Published online Apr 14, 2009. doi: 10.3748/wjg.15.1774
Revised: March 12, 2009
Accepted: March 19, 2009
Published online: April 14, 2009
Small bowel adenocarcinoma (SBA) in patients with Crohn’s disease (CD) is quite rare, difficult to diagnose without surgery, and has a poor prognosis. Here, we report a 48-year-old man with SBA and a 21-year history of CD who was diagnosed by a combination of positron emission tomography/computed tomography (PET/CT) and double-balloon enteroscopy (DBE). Since the age of 27 years, the patient had been treated for ileal CD and was referred to our hospital with persistent melena. Multiple hepatic tumors were found by CT. PET/CT detected an accumulation spot in the small bowel. DBE revealed an ulcerative tumor in the ileum about 100 cm from the ileocecal valve. An endoscopic forceps biopsy specimen showed poorly differentiated adenocarcinoma. There were some longitudinal ulcer scars near the tumor, and the chronic inflammation in the small bowel appeared to be associated with the cancer development. Previous reports suggest the risk of SBA in patients with CD is higher than in the overall population. Since early diagnosis is extremely difficult in these cases, novel techniques, such as PET/CT and DBE, may be expected to help in making a preoperative diagnosis of the development of SBA in CD.
- Citation: Kodaira C, Osawa S, Mochizuki C, Sato Y, Nishino M, Yamada T, Takayanagi Y, Takagaki K, Sugimoto K, Kanaoka S, Furuta T, Ikuma M. A case of small bowel adenocarcinoma in a patient with Crohn’s disease detected by PET/CT and double-balloon enteroscopy. World J Gastroenterol 2009; 15(14): 1774-1778
- URL: https://www.wjgnet.com/1007-9327/full/v15/i14/1774.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1774
Previous studies have documented that patients with inflammatory bowel disease (IBD) are at increased risk of developing colorectal and intestinal cancer[1–6]. However, because of its rarity, there is limited data on the precise cancer risk of small bowel adenocarcinoma (SBA) in patients with Crohn’s disease (CD). To date, recommendations for screening and surveillance have been supported by very limited data[7]. Even in extensive colitis, a recent report found that colonoscopy surveillance may not improve survival[8], and other tools are needed to detect cancer development in the early stages. Here, we report a patient with a 21-year history of CD who developed a SBA that was detected by 18F-fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) and double-balloon enteroscopy (DBE) without conventional intestinal examinations.
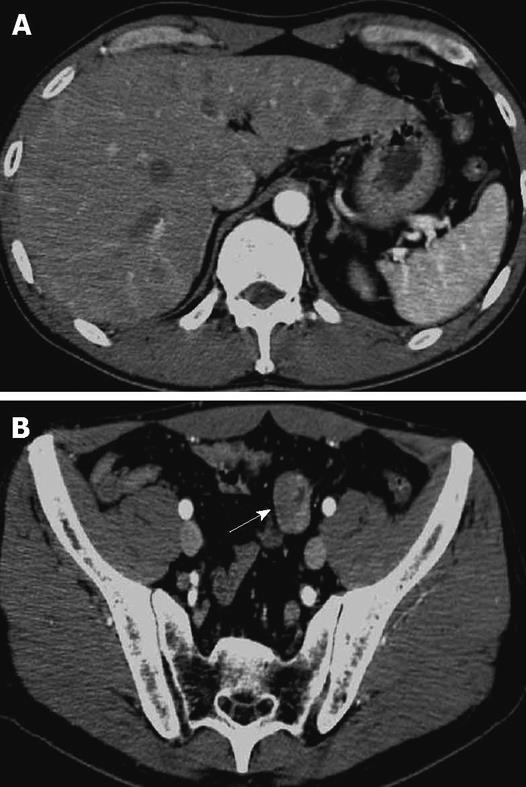
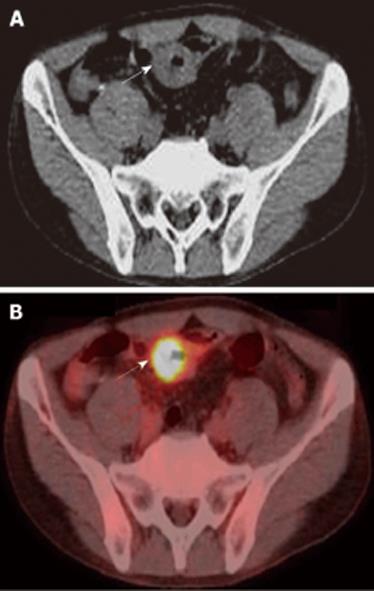
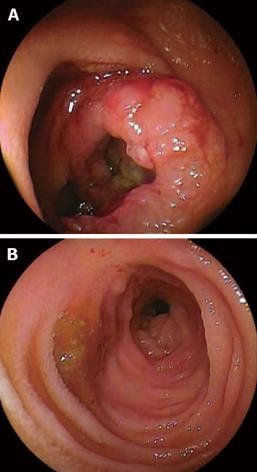
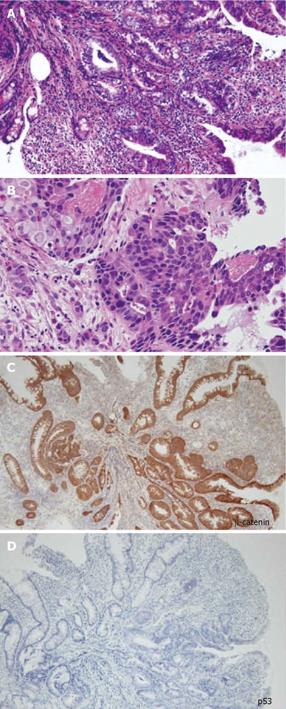
