Published online Apr 14, 2009. doi: 10.3748/wjg.15.1738
Revised: February 17, 2009
Accepted: February 24, 2009
Published online: April 14, 2009
AIM: To investigate the expression of α-fetoprotein (AFP), a cancer-associated fetal glycoprotein, and its involvement during rat colon development.
METHODS: Colons from Sprague-Dawley rat fetuses, young and adult (8 wk old) animals were used in this study. Expression levels of AFP in colons of different development stage were detected by reverse-transcriptase PCR (RT-PCR) and Western blotting. To identify the cell location of AFP in the developing rat colons, double-immunofluorescent staining was performed using antibodies to specific cell markers and AFP, respectively.
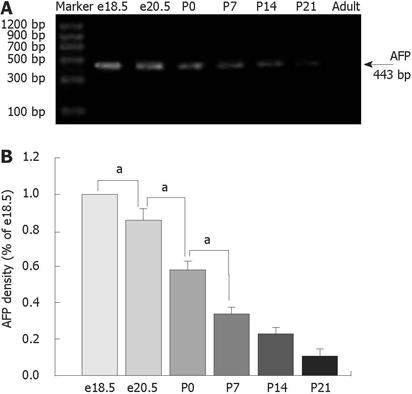
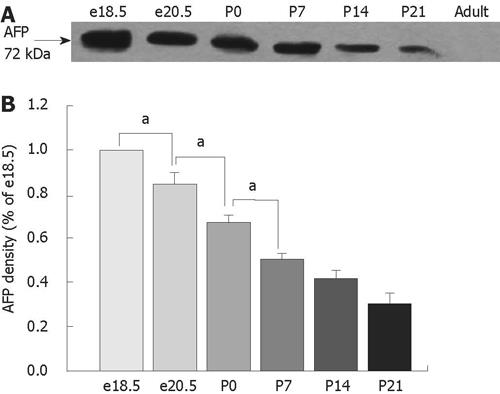
RESULTS: The highest levels of AFP mRNA were detected in colons of rats at embryonic day 18.5 (e18.5). Compared to e18.5 d, the AFP expression was significantly decreased during rat development [85% for e20.5, P < 0.05, 58% for postnatal day 0.5 (P0.5), P < 0.05, 37% for P7, P < 0.05, 24% for P14, P < 0.05, and 11% for P21, P < 0.05] and undetected in adult rats. Only the 72-kDa isoform of AFP was detected by Western blotting, the expression pattern was similar to AFP mRNA and conformed to the results of mRNA expression. The AFP positive staining was identical to different distribution patterns in fetuses, young and adult animals and positive staining for both AFP and vimentin was overlapped in mesenchymal cells at each stage tested.
CONCLUSION: This study has for the first time demonstrated that AFP is localized in the mesenchyme of rat colon from the embryo to the weaning stage by immunofluorescence and presents 72-kDa isoform in the developing rat colons by Western blotting. The dynamic expression of AFP in the various developmental stages of the colon indicates that AFP might be involved in many aspects of colon development.
- Citation: Liu XY, Dong D, Sun P, Du J, Gu L, Ge YB. Expression and location of α-fetoprotein during rat colon development. World J Gastroenterol 2009; 15(14): 1738-1743
- URL: https://www.wjgnet.com/1007-9327/full/v15/i14/1738.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1738
The mammalian gut epithelium is a highly organized and dynamic system that requires continuous, controlled proliferation and differentiation throughout life. Identification of the growth factors controlling these processes is crucial since the molecular mechanisms regulating organogenesis are often the same as those necessary for repair following injury. Furthermore, mis-regulations of embryonic signaling pathways are often associated with neoplastic diseases.
We assumed that accurate transcriptional profiles over gut development interval could provide fundamental information about underlying mechanisms, and characterized candidate regulators of cell interactions and mucosal differentiation. This resource also can be applied to address long-standing questions about reactivation of fetal genes in cancer.
