Published online Mar 21, 2009. doi: 10.3748/wjg.15.1346
Revised: January 12, 2009
Accepted: January 19, 2009
Published online: March 21, 2009
AIM: To investigate the effects of curcumin on the expression of peroxisome proliferator-activated receptorδ (PPARδ) and related genes in HT-29 cells.
METHODS: HT-29 cells were treated with curcumin (0-80 &mgr;mol/L) for 24 h. The effects of curcumin on the morphology of HT-29 cells were studied by Hoechst 33342 staining. The activity of caspase-3 was determined using DEVD-pNA as substrate. The levels of peroxisome PPARδ, 14-3-3epsilon and vascular endothelial growth factor (VEGF) in HT-29 cells were determined by Western blotting analysis and their mRNA expression was determined by real-time quantitative RT-PCR.
RESULTS: Treatment with 10-80 &mgr;mol/L curcumin induced typical features of apoptosis and activated the caspase-3 in HT-29 cells. The expression of PPARδ, 14-3-3epsilon and VEGF was reduced and the activity of β-catenin/Tcf-4 signaling was inhibited by curcumin treatment.
CONCLUSION: Curcumin can induce apoptosis of HT-29 cells and down-regulate the expression of PPARδ, 14-3-3epsilon and VEGF in HT-29.
- Citation: Wang JB, Qi LL, Zheng SD, Wang HZ, Wu TX. Curcumin suppresses PPARδ expression and related genes in HT-29 cells. World J Gastroenterol 2009; 15(11): 1346-1352
- URL: https://www.wjgnet.com/1007-9327/full/v15/i11/1346.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1346
Peroxisome proliferator-activated receptors (PPARs) belong to the nuclear hormone receptor superfamily that enable the cell to respond to extracellular stimuli through transcriptional regulation of gene expression[12]. PPARs comprise three subtypes: PPARα, PPARδ and PPARγ. Many of the functions of PPARs are associated with pathways of lipid transport and metabolism[3–5]. Moreover, PPARs play important roles in cell replication, differentiation, tumorigenesis and apoptosis. For example, the expression of PPARδ is elevated in human and rat colorectal cancer cells when compared with normal colon epithelial cells[67]. PPARδ has also been implicated in the growth of other human cancers, including hepatocellular carcinoma, cholangiocarcinoma, breast cancer and prostate cancer[89].
14-3-3 proteins are anti-apoptotic and anti-inflammatory molecules in cells, which include at least 7 isoforms (β, γ, epsilon, η, ζ, σ, τ/θ)[10]. 14-3-3 can bind phosphorylated Bad, sequester Bad in the cytosol, and inhibit cytochrome c release, caspase-3 activation, and the apoptosis of cells. PPARδ can induce the expression of 14-3-3epsilon protein. Elevated 14-3-3epsilon augments Bad sequestration and prevents Bad-triggered apoptosis[10]. C/EBPβ protein is a mediator of PPARδ-dependent 14-3-3epsilon gene regulation in human endothelial cells. PPARδ can regulate the expression of C/EBPβ protein, which can bind to the C/EBP response element located at -160/-151 of the 14-3-3epsilon gene[11]. PPARδ can directly bind to PPAR response elements located between -1426 and -1477 of the 14-3-3epsilon promoter region, thereby activating 14-3-3epsilon promoter activity and protein expression[10]. PPARδ can also regulate the expression of vascular endothelial growth factor (VEGF), which can promote colon tumor epithelial cell survival[1213].
Curcumin is an important polyphenol extracted from the rhizomes of Curcuma longa L. Several studies have shown that curcumin exerts antioxidant, anti-inflammatory, anti-carcinogenic and chemopreventive activities on many tumor cells[14]. Curcumin can also down-regulate the activity of the β-catenin/Tcf signaling pathway[1516]. Curcumin affects the expression of the target genes of β-catenin/Tcf signaling pathway, such as c-Myc, cyclin D1 and c-Jun. PPARδ has been identified as another β-catenin/Tcf-regulated gene[6]. However, the effect of curcumin on the expression of PPARδ remains unknown. In this study, we investigated the effects of curcumin on the expression of PPARδ and related genes such as 14-3-3epsilon and VEGF. The results showed that curcumin could inhibit the expression of PPARδ and induce the down-regulation of the related genes, including 14-3-3epsilon and VEGF.