A 48-year-old man who was diagnosed with ileal CD as a 27-year-old was referred to our hospital with persistent melena. His family history was negative for this disease. He had no evidence of gluten intolerance. He had been treated with 5-aminosalicylates (5-ASAs) combined with an elemental-diet (ED) therapy and had several hospitalizations for temporary melena in the past two decades. He had not agreed to have an intestinal examination at regular intervals. In January 2007, he was examined by abdominal CT due to persistent melena for 1 year, and multiple metastatic tumors were discovered in the liver (Figure 1A). Blood tests showed a slight anemia (Hb 11.8 g/dL), but he was negative for inflammatory factors. The serum carcinoembryonic antigen (CEA) was elevated (31.6 ng/mL; normal range, < 2.5 ng/mL), and wall thickening in part of the ileum was detected by CT (Figure 1B). PET/CT showed accumulations in the multiple hepatic tumors and the wall thickening of the ileum (Figure 2). DBE showed an ulcerative tumor in the ileum about 100 cm away from the ileocecal valve (Figure 3A). There were some longitudinal ulcer scars near the tumor (Figure 3B). An endoscopic forceps biopsy specimen showed microscopically poorly-differentiated adenocarcinoma (Figure 4A and B). Immunohistochemistry of the tumor cells was negative for p58 and positive for β-catenin (Figure 4C and D). Accordingly, he was diagnosed with SBA with hepatic metastases. Although he was treated by chemotherapy with S-1 and cisplatin (CDDP), there was no effective response. He died 4 mo after the diagnosis because of liver failure due to progression of the hepatic metastases.
Primary SBA is rare and the incidence is reported to be 1 to 5% of all gastrointestinal tract malignancies[910], and the most important known risk factor for this malignant tumor is previous CD[511]. SBA in CD was reported for the first time by Ginzburg in 1956[12]. The risk for SBA is reportedly higher in patients with CD than in the overall population. Recent meta-analysis revealed the relative risk of SBA in patients with CD as 28.4 (95% CI, 14.5-55.7)[13] to 33.2 (95% CI, 15.9-60.9)[14]. In clinical settings, SBA in CD is difficult to diagnose, and most previous cases were diagnosed after surgery without suspicion of malignancy. Therefore, novel tools are needed to detect and survey the development of this cancer. In our case, we were able to diagnose SBA non-surgically by a novel approach using PET/CT and DBE.
Based on previous reports, Dossett et al[3] summarized the 154 cases of SBA in CD reported in Europe and America. SBA in CD occurred more frequently in males than females (M:F ratio of 2.4:1). The age at diagnosis ranged from 21 to 86 years (mean age, 51.3), and the average duration of CD was 24.5 years (0-45 years)[3]. SBA in CD was observed at a younger age[1] as compared with de novo cancers that were found in 60-69-year-olds[15]. Tumors were found in the ileum at a rate of 75%. The presence of previously bypassed segments of the intestine was 20.3%. Obstruction was the most common manifestation (76%), whereas hemorrhage, fistula, and perforation were observed in 3.9%, 3.9%, and 5.4% of cases, respectively. The majority of diagnoses were made at the time of operation (35.4%) or postoperatively (61.5%). Only 3.1% of the cases could be diagnosed preoperatively. Survival rates after 1 and 2 years were 49.6% and 27%, respectively[3].