A number of transcription factors, growth factors, and their receptors have been found to be expressed in the gastrointestinal epithelium or mesenchyme. However, little is known about their specific functions in gastrointestinal development. For those factors where a mutation has been generated by gene targeting, gastrointestinal development either proceeds normally[1–3] or the embryos die too early to allow assessment of the gene functions in gut development[45].
Mammalian α-fetoprotein (AFP) is a single-chain glycoprotein with molecular mass ranging from 66 to 72 kDa and 3%-5% carbohydrate (glycan) content. This protein, which expresses at high levels in the fetal liver and yolk sac, constitutes 0.1% of the total mRNA in the fetal gut[6]. At birth, AFP mRNA declines precipitously in both liver and gut to levels that are barely detectable[7]. The gut development during late gestation and early neonatal period is accompanied by changes in the synthesis of AFP[8], and abundance declines significantly during gut development. In this case, AFP is considered as an important growth factor with a specific function in gastrointestinal development.
The ontogeny of AFP gene expression has been examined in the fetal and adult mouse gastrointestinal tract[9] to understand the basis of the ontogeny of AFP transcription in the gut and its regulatory elements. However, little is known about the expression pattern of AFP genes or its involvement during rat colon development.
Colons from Sprague-Dawley rat fetuses embryonic day 18.5 (e18.5 and e20.5 gestation), young (0 d and 1, 2 and 3 wk old) and adult (8 wk old) animals were used in this study. Five rats were used at each age stage. The embryonic age was determined according to Kaufman[10]. Mating was performed by housing a male and a female rat together in the same cage overnight. The presence of a vaginal plug was determined the next morning (0.5 d gestation). Rats were housed in plastic cages in an air-conditioned and light controlled room at 24 ± 2°C and 60% ± 5% humidity. The study protocol was approved by the Nanjing Medical University Animal Care and Use Committee.
Total RNA was extracted from tissues at each time point with TRIZOL reagent (Invitrogen Life Technologies, Burlington, Ontario, Canada), according to the manufacturer’s instructions. The quality of the RNA was verified by agarose gel electrophoresis using ethidium bromide staining. For each PCR, 2 &mgr;g DNA-free total RNA with oligo (deoxythymidine) primers and reverse transcriptase were used. PCR was performed in 50-&mgr;L reactions containing 2.5 ng cDNA, 1 &mgr;L each primer pair, and 25 &mgr;L Premix Taq (TaKaRa, CA, USA). PCR was carried out in a T-gradient Biometra PCR thermal cycler (Montreal Biotech Inc., Kirkland, Quebec, Canada) to determine the annealing temperature for each pair of primers[11]. The AFP primer pairs used were: 5’-GCTGAACCCAGAGTACTGCAC-3’ (forward), and 5’-GACACGTC GTAGATGAACGTG-3’ (reverse). Amplification reactions were carried out for 30 cycles at 94°C for 30 s, 58.4°C for 30 s and at 72°C for 1 min.
The amplified products were 443 bp and analyzed on 1% agarose gels and visualized by ethidium bromide staining. Controls omitting RT cDNA or DNA polymerase showed no reaction bands. The data were normalized by 18S RNA.