RPMI-1640, fetal bovine serum (FBS), penicillin, streptomycin, and trypsin were purchased from GIBCO. Curcumin, sodium dodecylsulfate (SDS), phenylmethyl-sulfonylfluoride (PMSF), DNaseI and bovine serum albumin (BSA) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Anti-VEGF, anti-Tcf-4 and horseradish peroxidase-conjugated goat anti-rabbit antibodies were obtained from Epitomics. Anti-14-3-3epsilon, anti-PPARδ antibody and protein A/G plus-agarose were provided by Santa Cruz Biotechnology. Nitrocellulose membrane and the enhanced chemiluminescence (ECL) detection system were purchased from Amersham (USA). PrimeScript 1st strand cDNA Synthesis Kit and PCR Kit were from Takara, Japan. Caspase-3 assay kit and nuclear and cytoplasmic protein extraction kit were purchased from Beyotime Biotech, China. Other reagents used were of analytical grade and procured locally.
The human colon cancer cell line HT-29 was obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI 1640, supplemented with 10% FBS, 100 U/mL penicillin and 100 &mgr;g/mL streptomycin at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Upon reaching 70%-80% confluence, the cells were exposed to 0-80 &mgr;mol/L curcumin for 24 h.
Caspase-3 activities were measured as previously described[17]. Briefly, cells were lysed in a buffer containing 5 mmol/L Tris (pH 8), 20 mmol/L EDTA, and 0.5% Triton-X 100. Reaction mixture contained 20 mmol/L HEPES (pH 7.0), 10% glycerol, 2 mmol/L dithiothreitol, 50 &mgr;g protein per condition, and 200 &mgr;mol/L DEVD-pNA as substrate. After incubation for 24 h at 37°C, the absorbance in each well was measured at 405 nm with a microplate ELISA reader.
Chromatin condensation was detected by nuclear staining with Hoechst 33342[18]. After treatment with 0-80 &mgr;mol/L curcumin for 24 h, cells were harvested and washed with PBS three times. Then, the cells were stained with 1 &mgr;L of Hoechst 33342 (5 mg/mL, Sigma) in 1 mL basal medium and incubated at room temperature in the dark for 15 min. Stained cells were imaged under a fluorescent microscope using 350 nm stimulation and 460 nm emission.
Total RNA was isolated using Trizol Isolation Reagent (Invitrogen, USA). RNA integrity was confirmed by denaturing agarose gel electrophoresis, and the concentration was quantified by measuring the optical density (OD) at 260 nm. One microgram total RNA was used for DNaseI treatment (Sigma) and subsequent cDNA synthesis. Reverse-transcription was performed with PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Japan) according to the manufacturer’s instructions. Real-time qPCR was performed on the ABI 7500 Real Time PCR System using SYBR Premix Ex Taq II (Takara, Japan) for analyzing expression of genes. Table 1 shows the primers used for real-time quantitative RT-PCR. The amplification reactions were performed under the following PCR conditions: one cycle at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, 59°C for 15 s and 72°C for 30 s. mRNA fold changes in target genes relative to the endogenous GAPDH control were calculated as suggested by Schmittgen et al[19]. Each reaction was performed in triplicate.