In the present case, there were some longitudinal ulcer scars near the tumor, suggesting that chronic inflammation is associated with the development of SBA. Because it is well known that a combination of immunohistochemically strong p53 expression and absent or weak β-catenin expression in ulcerative colitis (UC) patients is evidence for colitis-associated dysplastic lesions[16], we examined the immunohistochemical characteristics of the tumor[17]. In this case, there was no detectable p53 expression, but strong β-catenin expression. Chemotherapy and radiation for SBA have produced disappointing results, and only a few studies have evaluated chemotherapy for unresectable SBA in CD[18]. There have been no controlled studies recommending an effective treatment regimen. Although we treated this patient by systemic chemotherapy with S-1 and CDDP in agreement with the patient, the therapy was not effective and did not seem to prolong the survival time.
There are several previous reports of long-standing risk factors for SBA, including onset of the disease before the age of 30 years, presence of a bypassed segment, chronic active course with stricture and fistulas, male gender, and smoking[1–351119–21]. Corticosteroid and azathioprine therapy to treat CD are also considered potential risk factors. However, 5-ASAs prevent the development of intestinal adenocarcinoma in IBD[122–25]. There is also a theoretical risk for an increased rate of malignancies due to antagonism of TNF-α, but to date, there is no clear proof of such an effect[2627].
Recommendations for screening and surveillance of SBA in CD have been supported by only very limited data[8]. The overall risk of colorectal cancer for UC patients is estimated to be 3.7% (95% CI, 3.2%-4.2%); 2% at 10 years, 8% at 20 years, and 18% at 30 years[28], whereas reported cancerin CD is 0.63%-3.1%[29]. Although diagnostic investigations using conventional modalities such as small bowel series, double-contrast enteroclysis, and upper and lower gastrointestinal endoscopies have been performed on patients with a high cancer risk, a recent report found that colonoscopy surveillance may not improve survival even in studying extensive colitis, thus other tools are needed to detect cancer development[8]. CT and MRI are now considered the common imaging modality of choice for abdominal malignancy. Endoscopy of the small intestine, especially capsule endoscopy and DBE, are promising new diagnostic tools[30]. Capsule endoscopy is not invasive, but has a risk of retention for patients with CD who have a stenosis in the intestinal tract[3132]. DBE is a new endoscopic method that provides complete visualization, the ability to biopsy the small bowel, and provides diagnostic and therapeutic information[33].
The recent development of innovative im In this case, PET/CT was effective for the discovery of a small bowel tumor at the initial examination. Although the localization of lesions may not be completely accurate, PET/CT provides accurately fused morphological and functional imaging within a single examination. Today, PET imaging has a high sensitivity for detecting colorectal cancer (CRC) and is superior to conventional CT staging when CRC patients are assessed for local and distant metastases[3435]. PET/CT also offers a better non-invasive tool for identifying and localizing active intestinal inflammation in patients with CD[36]. Although PET/CT is not able to replace conventional studies due to its high cost, it may be useful when conventional studies cannot be performed or are not completed in CD patients. In this case, the tumor was detected at an advanced stage with multiple liver metastases. Although it is still uncertain whether combining PET/CT and DBE will enable us to make an early diagnosis for SBA, this case certainly indicates the possibility of combining PET/CT and DBE without conventional modalities, such as a small bowel series, to detect a primary malignant tumor of the small bowel without surgery. One strategy to monitor high-risk patients might be to perform PET/CT at an initial examination, and then conduct a DBE examination for the patient who has abnormal regions suspected as being malignant.