The tissues were homogenized in a lysis buffer containing 50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 5 mmol/L EDTA, 10 mmol/L NaF, 1 mmol/L sodium orthovanadate, 1% Triton X-100, 0.5% sodium deoxycholate, 1 mmol/L phenylmethylsulfonyl fluoride and Complete Protease Inhibitor Cocktail (Roche, Mannheim, Germany). The lysate was then centrifuged at 12 000 ×g for 25 min at 4°C. The total protein concentration of each sample was analyzed by BCA Protein Assay Kit (Pierce, Rockford, IL, USA). An equal amount of protein samples, 60 &mgr;g, from each specimen was boiled in 3 × loading buffer (10 mmol/L Tris-HCl, pH 6.8 including 3% SDS, 5% β-mercaptoethanol, 20% glycerol and 0.6% bromophenol blue) for 3 min and separated by 12.5% SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). After transfer, membranes were blocked with 5% fat-free milk in Tris-buffered saline plus 0.05% Tween 20 (TBS-T) overnight at 4°C. The membranes were then incubated with the primary antibody (sc-8108, an affinity purified goat polyclonal antibody raised against a peptide mapping at the C-terminus of AFP, diluted 1:500; Santa Cruz, Biotechnology CA, USA) for 2 h at room temperature. After washing in TBS-T three times, the membranes were incubated with the peroxidase-linked rabbit antigoat IgG conjugates (Santa Cruz Biotechnology) for 1 h at room temperature. At the end, they were washed again in TBS-T, incubated in enhanced chemiluminescence reagents (Pierce) for 2 min, and exposed to X-Omat BT film (Eastman Kodak, Rochester, NY, USA). Signal intensity was quantified using a Bio-Rad image analysis system and the results were normalized to band intensities at e18.5. Loading controls of presumably and constantly expressed proteins such as β-actin were used; however, their variability and increase in development precluded their use[12]. For negative controls, the primary antibody was omitted.
Tissues were fixed in 4% paraformaldehyde overnight at 4°C followed by a standard protocol of dehydration and paraffin embedding, and 5-&mgr;m sections were cut. The paraffin sections were deparaffinized in xylene and rehydrated in graded ethanol and distilled water. The non-specific binding sites were blocked in 1% bovine serum albumin (BSA) for 30 min. For AFP and vimentin double immunofluorescence, the goat anti-AFP primary polyclonal antibody was applied and revealed using fluorescein isothiocyanate (FITC)-labeled rabbit antigoat IgG (1:400, sc-2777; Santa Cruz Biotechnology). Mouse anti-vimentin primary monoclonal antibody (1:1000, CBL202; Chemicon International, Inc. Temecula, CA, USA) was then applied and revealed by rhodamine-labeled anti-mouse IgG (1:400, AP192C; Chemicon International, Inc.). Sections were placed in gel aqueous mounting medium (G0918; Sigma, St. Louis, MO, USA) with a cover glass and were examined under an Olympus BX51 microscope (Olympus Optical, Tokyo, Japan). Controls were treated by omitting the primary or secondary antibody. No staining was observed under the negative control conditions. Images were taken at a magnification × 200.
All experiments were done in triplicate. Analysis of the experimental data was carried out using PDQuest 7.0 software (Bio-Rad Laboratories) and one-way analysis of variance and paired t test were used. Data are presented as mean ± SD. P < 0.05 was considered statistically significant.
We carried out RT-PCR and Western blotting to detect the expression of AFP using samples extracted from the colons of fetal e18.5 and e20.5, postnatal day 0 (P0), P7, P14 and P21 and adult rats. As shown in Figure 1, the highest levels of AFP mRNA were detected in colons of rats at e18.5. The AFP mRNA levels in colons declined steadily during rat development and were undetected in adult rats (P < 0.05) (Figure 1). The level of AFP mRNA in e20.5 colon was significantly decreased compared with that in e18.5 colon. AFP mRNA in P0 colon was lower than those in e20.5 colon (P < 0.05), and AFP mRNA in P7 colon was also lower than in P0 (P < 0.05). There was no difference in the levels of AFP mRNA between P14 and P21. The AFP protein had four isoforms: 72, 60, 48 and 37 kDa. In our study, only the 72-kDa isoform of AFP was detected in rat colon (Figure 2A). From the results of the densitometric quantification (Figure 2B), it was seen that the total AFP was the highest at e18.5, after which expression decreased steadily, being the lowest in the adult colons. This result was similar to those of AFP mRNA expression.
