Published online Mar 21, 2009. doi: 10.3748/wjg.15.1339
Revised: January 12, 2009
Accepted: January 19, 2009
Published online: March 21, 2009
AIM: To investigate the antioxidant activity of chito-oligosaccharides (COSs) on pancreatic islet cells in diabetic rats induced by streptozotocin.
METHODS: The antioxidant effect of COSs on pancreatic islet cells was detected under optical microscopy and with colorimetric assay and gel electrophoresis. The activities of glutathione peroxidase and superoxide dismutase, total antioxidant capacity, and content of malondialdehyde in serum and tissue slices of pancreas were examined after 60 d to determine the effect of COSs in streptozotocin-induced diabetes in rats.
RESULTS: COSs can prohibit the apoptosis of pancreatic islet cells. All concentrations of COSs can improve the capability of total antioxidant capacity and activity of superoxide dismutase and decrease the content of malondialdehyde drastically. Morphological investigation in the pancreas showed that COSs have resulted in the reduction of islets, loss of pancreatic cells, and nuclear pyknosis of pancreatic cells.
CONCLUSION: COSs possess various biological activities and can be used in the treatment of diabetes mellitus.
- Citation: Yuan WP, Liu B, Liu CH, Wang XJ, Zhang MS, Meng XM, Xia XK. Antioxidant activity of chito-oligosaccharides on pancreatic islet cells in streptozotocin-induced diabetes in rats. World J Gastroenterol 2009; 15(11): 1339-1345
- URL: https://www.wjgnet.com/1007-9327/full/v15/i11/1339.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1339
Chitosan’s properties allow it to rapidly clot blood, and it has recently gained approval in the USA for use in bandages and other hemostatic agents[1]. It has been further suggested that chitosan inhibits uptake of dietary lipids by increasing the thickness of the intestinal lumen boundary layer, a proposal again supported by numerous animal experiments that chito-oligosaccharides (COSs) possess various biological activities and can be used in diabetes treatment. Unlike high molecular weight chitosan, COSs, which are readily soluble in water due to their shorter chain and free amino groups in D-glucosamine units, and easily absorbed through the intestine, can quickly get into the blood flow and have versatile functional properties[2–4].
Pancreatic beta-cell response to nutrient excess occurs by hypertrophy of existing β cells that increases insulin production and then formation of new β cells from progenitor cells. Failure of pancreatic β cells to adequately expand in settings of increased insulin demand ends up in hyperglycemia[5]. Streptozotocin (STZ) selectively kills insulin-secreting β cells, and is widely used to generate animal models of diabetes. Previous studies have suggested that streptozotocin toxicity results from its N-nitrosourea moiety releasing nitric oxide and possessing DNA alkylating activity. More recently, it has also been proposed that streptozotocin induces apoptosis by inhibiting O-GlcNAcase, an enzyme that, together with O-GlcNAc transferase, is important for dynamic intracellular protein O-glycosylation[6].
In the present study, soluble COSs with low molecular weight were prepared by enzymatic hydrolysis of chitosan with chitosanase. The purpose of this study was to examine the antioxidant effect of chito-oligosaccharides on pancreatic islet cells and pancreatic islets in rats with STZ-induced diabetes.
Chitosan (90% deacetylated, Mr: 500000) was purchased from Jinan Haidebei Marine Bioengineering Co., Ltd (Shangdong, China). Chito-oligosaccharides were prepared by enzymatic hydrolysis of chitosan with chitosanase. NIT-1 cell line was from Institute of Medicine, Ocean University of China. Male Wistar rats (200 ± 20 g) were from Laboratory Animal Centre, Institute of Medicine, Ocean University of China. STZ was from Sigma Chemical Co. Tissue-culture medium and reagents were from Gibco. All chemicals were purchased from Sigma Chemical unless otherwise stated.
NIT-1 cell line, a widely used β cell line for insulin secretion studies, was established from non-obese diabetic (NOD) mice transgenic for the SV40 T antigen under control of the insulin promoter, and cultured in DMEM containing 10% FBS and antibiotics (100 IU/mL of penicillin and 100 &mgr;g/mL of streptomycin). All cultures were kept at 37°C in 95% O2 and 5% CO2. The medium was changed every 2 d.