| Apoptosis modulator | Forward primer (5’→3’) | Reverse primer (5’→3’) |
| PPARδ | GCAGGCTCTAGAATTCCATC | GTGCAGCCTTAGTACATGTC |
| VEGF | AGGAGGAGGGCAGAATCATCA | CTCGATTGGATGGCAGTAGCT |
| 14-3-3epsilon | GAGCGATACGACGAAATGGT | CCTTGGACTCGCCAGTGTTAG |
| GAPDH | GGCAAATTCAACGGCACAGT | AGATGGTGATGGGCTTCCC |
Western blotting analysis was done as previously described with minor modifications to detect the expressions of PPARδ, 14-3-3epsilon, and VEGF protein[20]. The total cellular protein and the nuclear protein were extracted according to the instructions of nuclear and cytoplasmic extraction reagents kit (Beyotime, Haimen, China). The lysates were used to estimate their protein content with BCA protein assay. Fifty micro-grams of protein from each sample was subjected to SDS-PAGE. After electrophoresis, proteins were electroblotted to a Hybond-C Extra nitrocellulose membrane (Amersham, USA). The membrane was blocked at room temperature with 5% non-fat dry milk in TBS containing 0.3% Tween (TBS-T). The membrane was washed three times with TBS-T and incubated overnight at 4°C with the primary antibody, anti-PPARδ (1:500), anti-14-3-3epsilon (1:2000), and anti-VEGF (1:1000), followed by 1 h incubation with a 1:5000 dilution of the appropriate horseradish-peroxidase-conjugated secondary antibody. After incubation, the membrane was washed with TBS-T for three times, the antigen-antibody complexes were visualized by enhanced chemiluminescence and exposure to X-ray film for 0.5 up to 30 min.
The immunoprecipitation was done as previously described with minor modifications[16]. The nuclear lysates containing 500 &mgr;g protein were incubated with 5 &mgr;g primary antibody overnight at 4°C. Fifty microliters of protein A/G plus-agarose (Santa Cruz Biotechnology) was added and the complex was incubated at 4°C overnight. The beads were washed three times with high salt buffer (1 mol/L Tris-HCl, pH 7.4, 0.50 mol/L NaCl, and 1% Nonidet P-40) and twice with lysis buffer to eliminate non-specific binding. The immunoprecipitated complexes were released with 2 × sample buffer for Western analysis.
Transient transfection was performed using Fugene 6 Transfection Reagent (Roche) in accordance with the manufacturer’s instructions. Briefly, HT-29 cells were seeded at a density of 2 × 105 cells per well in six-well plates. After 24 h, cells were transfected with 0.5 &mgr;g luciferase reporter constructs (TOPflash or FOPflash, respectively) and 0.5 &mgr;g β-galactosidase gene. Three hours after transfection, the cells were treated with 0-80 &mgr;mol/L curcumin and the incubation was continued for 24 h. Then, the cells were collected and resuspended in Luciferase Reporter Lysis Buffer (Promega, USA). The cell lysates were centrifuged and aliquots (70 &mgr;L) of the supernatant were assayed for the activity of luciferase and galactosidase. Reporter activity was normalized for variations in transfection efficiency using β-galactosidase as an internal control. Experiments were performed three times independently.
Results are presented as mean ± SE. Comparisons between multiple groups were performed using the one-way ANOVA followed by Dunnett’s test. Differences were considered to be significant at P < 0.05.
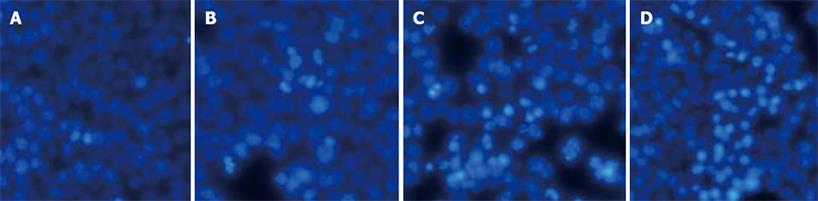
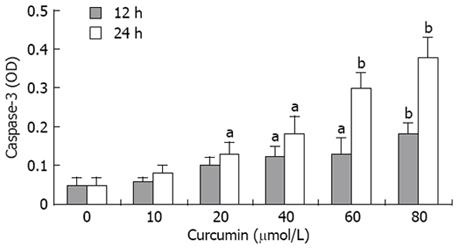
Hoechst 33342 staining assay was performed to observe the effects of curcumin on cell nuclear morphology. As shown in Figure 1, the control cells displayed intact nuclear structure, while nuclei with chromatin condensation and formation of apoptotic bodies were seen in cells incubated with curcumin in a dose-dependent fashion (Figure 1A-D). Caspase-3 activation is an important marker of apoptosis, so we determined the activities of caspase-3 in HT-29 treated with curcumin for 12 or 24 h. Caspase-3 activities were highly elevated by curcumin at the concentration of 20-80 &mgr;mol/L (Figure 2).