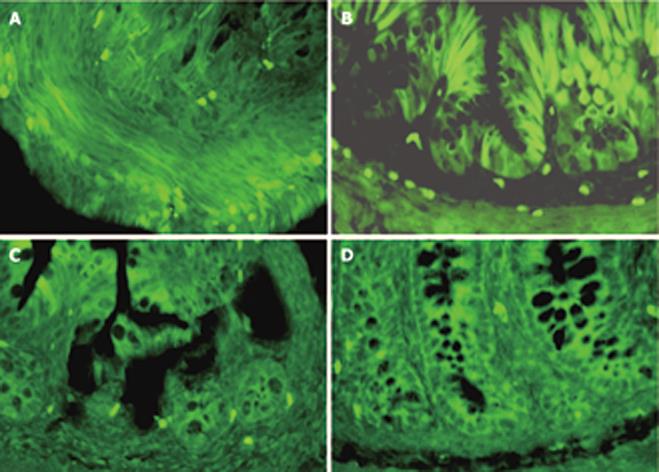
In e18.5 fetus, the colonic mucosa was lined by a stratified epithelium and AFP positive staining was detected in the epithelium and mesenchymal tissue (Figure 3A). In e20.5 fetus, the epithelium has transformed into a simple columnar one. The AFP positive cells located at the bottom of crypt-like structure and the number of positive cells decreased markedly at this time (Figure 4A). After birth, positive cells were scattered on the epithelium during the first 7 d (Figure 4B). By P14 and P21, when the adult crypt structure replaced the villi, positive cells became largely restricted to the base of the crypts, no positive staining of AFP in the adult colonic epithelium was observed (Figure 3C and D).
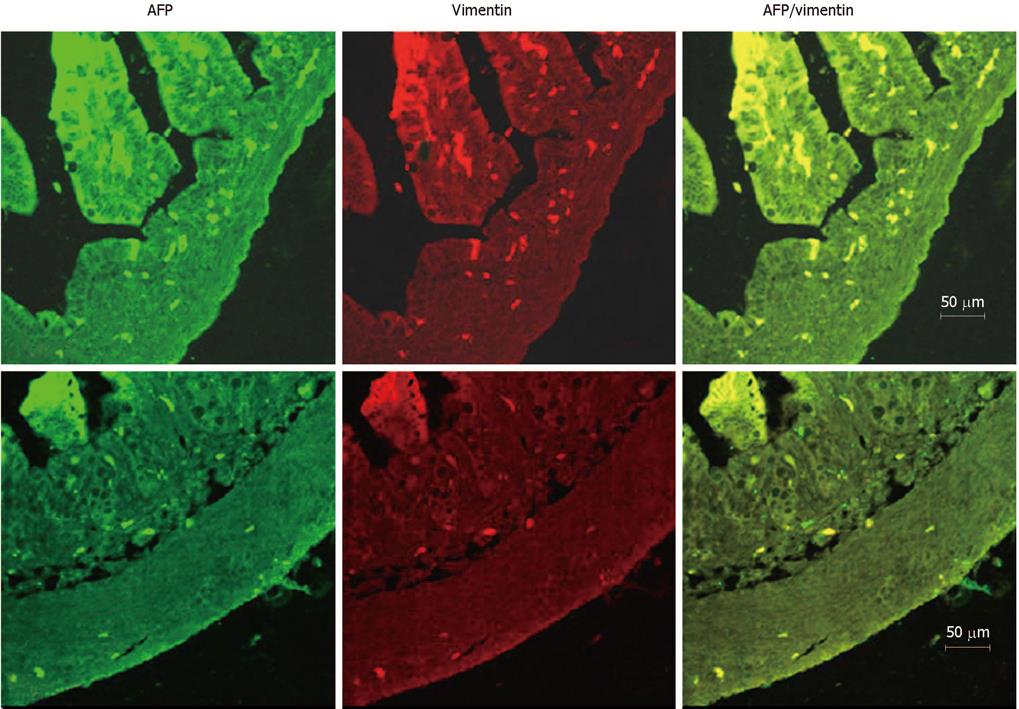
The pattern of AFP-expressing cells and mesenchymal cells were very similar, which suggested a relationship between them. To identify the cells, an antibody against vimentin, which has been used as a marker for the mesenchymal cells, was used. Double-immunofluorescent staining for the vimentin and AFP showed a complete overlap between the AFP positive cells and the antibody staining for vimentin at each stage tested (Figure 4A and B).