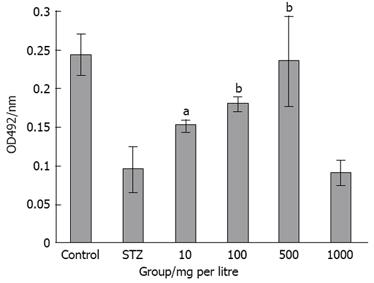
MTT assay: COS was dissolved in Dulbecco’s modified Eagle’s medium (DMEM) without FBS and then diluted the medium to 10, 100, 500, 1000 and 2000 mg/L degrades. The structure and function of cultured pancreatic β cells were observed under inverted phase contrast microscope. MTT assay was used to estimate the cell viability. Pancreatic β cells were digested by 0.25% trypsin until appearance of unicellular suspension. Then, 100-&mgr;L suspensions with the concentration of 5.0 × 104 cells/mL were transplanted into 96-well plates. Each well had eight parallels. After incubating for 24 h, the medium was changed with COSs at different concentrations. Cells were divided into normal control group; positive control group with NIT-1 cells treated with STZ; and COS protective group with NIT-1 cells treated with different concentrations of COS for 0.5 h, and then cultured with 2 mmol/L STZ for 12 h. Cells were incubated with 0.5 mg/mL of MTT in the last 4 h of the culture period (48 h). The medium was then decanted, 200 &mgr;L DMSO was added to the wells, and the absorbance was determined at 492 nm using an ELISA reader. The proliferation rate was calculated (PR = A/A0 × 100 %).
DNA gel electrophoresis: Groups were divided as described above. After treatment, DNA was extracted from the cultured cells, washed in Tris-HCl for 3 times, and 0.5 mL STE extraction buffer (0.5 mol/L NaCl, 100 mmol/L Tris-HCl, 10 mmol/L EDTA, 1% SDS, 100 &mgr;g/mL protease K, pH = 8.0) was added, and the cells were re-suspended. The suspension was incubated in a water bath at 65°C for 30 min with occasional shaking, cooled to room temperature and incubated with chloroform/isoamylalcohol (24:1) for 10 min. After centrifugation at 5600 ×g for 10 min, the supernatant was mixed with dehydrated alcohol and incubated at -20°C for 2 h. Then, the mixture was centrifuged at 8600 ×g for 15 min. The DNA precipitation was washed with 70% ethanol twice and dissolved in sterilized TE (1 mol/L Tris-HCl, 0.1 mol/L EDTA, pH = 8.0). The DNA was examined in 1.2% agarose gel containing ethidium bromide (0.4 &mgr;g/mL) for about 1 h at 5 V/cm. The gel was photographed under ultraviolet light (254 nm) using GDS-760 image-analysis system (UVP).
Single cell gel electrophoresis (SCGE): After treatment, cells were washed in DMEM without serum, resuspended in PBS to a concentration of 2 ×105 cells/10 &mgr;L, mixed with 1% Low Melting Agarose (LMA), spread on a slide coated with a layer of 0.5% Normal Melting Agarose and covered with another layer of LMA. Slides were immersed in lysis solution at 4°C for 1 h. After lysis, the slides were placed in a horizontal gel electrophoresis tank filled with fresh alkaline electrophoresis buffer ( 300 mmol/L NaOH, 1mmol/L Na2-EDTA, pH 13) and incubated in the solution for 20 min at 4°C. Electrophoresis (1 V/cm and 300 mA) was conducted at 4°C for 20 min, using a Bio-Rad 300 power supply. Once completed, the slides were washed 3 times with a neutralizing solution (0.4 mol/L Tris-HCl, pH = 7.5) and stained with ethidium bromide (5 &mgr;g/mL). The slides were examined at 200 × magnification under a fluorescent microscope equipped with an excitation filter of 515-560 nm and a barrier filter of 590 nm. Twenty cells were selected randomly from each group as described above and analyzed by the Casp computerized image analysis system. To quantify the DNA damage, four different comet parameters were evaluated: tail length, tail DNA%, tail moment and olive tail moment.