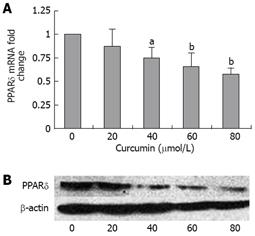
PPARδ plays important roles in the growth and proliferation of many cancer cells[8]. PPARδ has been identified as one of the down-streaming targets of the β-catenin/Tcf-4 pathway[6]. We studied whether curcumin affected the expression of PPARδ in HT-29 cells. HT-29 cells were incubated with curcumin at different concentrations. PPARδ gene expression at the mRNA level was detected by quantitative RT-PCR. The results showed that 40-80 &mgr;mol/L of curcumin could significantly reduce the level of PPARδ in HT-29 cells (Figure 3A). Whole-cell extracts were prepared and analyzed by Western blotting. Curcumin reduced the level of PPARδ protein in a dose-dependent manner (Figure 3B).
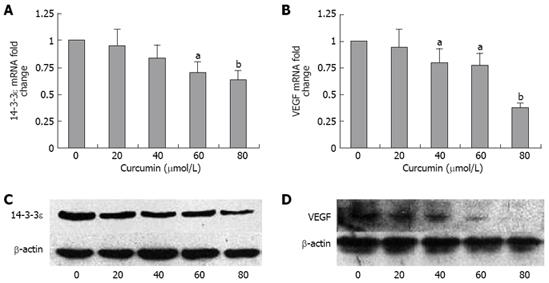
14-3-3epsilon plays important roles in protecting cells from apoptosis[21]. It harbors three contiguous PPAR response elements (PPREs), which are the responsive promoter region of 14-3-3epsilon activation. Deletion of PPREs abrogated PPARδ-mediated 14-3-3epsilon to PPARδ up-regulation[10]. On the basis of this study, we hypothesized that curcumin could inhibit the expression of 14-3-3epsilon in HT-29 cells. The real-time quantitative PCR showed that treatment with 60 and 80 &mgr;mol/L curcumin for 24 h could markedly reduce the 14-3-3epsilon mRNA level in HT-29 cells (Figure 4A). The Western blotting showed that curcumin treatment also inhibited the 14-3-3epsilon protein expression at the concentration of 20-80 &mgr;mol/L (Figure 4C).
VEGF can stimulate endothelial cell proliferation and prevents apoptosis in the endothelial cells of newly formed vessels[22]. VEGF has been identified as one of the potential targets of PPARδ in colorectal cancer (CRC) cells[13]. We hypothesized that curcumin can decrease the expression of VEGF. The real-time quantitative RT-PCR showed that 40-80 &mgr;mol/L of curcumin could significantly down-regulate the expression of VEGF (Figure 4B). Consistent with the VEGF mRNA level, VEGF protein was also decreased by curcumin at the concentrations of 20-80 &mgr;mol/L (Figure 4D).
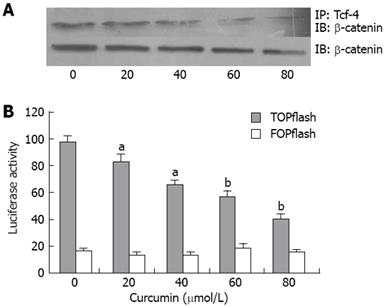
The association of β-catenin with Tcf-4 is required for activation of β-catenin/Tcf signaling, so we determined the level of the β-catenin/Tcf-4 complex in the nucleus of HT-29 cells. We used anti-Tcf-4 antibody to co-immunoprecipitate the complex of Tcf-4 and β-catenin from nuclear extracts and determined the amount of β-catenin by immunobloting. Curcumin markedly dereased the level of β-catenin/Tcf-4 complex (Figure 5A). However, the level of total β-catenin in the nucleus was not significantly affected by incubation of curcumin (Figure 5A). These results suggested that curcumin could inhibit β-catenin associated with Tcf-4 in the nucleus. Because PPARδ is a target gene of β-catenin/Tcf-4, curcumin may decrease the expression of PPARδ through blocking the β-catenin/Tcf-4 signaling pathway.