AFP is known to be associated with the successful completion of term pregnancies in mammals and even minute amounts of AFP may still be necessary during human pregnancy[13]. The capability of both up and down modulation of growth and differentiation as a dose-dependent function of AFP has been demonstrated in a multitude of cell types including placental, ovarian, uterine, lymphoid, epidermal, endothelial, testicular, breast, and liver[14–18]. The rat colon undergoes rapid growth and differentiation during the last few days of gestation and the first 2 wk after birth[19–22]. This maturation process is accompanied by changes in the composition of AFP, which indicates that AFP might be involved in many aspects of colon development. Liu et al[23] studied the changes of the protein levels of AFP in rat pancreas during development also by Western blot analysis and immunohistochemistry. Their results have demonstrated that the expression of AFP protein in the rat pancreas was increased in e18.5 rats and down-regulated after birth. They found a the similar possibility to ours, that the pancreatic cells, which went through dramatic growth, differentiation and proliferation, result from the dynamic expression of AFP in the various developmental stages[23].
The genetic variants of rat AFP mRNA consist of sizes ranging from 2.2 to 1.35 kb, representing translated proteins ranging from 72 kDa down to 37 kDa, respectively[24]. The smaller AFP isoforms are found to be truncated from the amino-terminal end. Molecular variants of AFP expressed in liver have long been reported in biomedical studies. All isoforms of the 72-kDa, 60-kDa and 48-kDa AFP protein may be involved in different aspects of liver cell behavior[2526]. Using a polyclonal antibody against a peptide mapping at the C terminus of AFP[27–29], we found that only the 72-kDa isoform in the developing rat colons was detected by Western blotting. These results indicate that the 72-kDa isoform of AFP, with the highest expression in embryonic and regenerated liver, may also play an important role in colon cell proliferation and organ maturation.
Tyner et al[9] have reported that AFP is expressed in a subset of enteroendocrine cells expressing chromogranin A, which suggested that they could be of enteroendocrine origin. AFP is a soluble glycoprotein that is able to bind many ligands including fatty acids, metals, steroids (estrogens), thyroxin, and tryptophan[630]. The identity of the cells that express AFP in the colon cells was tested by double antibody staining with antibodies to chromogranin A (an enteroendocrine cell marker), cytokeratin (a epithelial cell marker), proliferating cell nuclear antigen (a proliferative cell marker) and vimentin (a mesenchymal cell marker and AFP) at each stage in rat development, respectively (data not shown). Only an overlap of positive staining of AFP and vimentin was found in the same cell, indicating that AFP is indeed expressed and produced in mesenchymal cells. Liu et al[23] reported that, in rat developing pancreas, AFP was also co-expressed with the vimentin, which was similar to our results. These results demonstrated that mesenchymal cell-derived AFP can act as a potent paracrine regulator of colon cell proliferation and organ maturation. The epithelial-mesenchymal interactions play an essential role in the control of gastrointestinal epithelial growth and differentiation not only in fetal stages, but also in adults[3132], but the mechanism has not been fully understood. Characterization of AFP expression in mesenchymal cells may help us discern a function in the gastrointestinal tract.