Male Wistar rats were rendered diabetic by intraperitoneal injection of STZ at 65 mg/kg body weight. The rats whose fasting blood glucose level was above 11.11 mmol/L were used for experiments after 7 d. Then, the rats were randomly divided into metformin treatment group, positive control group and diabetes mellitus (DM) and COS treatment groups. Test samples were given intragastrically with gavage needle for 60 d. COS treatment groups were given COSs orally at the concentrations of 250, 500 and 1500 mg/kg body weight daily. Normal control group and DM group received an equal volume of distilled water, about 10 mL/kg body weight daily. The metformin treatment group was given metformin at a concentration of 200 mg/kg body weight. After 60 d, blood samples were collected by orbital sinus puncture after lightly anesthetizing the rats with ether. Serum was separated from the blood samples by centrifugation (5000 r/min, 10 min) and the activity of glutathione peroxidase (GPH-PX) and superoxide dismutase (SOD), the capability of total antioxidant capacity (T-AOC) and the content of malondialdehyde (MDA) were measured. Pancreas pathologic changes were observed under microscope after the small lumps (3 mm) were fixed in 4% paraformaldehyde in 0.1 mol/L cacodylate buffer (pH 7.2), embedded in paraffin, and stained with hematoxylin-eosin.
ANOVA was performed with Duncan’s multiple range tests. SAS was used to compare the means (SAS Institute, Inc., Cary, NC, USA). P < 0.05 was considered statistically significant
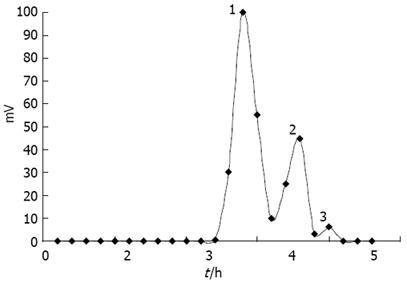
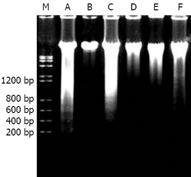
COSs were prepared by enzymatic hydrolysis of chitosan. Components of the hydrolysis product were separated by Sephadex G-25 (Figure 1), and component 1 analyzed by TSK-GEL G3000PWXL had an average molecular weight of 1200 u, and a degree of deacetylation of 90% by the first derivative method of UV spectrometry.
The effect of COS on pancreatic islet cell viability was assessed by MTT assay (Figure 2). The degree of cell damage decreased with the increase of COS concentration (10-500 mg/L) and was significantly higher compared with the positive control group (Figure 3). The maximum protective effect on cell viability was achieved by 500 mg/L COS among the four samples (Figure 4).
Through DNA ladder (Figure 5) detection, we found that the injury degree of DNA fragmentation became more serious with the decrease in COS concentration (500-10 mg/L). As shown in Figure 5, the total DNA of the normal control group was a complete band and non-perceptible. An obvious DNA ladder could be seen in the positive control group treated with STZ. However, the DNA ladder disappeared with the increasing concentration of COS (10-500 mg/L). And the DNA ladder was more obviously decreased with 500 mg/L COS.
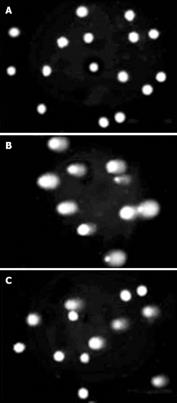
STZ can damage the DNA of the NIT-1 cells. The exposure to STZ produced significant DNA migration in the positive control group (Figure 6B). On the contrary, induction of DNA damage was rarely observed in the COS-treated group (500 mg/L) (Figure 6C). Similar differences in DNA damage between other COS groups (10-500 mg/L) and positive control group were observed after treatment with STZ. The concentration-dependent protective effect was found with COS at 10-500 mg/L, and the degree of DNA damage was decreased.
The results showed that exposure to STZ induced the breakage of DNA strands. COS can inhibit NIT-1 cell damage induced by STZ, and enhance the protective action of pancreatic β cells significantly (Table 1). All concentrations of COS could decrease the cells treated by STZ and had apparent dosage-dependent effects. The pretreatment of COS (500 mg/L) decreased the cells in tail length, tail DNA%, tail moment and olive tail moment significantly (P < 0.01).