PPARδ has been identified as a target of the β-catenin/Tcf signaling pathway[6]. We hypothesized that curcumin can inhibit the expression via the β-catenin/Tcf signaling pathway. We transfected HT-29 cells with either TOPflash or FOPflash. The cells were incubated with 0-80 &mgr;mol/L curcumin for 24 h and the luciferase activity was determined. As a result, curcumin could decrease the transcriptional activity of β-catenin/Tcf-4 signaling pathway in HT-29 cells (Figure 5B).
Curcumin can induce apoptosis of many cell lines. However, the mechanism is still unclear. In this study, we demonstrated that curcumin could induce the apoptosis of HT-29 cells and down-regulate the expression of PPARδ, 14-3-3epsilon and VEGF, which suggested that curcumin-induced apoptosis was attributed to the inhibition of the expression of PPARδ, 14-3-3epsilon or VEGF.
The PPARs are ligand-activated transcription factors that are members of the nuclear hormone receptor superfamily. PPARs form heterodimers with the retinoic X receptor and bind to DNA in correspondence to specific PPRE located in the promoter of target genes[23]. The PPAR subfamily includes PPARα, PPARδ (or β) and PPARγ, which share extensive structural homology[23]. Studies have shown that PPARα and PPARγ play important roles in such physiological processes as fatty acid metabolism, glucose metabolism, immunity, and cellular differentiation[2425]. However, the physiological role of PPARδ is less studied. Recently, PPARδ has been found to be related to carcinogenesis[2627]. Curcumin has been proved to inhibit the growth and differentiation of many cancer cell lines[2829]. Administration of 30 mg/kg curcumin by intraperitoneal injection for 2 wk could significantly improve the expression of PPARγ in colonic tissues of rats[30]. To the best of our knowledge, the effects of curcumin on PPARδ have not been investigated. In this study, we found that curcumin could inhibit the expression of PPARδ in HT-29 cells. We hypothesize that there are two possible reasons. First, He et al[6] have identified PPARδ as a β-catenin/Tcf-regulated gene. In this study, curcumin could inhibit the β-catenin associated with Tcf-4 and down-regulate the activity of the β-catenin/Tcf-4 signaling pathway, which is in agreement with the studies of Jaiswal et al and Park et al[1516]. These studies indicate that curcumin may reduce the expression of PPARδ by blocking the β-catenin/Tcf-4 signaling pathway. Second, PPARδ can also be up-regulated by oncogenic K-Ras[31], suggesting that curcumin may reduce the expression of PPARδ by other signaling pathways. We will further study the mechanism that curcumin inhibits the expression of PPARδ.
14-3-3 are cytosolic proteins serving as a scaffold to interact with a large number of proteins[32]. They may protect cells from apoptosis through their binding and sequestering phosphorylated Bad in cytosol[21]. Seven isoforms of 14-3-3 proteins have been identified in mammalian cells[10]. It has been indicated that 14-3-3epsilon is a target gene of PPARδ, which can bind to the PPRE upstream of 14-3-3epsilon promotor region[10]. We found that curcumin decreased the expression of 14-3-3epsilon in HT-29 cells. To the best of our knowledge, curcumin-induced down-regulation of 14-3-3epsilon has not reported previously.
VEGF is also up-regulated by the activation of PPARδ in colon carcinoma cells[12]. Several studies have indicated that curcumin can down-regulate the expression of VEGF in different cell lines[3334]. In the present study, we found that curcumin could inhibit the expression of VEGF in HT-29 cells. VEGF plays important roles in tumor angiogenisis[35]. Our results suggest curcumin may inhibit the angiogenisis of colorectal tumors.