Cancer cells display immature features and dysregulated gene expression, with attenuation of tumor suppressors and aberrant expression of genes that are inactive in normal adult tissues. Since many down-regulated genes during development are re-expressed in tumors, understanding their respective cellular roles can provide information about both development and cancer biology. Despite some recent advances[33], the extent of embryonic gene expression by tumor cells and the significance of this phenomenon are still unknown. AFP is one such gene and is reactivated in human tumors of the same fetal origin. Further studies are necessary to identify elements in the AFP gene that contribute to its expression. Numerous data support the hypothesis that AFP repression is a part of a global scheme of liver differentiation prematurely activated by glucocorticoids[34]. The involvement of glucocorticoids in AFP expression in the gut remains an open question. Studies are now in progress in our laboratory using animal and tissue culture models.
In summary, our present study has, for the first time, demonstrated that AFP is localized to the mesenchyme in rat colon from the embryo to the weaning stage (up to 21 d after birth) by immunofluorescence, and presents a 72-kDa isoform in the developing rat colon by Western blotting. The dynamic expression of AFP in the various developmental stages of the colon indicates that AFP might be involved in many aspects of colon development. The exact function of AFP in colon development remains to be determined.
Mammalian α-fetoprotein (AFP) is a single-chain glycoprotein, developmentally down-regulated and re-expressed in tumors. The authors assumed that its accurate profiles over gut developmental interval could provide fundamental information about underlying mechanisms. This resource also can be applied to address longstanding questions about reactivation of fetal genes in cancer.
This study demonstrated that AFP presented a 72-kDa isoform and localized to the mesenchyme in the developing rat colon. The expression of AFP in the various developmental stages is dynamical. AFP might be involved in many aspects of colon development.
This animal model is a useful tool for the studies of gastrointestinal mucosal proliferation and differentiation mechanism in vivo. The authors will identify elements in the AFP gene that contribute to its expression.
AFP is a single-chain glycoprotein with molecular mass ranging from 66 to 72 kDa and a 3%-5% carbohydrate (glycan) content.
The study investigated the expression of AFP and its involvement during rat colon development. It was well designed and conducted adequately.
| 1. | Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693-699. |
| 2. | Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell. 1993;75:59-72. |
| 3. | Luetteke NC, Qiu TH, Peiffer RL, Oliver P, Smithies O, Lee DC. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993;73:263-278. |
| 4. | Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE Jr. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575-588. |
| 5. | Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, Gherardi E, Birchmeier C. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373:699-702. |
| 6. | Tilghman SM. The structure and regulation of the alpha-fetoprotein and albumin genes. Oxf Surv Eukaryot Genes. 1985;2:160-206. |
| 7. | Tilghman SM, Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci USA. 1982;79:5254-5257. |
| 8. | Angeletti RH. Chromogranins and neuroendocrine secretion. Lab Invest. 1986;55:387-390. |
| 9. | Tyner AL, Godbout R, Compton RS, Tilghman SM. The ontogeny of alpha-fetoprotein gene expression in the mouse gastrointestinal tract. J Cell Biol. 1990;110:915-927. |
| 10. | Kaufman MH. The Atlas of Mouse Development. London: Academic press 1992; 8. |
| 11. | Yashpal NK, Li J, Wang R. Characterization of c-Kit and nestin expression during islet cell development in the prenatal and postnatal rat pancreas. Dev Dyn. 2004;229:813-825. |
