenterology. 1999;46:2658-2661. |
| 6. | Morse MA, Stoner GD. Cancer chemoprevention: principles and prospects. Carcinogenesis. 1993;14:1737-1746. |
| 7. | Lu PJ, Yang C, Lin CN, Li CF, Chu CC, Wang JJ, Chen JY. Shiunko and acetylshikonin promote reepithelialization, angiogenesis, and granulation tissue formation in wounded skin. Am J Chin Med. 2008;36:115-123. |
| 8. | Wang JP, Tsao LT, Raung SL, Hsu MF, Kuo SC. Investigation of the inhibition by acetylshikonin of the respiratory burst in rat neutrophils. Br J Pharmacol. 1997;121:409-416. |
| 9. | Pietrosiuk A, Furmanowa M, Skopińiska-Rózewska E, Sommer E, Skurzak H, Bany J. The effect of acetylshikonin isolated from Lithospermum canescens roots on tumor-induced cutaneous angiogenesis. Acta Pol Pharm. 2004;61:379-382. |
| 10. | Zeng Y, Luo G, Yang W, Zhou L. Inhibitory effect of acetylshikonin injection on mouse S180 sarcoma. Zhongyao Yaoli Yu Linchuang. 2008;24:22-23. |
| 11. | Luo G, Guan X, Zhou L. Apoptotic effect of citrus fruit extract nobiletin on lung cancer cell line A549 in vitro and in vivo. Cancer Biol Ther. 2008;7:966-973. |
| 12. | Qu ZH, Zhang XL, Tang TT, Dai KR. Promotion of osteogenesis through beta-catenin signaling by desferrioxamine. Biochem Biophys Res Commun. 2008;370:332-337. |
| 13. | Zeng Y, Luo G, Yang W, Zhou L, Xie H. Antitumor effect and mechanism of AKT injection. Sichuan Daxue Xuebao (Medical Science Edition). 2008;39:500-502. |
| 14. | Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16:199-209. |
| 15. | Chen X, Yang L, Zhang N, Turpin JA, Buckheit RW, Osterling C, Oppenheim JJ, Howard OM. Shikonin, a component of chinese herbal medicine, inhibits chemokine receptor function and suppresses human immunodeficiency virus type 1. Antimicrob Agents Chemother. 2003;47:2810-2816. |
| 16. | Wu Z, Wu L, Li L, Tashiro S, Onodera S, Ikejima T. p53-mediated cell cycle arrest and apoptosis induced by shikonin via a caspase-9-dependent mechanism in human malignant melanoma A375-S2 cells. J Pharmacol Sci. 2004;94:166-176. |
| 17. | Yang F, Chen Y, Duan W, Zhang C, Zhu H, Ding J. SH-7, a new synthesized shikonin derivative, exerting its potent antitumor activities as a topoisomerase inhibitor. Int J Cancer. 2006;119:1184-1193. |
| 18. | Papageorgiou VP, Assimopoulou AN, Couladouros EA, Hepworth D, Nicolaou KC. The chemistry and biology of alkannin, shikonin, and related naphtazarin natural products. Angew Chem Int Edit. 1999;38:270-300. |
| 19. | Liu J, Zhou W, Li SS, Sun Z, Lin B, Lang YY, He JY, Cao X, Yan T, Wang L. Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogues. Cancer Res. 2008;68:8871-8880. |
| 20. | Mao X, Yu CR, Li WH, Li WX. Induction of apoptosis by shikonin through a ROS/JNK-mediated process in Bcr/Abl-positive chronic myelogenous leukemia (CML) cells. Cell Res. 2008;18:879-888. |
| 21. | Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322-1326. |