The recent development of innovative imaging techniques involving PET/CT and DBE has opened a new area in the exploration of the small bowel in CD patients. Each of these techniques is characterized by its own profile of favorable and unfavorable features[30]. Future clinical studies are expected to demonstrate strategies to monitor SBA in CD patients.
| 1. | Solem CA, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Small intestinal adenocarcinoma in Crohn’s disease: a case-control study. Inflamm Bowel Dis. 2004;10:32-35. [Cited in This Article: ] |
| 2. | Jess T, Winther KV, Munkholm P, Langholz E, Binder V. Intestinal and extra-intestinal cancer in Crohn’s disease: follow-up of a population-based cohort in Copenhagen County, Denmark. Aliment Pharmacol Ther. 2004;19:287-293. [Cited in This Article: ] |
| 3. | Dossett LA, White LM, Welch DC, Herline AJ, Muldoon RL, Schwartz DA, Wise PE. Small bowel adenocarcinoma complicating Crohn’s disease: case series and review of the literature. Am Surg. 2007;73:1181-1187. [Cited in This Article: ] |
| 4. | Lewis JD, Deren JJ, Lichtenstein GR. Cancer risk in patients with inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:459-477. [Cited in This Article: ] |
| 5. | Munkholm P, Langholz E, Davidsen M, Binder V. Intestinal cancer risk and mortality in patients with Crohn’s disease. Gastroenterology. 1993;105:1716-1723. [Cited in This Article: ] |
| 6. | Persson PG, Karlén P, Bernell O, Leijonmarck CE, Broström O, Ahlbom A, Hellers G. Crohn’s disease and cancer: a population-based cohort study. Gastroenterology. 1994;107:1675-1679. [Cited in This Article: ] |
| 7. | Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378-389. [Cited in This Article: ] |
| 8. | Friedman S, Rubin PH, Bodian C, Goldstein E, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn’s colitis. Gastroenterology. 2001;120:820-826. [Cited in This Article: ] |
| 9. | Barclay TH, Schapira DV. Malignant tumors of the small intestine. Cancer. 1983;51:878-881. [Cited in This Article: ] |
| 10. | Mittal VK, Bodzin JH. Primary malignant tumors of the small bowel. Am J Surg. 1980;140:396-399. [Cited in This Article: ] |
| 11. | Lashner BA. Risk factors for small bowel cancer in Crohn’s disease. Dig Dis Sci. 1992;37:1179-1184. [Cited in This Article: ] |
| 12. | Ginzburg L, Schneider KM, Dreizin DH, Levinson C. Carcinoma of the jejunum occurring in a case of regional enteritis. Surgery. 1956;39:347-351. [Cited in This Article: ] |
| 13. | von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn’s disease. Dis Colon Rectum. 2007;50:839-855. [Cited in This Article: ] |
| 14. | Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097-1104. [Cited in This Article: ] |
| 15. | Negri E, Bosetti C, La Vecchia C, Fioretti F, Conti E, Franceschi S. Risk factors for adenocarcinoma of the small intestine. Int J Cancer. 1999;82:171-174. [Cited in This Article: ] |
| 16. | Walsh SV, Loda M, Torres CM, Antonioli D, Odze RD. P53 and beta catenin expression in chronic ulcerative colitis--associated polypoid dysplasia and sporadic adenomas: an immunohistochemical study. Am J Surg Pathol. 1999;23:963-969. [Cited in This Article: ] |
| 17. | Odze RD. Adenomas and adenoma-like DALMs in chronic ulcerative colitis: a clinical, pathological, and molecular review. Am J Gastroenterol. 1999;94:1746-1750. [Cited in This Article: ] |
| 18. | Bruckner HW, Hrehorovich VR, Sawhney HS, Meeus SI, Coopeman AM. Chemotherapeutic management of small bowel adenocarcinoma associated with Crohn’s disease. J Chemother. 2006;18:545-548. [Cited in This Article: ] |
| 19. | Partridge SK, Hodin RA. Small bowel adenocarcinoma at a strictureplasty site in a patient with Crohn’s disease: report of a case. Dis Colon Rectum. 2004;47:778-781. [Cited in This Article: ] |