| Group | Concentration (mg/L) | Tail length (&mgr;m) | Tail DNA (%) | Tail moment | Olive tail moment |
| Control | 7.64 ± 6.92b | 0.83 ± 1.16b | 0.13 ± 0.24b | 0.32 ± 0.47b | |
| STZ | 80.50 ± 31.72 | 29.93 ± 15.93 | 28.01 ± 22.34 | 25.73 ± 18.52 | |
| COS | 10 | 49.90 ± 33.83a | 28.41 ± 23.35 | 20.69 ± 21.67 | 17.10 ± 14.73 |
| 100 | 34.67 ± 41.72b | 19.77 ± 24.28 | 13.86 ± 20.36 | 11.15 ± 15.40a | |
| 500 | 23.00 ± 20.79b | 6.56 ± 7.57b | 2.90 ± 4.12b | 3.64 ± 4.54b | |
| 1000 | 51.00 ± 15.44a | 20.14 ± 10.51 | 11.48 ± 9.42 | 13.06 ± 6.61a |
COSs at all concentrations improved the capability of T-AOC and activity of SOD and decreased the content of MDA in serum drastically (Table 2). The capability of T-AOC was enhanced and the content of MDA decreased with COS in a dosage-dependent manner.
| Group | Concentration (mg/kg) | T-AOC (U/mL) | GPH-PX (U/mL) | MDA (nmol/mL) | SOD (U/mL) |
| Control | 12.10 ± 2.58b | 468.61 ± 4.35 | 6.21 ± 0.53b | 182.51 ± 15.73b | |
| DM | 5.94 ± 1.70 | 467.363 ± 4.17 | 13.82 ± 1.69 | 116.67 ± 2.19 | |
| COS-H | 1500 | 10.02 ± 1.34b | 458.70 ± 4.47 | 6.03 ± 0.93b | 136.89 ± 13.66 |
| COS-M | 500 | 7.49 ± 1.03a | 461.82 ± 10.20 | 6.88 ± 1.08b | 141.65 ± 11.13a |
| COS-L | 250 | 6.96 ± 2.45a | 464.76 ± 8.79 | 7.2 ± 1.59b | 152.84 ± 13.52b |
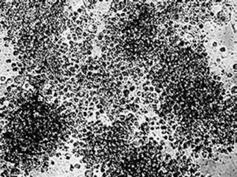
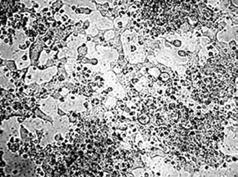
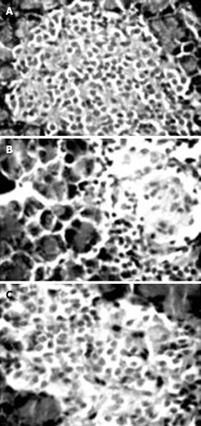
Morphological investigation of pancreas in the DM group showed a reduction of islets, β cell loss, and nuclear pyknosis of β cells to various degrees after 60 d of STZ injection (Figure 7B). While COSs of all concentrations could protect the pancreas against STZ, the morphological observation indicates that the cells treated with medium dosage (500 mg/kg) were most protected (Figure 7C), showing similar morphological features to those of the normal control group (Figure 7A).
It has been observed that the radical scavenging properties of COSs are dependent on their DD and molecular weight. Based on the results obtained from studies carried out using electron spin trapping techniques, COSs with low molecular weight have been identified to have a higher potential to scavenge different radicals[7]. In addition, highly deacetylated (90%) COS is more preferable to scavenge DPPH, hydroxyl, superoxide and carbon-centered radicals[8].
Recently, several approaches have been taken to prepare COSs, among which the enzymatic preparation methods have attracted great interest due to safety concerns. New improvements have been introduced to enzymatic production. Properties of COSs, such as DP, DA, charge distribution and nature of chemical modification to the molecule, strongly influence their observed biological activities. Therefore, molecular weight is considered as a principal characteristic of COSs that highly correlates with their biological activities. We have successfully obtained COSs with a DP of 7-9 by enzymatic hydrolysis and investigated their effects on pancreatic cell protection and their antioxidant activity in vivo and in vitro.