In summary, our study shows that curcumin can suppress the expression of PPARδ and the related genes such as 14-3-3epsilon and VEGF in HT-29 cells. The reasons why curcumin inhibits the expression of PPARδ and related protein are still unclear. Curcumin can down-regulate the activity of β-catenin/Tcf-4 signaling pathway, which suggests that curcumin may decrease the expression of PPARδ by the inhibition of β-catenin/Tcf-4 signaling activity in HT-29 cells.
Colorectal cancer is one of the leading causes of death and is a major public health problem in Western countries. Peroxisome proliferator-activated receptors (PPARs) play important roles in cell replication, differentiation, tumorigenesis, and apoptosis. The expression of PPARδ is elevated in human and rat colorectal cancer cells when compared with normal colon epithelial cells.
Curcumin can affect the expression of the target genes of β-catenin/Tcf signaling pathway, such as c-Myc, cyclin D1, c-Jun, etc. PPARδ has been identified as another β-catenin/Tcf-regulated gene. However, the effect of curcumin on the expression of PPARδ remains unknown. In this study, the authors demonstrate that curcumin could affect the expression of PPARδ and related genes such as 14-3-3epsilon and vascular endothelial growth factor (VEGF).
Recent studies have suggested the PPARδ play important roles in colorectal carcinogenesis. It activates the expression of 14-3-3epsilon, which can sequester the pro-apoptotic protein, Bad, to inhibit the apoptosis of cancer cells. It is the first report showing that the curcumin down-regulates the expression of PPARδ and 14-3-3epsilon.
PPARδ is found as a new target of curcumin to induce the apoptosis of HT-29 cells. New curcumin derivatives can be developed to efficiently inhibit the growth and differentiation of colorectal cancer cells by down-regulating the expression of PPARδ.
Curcumin is an important polyphenol extracted from the rhizomes of Curcuma longa L. PPARs belong to the nuclear hormone receptor superfamily that enable the cell to respond to extracellular stimuli through transcriptional regulation of gene expression, including PPARα, PPARδ, and PPARγ. 14-3-3 proteins are anti-apoptotic and anti-inflammatory molecules in cells, which include at least seven isoforms (β, γ, epsilon, η, ζ, σ, τ/θ). Elevated 14-3-3epsilon augments Bad sequestration and prevents Bad-triggered apoptosis.
Curcumin can induce apoptosis of many cell lines. However, the mechanism is still unclear. In this study, the authors demonstrated that curcumin could induce the apoptosis of HT-29 cells and down-regulate the expression of PPARδ, 14-3-3epsilon and VEGF. More importantly, the authors showed that curcumin could markedly lower the level of β-catenin/Tcf-4 complex without affecting the level of total β-catenin in nucleus after incubation of curcumin. These results suggested that curcumin could inhibit β-catenin associated with Tcf-4 in nucleus.
| 1. | Robinson-Rechavi M, Escriva Garcia H, Laudet V. The nuclear receptor superfamily. J Cell Sci. 2003;116:585-586. |
| 2. | Michalik L, Desvergne B, Wahli W. Peroxisome proliferator-activated receptors beta/delta: emerging roles for a previously neglected third family member. Curr Opin Lipidol. 2003;14:129-135. |
| 3. | Lowell BB. PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99:239-242. |
| 6. | He TC, Chan TA, Vogelstein B, Kinzler KW. PPARdelta is an APC-regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335-345. |
| 7. | Gupta RA, Tan J, Krause WF, Geraci MW, Willson TM, Dey SK, DuBois RN. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci USA. 2000;97:13275-13280. |
| 8. | Stephen RL, Gustafsson MC, Jarvis M, Tatoud R, Marshall BR, Knight D, Ehrenborg E, Harris AL, Wolf CR, Palmer CN. Activation of peroxisome proliferator-activated receptor delta stimulates the proliferation of human breast and prostate cancer cell lines. Cancer Res. 2004;64:3162-3170. |