| 20. | Christodoulou D, Skopelitou AS, Katsanos KH, Katsios C, Agnantis N, Price A, Kappas A, Tsianos EV. Small bowel adenocarcinoma presenting as a first manifestation of Crohn’s disease: report of a case, and a literature review. Eur J Gastroenterol Hepatol. 2002;14:805-810. [Cited in This Article: ] |
| 21. | Kaerlev L, Teglbjaerg PS, Sabroe S, Kolstad HA, Ahrens W, Eriksson M, Guénel P, Hardell L, Launoy G, Merler E. Medical risk factors for small-bowel adenocarcinoma with focus on Crohn disease: a European population-based case-control study. Scand J Gastroenterol. 2001;36:641-646. [Cited in This Article: ] |
| 22. | Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854-862. [Cited in This Article: ] |
| 23. | Moody GA, Jayanthi V, Probert CS, Mac Kay H, Mayberry JF. Long-term therapy with sulphasalazine protects against colorectal cancer in ulcerative colitis: a retrospective study of colorectal cancer risk and compliance with treatment in Leicestershire. Eur J Gastroenterol Hepatol. 1996;8:1179-1183. [Cited in This Article: ] |
| 24. | Ryan BM, Russel MG, Langholz E, Stockbrugger RW. Aminosalicylates and colorectal cancer in IBD: a not-so bitter pill to swallow. Am J Gastroenterol. 2003;98:1682-1687. [Cited in This Article: ] |
| 25. | Piton G, Cosnes J, Monnet E, Beaugerie L, Seksik P, Savoye G, Cadiot G, Flourie B, Capelle P, Marteau P. Risk factors associated with small bowel adenocarcinoma in Crohn’s disease: a case-control study. Am J Gastroenterol. 2008;103:1730-1736. [Cited in This Article: ] |
| 26. | Wenzl HH, Reinisch W, Jahnel J, Stockenhuber F, Tilg H, Kirchgatterer A, Petritsch W. Austrian infliximab experience in Crohn’s disease: a nationwide cooperative study with long-term follow-up. Eur J Gastroenterol Hepatol. 2004;16:767-773. [Cited in This Article: ] |
| 27. | Kronberger IE, Graziadei IW, Vogel W. Small bowel adenocarcinoma in Crohn’s disease: a case report and review of literature. World J Gastroenterol. 2006;12:1317-1320. [Cited in This Article: ] |
| 28. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [Cited in This Article: ] |
| 29. | Korelitz BI. Carcinoma of the intestinal tract in Crohn’s disease: results of a survey conducted by the National Foundation for Ileitis and colitis. Am J Gastroenterol. 1983;78:44-46. [Cited in This Article: ] |
| 30. | Saibeni S, Rondonotti E, Iozzelli A, Spina L, Tontini GE, Cavallaro F, Ciscato C, de Franchis R, Sardanelli F, Vecchi M. Imaging of the small bowel in Crohn’s disease: a review of old and new techniques. World J Gastroenterol. 2007;13:3279-3287. [Cited in This Article: ] |
| 31. | Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, Lichtiger S, Lewis BS. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol. 2006;101:2218-2222. [Cited in This Article: ] |
| 32. | Rondonotti E, Villa F, Mulder CJ, Jacobs MA, de Franchis R. Small bowel capsule endoscopy in 2007: indications, risks and limitations. World J Gastroenterol. 2007;13:6140-6149. [Cited in This Article: ] |
| 33. | Oshitani N, Yukawa T, Yamagami H, Inagawa M, Kamata N, Watanabe K, Jinno Y, Fujiwara Y, Higuchi K, Arakawa T. Evaluation of deep small bowel involvement by double-balloon enteroscopy in Crohn’s disease. Am J Gastroenterol. 2006;101:1484-1489. [Cited in This Article: ] |
| 34. | Veit-Haibach P, Kuehle CA, Beyer T, Stergar H, Kuehl H, Schmidt J, Börsch G, Dahmen G, Barkhausen J, Bockisch A. Diagnostic accuracy of colorectal cancer staging with whole-body PET/CT colonography. JAMA. 2006;296:2590-2600. [Cited in This Article: ] |
| 35. | Antoch G, Vogt FM, Freudenberg LS, Nazaradeh F, Goehde SC, Barkhausen J, Dahmen G, Bockisch A, Debatin JF, Ruehm SG. Whole-body dual-modality PET/CT and whole-body MRI for tumor staging in oncology. JAMA. 2003;290:3199-3206. [Cited in This Article: ] |
| 36. | Louis E, Ancion G, Colard A, Spote V, Belaiche J, Hustinx R. Noninvasive assessment of Crohn’s disease intestinal lesions with (18)F-FDG PET/CT. J Nucl Med. 2007;48:1053-1059. [Cited in This Article: ] |