Many studies have suggested that the apoptotic events are often induced by the accumulation of reactive oxygen species (ROS)[9]. The mechanisms of ROS damage to organisms mainly relate to the damage of DNA, especially the oxidative damage of mtDNA. The oxidative damage of mtDNA might cause some changes in the primary structure of DNA, such as base deletion, modification and insertion mutagenesis, among which base deletion is most universal. ROS can also induce base deletion, base modification, single and double-strand breakage, and interstrand DNA cross links in nuclear DNA. Most damage in DNA primary structure is investigated by DNA ladder and SCGE methods. A model of chemically induced islet cell death in vivo and in vitro is the STZ mediated islet cell destruction. STZ is an alkylating agent that causes DNA strand breakage in rat pancreatic islet cells. Recent studies strengthen the hypothesis that the toxic effects of STZ are in part mediated by NO, which is generated during its metabolism.
Our studies found obvious damage to DNA in the process of apoptosis induced by 2 mmol/L STZ. In the positive group treated with STZ only, the total DNA was degraded into approximately 180-200 bp and its multiples and a typical DNA ladder appeared on the agarose gel after electrophoresis. However, it could not be detected in apoptosis induced by STZ after treatment with different concentrations of COS. SCGE assay is a powerful technique for the detection of DNA damage at alkali-sensitive sites in eukaryotic cells. Also known as the “comet” assay, it is a rapid, simple and sensitive method to quantify DNA damage in a small number of cells. During electrophoresis under alkaline conditions, cells with damaged DNA display increased migration of DNA from the nucleus towards the anode. Broken DNA migrates farther in the electric field, and the cell resembles a “comet” with a brightly fluorescent head and a tail region, which increases as damage increases. Our study showed that the long-time exposure to STZ was capable of causing a significant increase in DNA damage. Conversely, no induction of DNA damage was observed in the COS-treated group (500 mg/L). Similar differences in DNA damage between other COS groups and positive control group were observed after treatment with STZ (2 mmol/L). The results indicate that COSs have prominent protective effects on the DNA damage in NIT-1 cells induced by STZ, and can prohibit the apoptosis of NIT-1 cells induced by STZ.
There is an effective defense mechanism of antioxidation in organisms, including SOD, CAT and GPH-PX, which can scavenge ROS effectively. Cells are in the normal condition when the balance is maintained between oxidation and antioxidation. Once the balance is disturbed, the function will be affected, leading to apoptosis. Scavengers of free radicals are preventive antioxidants. The presence of radical scavenging compounds can break the oxidative sequence at different levels. Therefore, there has been a growing interest in the identification of natural antioxidants from many natural sources to overcome the radical-mediated deleterious effects in biological systems. Many biological compounds, including carbohydrates, peptides and some phenolic compounds, have been identified as potent radical scavengers. It has been described in previous studies that COSs can cause a rapid increase in the anti-oxidant enzymes activity to scavenge reactive oxygen. Even though the precise mechanism of radical scavenging activity of COSs is not clear, it is often attributed to the reaction between the amino and hydroxyl groups attached to C-2, C-3 and C-6 positions of the pyranose ring and the unstable free radicals, to form stable macromolecule radicals. However, there are some discrepancies about hydroxyl radical scavenging activities of COSs and some of their derivatives. The latest studies have revealed that metal ion uptake ability of COSs has a great influence on their hydroxyl radical scavenging ability[10]. According to their results, hydroxyl radical scavenging potency of COSs is partly due to chelating ability of transition Fe2+, molecular charge properties and proton donation via hydroxyl and amino groups. The uptake of metal ions by COSs can proceed through different mechanisms, including chelation via electron pairs of amino groups and ion exchange mechanisms of protonated amino groups[11]. Therefore, it further strengthens the effect of DD on radical scavenging which is directly correlated with protonation of amine groups. However, there is not much information about the relationship between ion chelation and antioxidant properties of COSs to date.
Our study showed that COSs of all concentrations improved the capability of T-AOC and activity of SOD and drastically decreased the content of MDA. Morphological investigation of the pancreas showed that COSs have significant effects on the reduction of islets, pancreatic cell loss and nuclear pyknosis of pancreatic cells, and can protect the pancreas against STZ. The possible mechanism of COSs’ protective function in the pancreas is that they have strong in vivo antioxidative properties, so that they can scavenge directly the reactive free radicals such as hydroxyl radical and superoxide anion, and inhibit DNA damage, to protect pancreas against oxidative damage induced by STZ.