| 9. | Glinghammar B, Skogsberg J, Hamsten A, Ehrenborg E. PPARdelta activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2003;308:361-368. |
| 10. | Liou JY, Lee S, Ghelani D, Matijevic-Aleksic N, Wu KK. Protection of endothelial survival by peroxisome proliferator-activated receptor-delta mediated 14-3-3 upregulation. Arterioscler Thromb Vasc Biol. 2006;26:1481-1487. |
| 11. | Brunelli L, Cieslik KA, Alcorn JL, Vatta M, Baldini A. Peroxisome proliferator-activated receptor-delta upregulates 14-3-3 epsilon in human endothelial cells via CCAAT/enhancer binding protein-beta. Circ Res. 2007;100:e59-e71. |
| 12. | Wang D, Wang H, Guo Y, Ning W, Katkuri S, Wahli W, Desvergne B, Dey SK, DuBois RN. Crosstalk between peroxisome proliferator-activated receptor delta and VEGF stimulates cancer progression. Proc Natl Acad Sci USA. 2006;103:19069-19074. |
| 13. | Jaeckel EC, Raja S, Tan J, Das SK, Dey SK, Girod DA, Tsue TT, Sanford TR. Correlation of expression of cyclooxygenase-2, vascular endothelial growth factor, and peroxisome proliferator-activated receptor delta with head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2001;127:1253-1259. |
| 14. | Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci. 2005;1056:206-217. |
| 15. | Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414-8427. |
| 16. | Park CH, Hahm ER, Park S, Kim HK, Yang CH. The inhibitory mechanism of curcumin and its derivative against beta-catenin/Tcf signaling. FEBS Lett. 2005;579:2965-2971. |
| 17. | Piwocka K, Zabłocki K, Wieckowski MR, Skierski J, Feiga I, Szopa J, Drela N, Wojtczak L, Sikora E. A novel apoptosis-like pathway, independent of mitochondria and caspases, induced by curcumin in human lymphoblastoid T (Jurkat) cells. Exp Cell Res. 1999;249:299-307. |
| 18. | Chen XL, Cao LQ, She MR, Wang Q, Huang XH, Fu XH. Gli-1 siRNA induced apoptosis in Huh7 cells. World J Gastroenterol. 2008;14:582-589. |
| 19. | Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194-204. |
| 20. | Chen A, Xu J. Activation of PPAR{gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol. 2005;288:G447-G456. |
| 21. | Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell. 1996;87:619-628. |
| 22. | Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024-1028. |
| 23. | Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649-688. |
| 24. | Kallwitz ER, McLachlan A, Cotler SJ. Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J Gastroenterol. 2008;14:22-28. |
| 25. | Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507-514. |
| 26. | Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046-1052. |
| 27. | Brockman JA, Gupta RA, Dubois RN. Activation of PPARgamma leads to inhibition of anchorage-independent growth of human colorectal cancer cells. Gastroenterology. 1998;115:1049-1055. |
| 28. | Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett. 2000;483:78-82. |
| 29. | Sen S, Sharma H, Singh N. Curcumin enhances Vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem Biophys Res Commun. 2005;331:1245-1252. |
| 30. | Zhang M, Deng C, Zheng J, Xia J, Sheng D. Curcumin inhibits trinitrobenzene sulphonic acid-induced colitis in rats by activation of peroxisome proliferator-activated receptor gamma. Int Immunopharmacol. 2006;6:1233-1242. |
| 31. | Shao J, Sheng H, DuBois RN. Peroxisome proliferator-activated receptors modulate K-Ras-mediated transformation of intestinal epithelial cells. Cancer Res. 2002;62:3282-3288. |
| 32. | Tzivion G, Avruch J. 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem. 2002;277:3061-3064. |
| 33. | Chadalapaka G, Jutooru I, Chintharlapalli S, Papineni S, Smith R 3rd, Li X, Safe S. Curcumin decreases specificity protein expression in bladder cancer cells. Cancer Res. 2008;68:5345-5354. |
| 34. | Premanand C, Rema M, Sameer MZ, Sujatha M, Balasubramanyam M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Invest Ophthalmol Vis Sci. 2006;47:2179-2184. |
| 35. | Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550-563. |