| 12. | Yashpal NK, Li J, Wheeler MB, Wang R. Expression of {beta}1 integrin receptors during rat pancreas development--sites and dynamics. Endocrinology. 2005;146:1798-1807. |
| 13. | Sher C, Shohat M. Congenital deficiency of AFP and Down syndrome screening. Prenat Diagn. 1997;17:884-885. |
| 14. | Keel BA, Eddy KB, Cho S, May JV. Human alpha-fetoprotein purified from amniotic fluid enhances growth factor-mediated cell proliferation in vitro. Mol Reprod Dev. 1991;30:112-118. |
| 15. | Wang XW, Xie H. Alpha-fetoprotein enhances the proliferation of human hepatoma cells in vitro. Life Sci. 1999;64:17-23. |
| 16. | Cingolani N, Shaco-Levy R, Farruggio A, Klimstra DS, Rosai J. Alpha-fetoprotein production by pancreatic tumors exhibiting acinar cell differentiation: study of five cases, one arising in a mediastinal teratoma. Hum Pathol. 2000;31:938-944. |
| 18. | De Mees C, Laes JF, Bakker J, Smitz J, Hennuy B, Van Vooren P, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein controls female fertility and prenatal development of the gonadotropin-releasing hormone pathway through an antiestrogenic action. Mol Cell Biol. 2006;26:2012-2018. |
| 19. | Helander HF. Morphological studies on the development of the rat colonic mucosa. Acta Anat (Basel). 1973;85:155-176. |
| 20. | Eastwood GL, Trier JS. Epithelial cell proliferation during organogenesis of rat colon. Anat Rec. 1974;179:303-309. |
| 22. | Colony PC, Kois JM, Peiffer LP. Structural and enzymatic changes during colonic maturation in the fetal and suckling rat. Gastroenterology. 1989;97:338-347. |
| 23. | Liu L, Guo J, Yuan L, Cheng M, Cao L, Shi H, Tong H, Wang N, De W. Alpha-fetoprotein is dynamically expressed in rat pancreas during development. Dev Growth Differ. 2007;49:669-681. |
| 24. | Watanabe T, Jimenez-Molina JL, Chou JY. Characterization of a rat variant alpha-fetoprotein. Biochem Biophys Res Commun. 1992;185:648-656. |
| 25. | Petropoulos C, Andrews G, Tamaoki T, Fausto N. alpha-Fetoprotein and albumin mRNA levels in liver regeneration and carcinogenesis. J Biol Chem. 1983;258:4901-4906. |
| 26. | Chou JY, Savitz AJ. alpha-Fetoprotein synthesis in transformed fetal rat liver cells. Biochem Biophys Res Commun. 1986;135:844-851. |
| 27. | Wilkinson DS, Ogden SK, Stratton SA, Piechan JL, Nguyen TT, Smulian GA, Barton MC. A direct intersection between p53 and transforming growth factor beta pathways targets chromatin modification and transcription repression of the alpha-fetoprotein gene. Mol Cell Biol. 2005;25:1200-1212. |
| 28. | Cui R, Nguyen TT, Taube JH, Stratton SA, Feuerman MH, Barton MC. Family members p53 and p73 act together in chromatin modification and direct repression of alpha-fetoprotein transcription. J Biol Chem. 2005;280:39152-39160. |
| 29. | Cavin LG, Venkatraman M, Factor VM, Kaur S, Schroeder I, Mercurio F, Beg AA, Thorgeirsson SS, Arsura M. Regulation of alpha-fetoprotein by nuclear factor-kappaB protects hepatocytes from tumor necrosis factor-alpha cytotoxicity during fetal liver development and hepatic oncogenesis. Cancer Res. 2004;64:7030-7038. |
| 30. | Peters T Jr. Serum albumin. Adv Protein Chem. 1985;37:161-245. |
| 31. | Kedinger M, Simon-Assmann PM, Lacroix B, Marxer A, Hauri HP, Haffen K. Fetal gut mesenchyme induces differentiation of cultured intestinal endodermal and crypt cells. Dev Biol. 1986;113:474-483. |
| 32. | Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu ZX, Pringault E, Leon-Robine S, Louvard D, Walker WA. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA. 1996;93:7717-7722. |
| 33. | Kho AT, Zhao Q, Cai Z, Butte AJ, Kim JY, Pomeroy SL, Rowitch DH, Kohane IS. Conserved mechanisms across development and tumorigenesis revealed by a mouse development perspective of human cancers. Genes Dev. 2004;18:629-640. |
| 34. | Houart C, Szpirer J, Szpirer C. The alpha-foetoprotein proximal enhancer: localization, cell specificity and modulation by dexamethasone. Nucleic Acids Res. 1990;18:6277-6282. |