The highly increasing prevalence of obesity and type 2 DM in the general population makes non-alcoholic fatty liver disease (NAFLD) the most common diagnosis in clinical practice[12]. Our study provided evidence for the protective effects of COSs in pancreastic β-cells against STZ in vitro and confirmed the usefulness of COSs in improving antioxidant ability and protecting the pancreas islet of STZ-induced diabetes in rats in vivo. These biological activities have considerable potential in DM and NAFLD.
Chitooligosaccharide (COS) possesses various biological activities and has been shown to be particularly useful in diabetes treatment. Several methods have been recently used to prepare COS, and enzymatic preparation methods capture a great interest due to their safety and non-toxicity.
Pancreatic β cells maintain the blood glucose concentration within a narrow range by modulating insulin secreton rate in response to the glucose levels in the blood. Proper insulin secretion requires the coordinated functioning of numerous β cells that form pancreatic islets. This coordination depends on a network of communication mechanisms whereby β cells interact with extracellular signals and adjacent cells via connexin channels. This study indicated that COSs improved the antioxidant ability and protection of the pancreas islet of streptozotocin (STZ)-induced diabetes in rats in vivo.
Soluble COSs with lower molecular weight were prepared by enzymatic hydrolysis of chitosan with chitosanase. This study indicated that COSs could improve the antioxidant ability and protect the pancreas islet against STZ-induced diabetes in rats in vivo.
This study indicated that COS could improve the antioxidant ability and protect the pancreas islets of STZ-induced diabetes in rats in vivo. These biological activities have considerable potential in diabetes mellitus treatment.
COS is a polycationic copolymer consisting of β-1, 4-linked 2-acetamido-D-glucose and β-1, 4-linked 2-amino-D-glucose units. COS obtained by hydrolysis or degradation of chitosan, is not only water-soluble but also more effective than chitosan. NIT-1 cell line, a widely used β cell line in insulin secretion studies, is established from non-obese diabetic mice transgenic for the SV40 T antigen under the control of insulin promoter.
| 1. | Ikeda I, Tomari Y, Sugano M. Interrelated effects of dietary fiber and fat on lymphatic cholesterol and triglyceride absorption in rats. J Nutr. 1989;119:1383-1387. |
| 2. | Fernandes JC, Tavaria FK, Soares JC, Ramos OS, João Monteiro M, Pintado ME, Xavier Malcata F. Antimicrobial effects of chitosans and chitooligosaccharides, upon Staphylococcus aureus and Escherichia coli, in food model systems. Food Microbiol. 2008;25:922-928. |
| 3. | Oliveira EN Jr, El Gueddari NE, Moerschbacher BM, Peter MG, Franco TT. Growth of phytopathogenic fungi in the presence of partially acetylated chitooligosaccharides. Mycopathologia. 2008;166:163-174. |
| 4. | Jung WK, Moon SH, Kim SK. Effect of chitooligosaccharides on calcium bioavailability and bone strength in ovariectomized rats. Life Sci. 2006;78:970-976. |
| 5. | Chang-Chen KJ, Mullur R, Bernal-Mizrachi E. Beta-cell failure as a complication of diabetes. Rev Endocr Metab Disord. 2008;9:329-343. |
| 6. | Pathak S, Dorfmueller HC, Borodkin VS, van Aalten DM. Chemical dissection of the link between streptozotocin, O-GlcNAc, and pancreatic cell death. Chem Biol. 2008;15:799-807. |
| 7. | Park PJ, Je JY, Kim SK. Free radical scavenging activity of chitooligosaccharides by electron spin resonance spectrometry. J Agric Food Chem. 2003;51:4624-4627. |
| 8. | Je JY, Park PJ, Kim SK. Free radical scavenging properties of hetero-chitooligosaccharides using an ESR spectroscopy. Food Chem Toxicol. 2004;42:381-387. |
| 9. | Xie W, Xu P, Liu Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg Med Chem Lett. 2001;11:1699-1701. |
| 10. | Huang R, Mendis E, Kim SK. Factors affecting the free radical scavenging behavior of chitosan sulfate. Int J Biol Macromol. 2005;36:120-127. |
| 11. | Guzman J, Saucedo I, Revilla J, Navarro R, Guibal E. Copper sorption by chitosan in the presence of citrate ions: influence of metal speciation on sorption mechanism and uptake capacities. Int J Biol Macromol. 2003;33:57-65. |
| 12. | Tarantino G. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? World J Gastroenterol. 2007;13:4669-4672. |