Published online Mar 14, 2009. doi: 10.3748/wjg.15.1246
Revised: November 13, 2008
Accepted: November 20, 2008
Published online: March 14, 2009
AIM: To study the morphology and ontogeny of dendritic cells of Peyer’s patches in rats at different development periods.
METHODS: The morphometric and flow cytometric analyses were performed to detect all the parameters of villous-crypts axis and the number of OX62+DC, OX62+CD4+SIRP+DC, and OX62+CD4-SIRP-DC in the small intestine in different groups of rats. The relationship between the parameters of villous-axis and the number of DC and DC subtype were analyzed.
RESULTS: All morphometric parameters changed significantly with the development of pups in the different age groups (F = 10.751, 12.374, 16.527, 5.291, 3.486; P = 0.000, 0.000, 0.000, 0.001, 0.015). Villous height levels were unstable and increased from 115.24 &mgr;m to 140.43 &mgr;m as early as 3 wk postpartum. Villous area increased significantly between 5 and 7 wk postpartum, peeked up to 13817.60 &mgr;m2 at 7 wk postpartum. Villous height and crypt depth ratios were relatively stable and increased significantly from 2.80 ± 1.01 to 4.54 ± 1.56, 9-11 wk postpartum. The expression of OX62+DC increased from 33.30% ± 5.80% to 80% ± 17.30%, 3-11 wk postpartum (F = 5.536, P = 0.0013). OX62+CD4+SIRP+DC subset levels detected in single-cell suspensions of rat total Peyer’s patch dendritic cells (PP-DCs) increased significantly from 30.73% ± 5.16% to 35.50% ± 4.08%, 5-7 wk postpartum and from 34.20% ± 1.35% to 43.60% ± 2.07% 9-11 wk postpartum (F = 7.216, P = 0.005).
CONCLUSION: This study confirms the age-related changes in villous-crypt axis differentiation in the small intestine. Simultaneously, there are also development and maturation in rat PP-DCs phenotypic expression. Furthermore, the morphological changes of intestinal mucosa and the development of immune cells (especially DC) peaked at 9-11 wk postpartum, indicating that the intestinal mucosae reached a relatively mature state at 11 wk postpartum.
- Citation: Zhou YJ, Gao J, Yang HM, Zhu JX, Chen TX, He ZJ. Morphology and ontogeny of dendritic cells in rats at different development periods. World J Gastroenterol 2009; 15(10): 1246-1253
- URL: https://www.wjgnet.com/1007-9327/full/v15/i10/1246.htm
- DOI: https://dx.doi.org/10.3748/wjg.15.1246
Different development periods have been suggested to play a role in controlling the development of gastrointestinal mucosal immune responses, which induce the intestinal mucosal immunity reflected differently over different development periods in children. As the lymphoid tissue is the primary site for the induction of mucosal immune responses. The morphometry of the villous-crypt axis in the small intestine reflects the function and adaptation of intestinal mucosal barriers. It was therefore of interest to investigate the potentially disparate phenotypic expression of dendritic cells (DCs) and the morphology of intestinal mucosa found at different periods as a basis for determining the mechanisms that are apparently critical in intestinal mucosal immunity.
DCs were first identified in 1973 by Steinman and Cohn[12] and were found in two locations in the intestinal mucosa: the Peyer’s patches (PPs) and lamina propria (LP). PP-DCs were isolated by Spalding in 1983[3]. It is now clear that PP-DCs may be unique in their ability to induce the differentiation of T cells that produce important cytokines such as IL-4 and IL-10 and other cytokines, including TGF-β, which is important for B cell differentiation and bystander suppression after oral antigen feeding[45]. PP-DCs primarily perform two important tasks: (1) uptake of antigen after its transcytosis across the follicle-associated epithelium (FAE), which is mediated by immature DCs located largely in the subepithelial dome (SED); (2) T and B cell activation by mature DCs, which are found in the SED. T and B cells activated in PPs are “imprinted” and back to the gut due to the unique ability of PP-DCs to induce lymphocytes to regulate immunity[6–9].
DC studies to date have been primarily carried out in mice in vitro. Little literature exists about rat DCs and of DCs in vivo, as current techniques for DC isolation are likely to induce phenotypic changes in DC. However, rats are of great value as experimental animals, especially in the field of nutrition. Additionally, some important findings about DC have been achieved through animal experiments. We therefore chose the rat model as the experimental object to detect the statement in vivo. In previous studies, DCs in mice had been divided into two subsets: CD8αα+ and CD8αα -DC[10]. CD8αα+ and CD8αα-DC preferentially activate T cells toward Th1 and Th2 differentiation, respectively[11]. CD8αα+DC constitutively cross-presents antigens to T cells, while CD8αα-DC does so upon their activation[12]. Kelsall et al[13] also identified two distinct subsets of DCs in 6 to 8-wk-old murine PP. One population of DCs was positioned to capture antigens transported by overlying M cells, while the other subset activated native T cells to become effector cells. Recently, four different subsets of DCs have been described in mouse PPs[1415]. Conversely, progress in the studies of rat DC is slow because of the relative shortage of reagents. CD11C, MHC-class II, and αepsilon-intergin (OX62 antigen) expression is routinely detected in rats to define DC in peripheral and lymphoid tissues. αepsilon-intergin expression is the strongest in mucosa-associated DC[1617]. Additionally, as early as in 1980, rat DCs were subdivided on the basis of differential expression of CD4 and a member of the SIRP (signal inhibitory regulatory protein) family of molecules (detected by OX41)[1819]. The existence of CD4-/SIRP- and CD4+/SIRP+DC subsets from the small intestine has recently been described[20]. Some reports[20] have shown that 12 to 16-wk-old rat intestinal and hepatic lymph DC is αE2 interginhi (OX-62) and includes two subsets: (1) signal regulatory protein α (SIRPα)hi/low and (2) CD4hi/low, which most likely represents murine CD8αα-/+DC. In both lymph and the spleen, SIRP+/CD4+ DCs are more potent than SIRP-/CD4- DCs in the activation of allogeneic CD4+ and CD8+ T cells, naïve Ag-specific CD4+ T cells in vivo, and sensitized Ag-specific CD4+ T cells in vitro. To date, few studies have been conducted in rat PP-DCs at different development periods, our work focused on the morphometry of the villous-crypt axis in the small intestine as well as PP-DC differentiation and maturation in rats during ontogeny. Flow cytometry was used with emphasis laid on the implications of PP-DC interactions with intestinal mucosal immunity.
Our current study was to gain a better understanding of the expression and activation of PP-DCs in Sprague-Dawley rats. Single-cell suspension from the PP of the small bowel was isolated via collagenase A digestion. By collecting and manipulating these cells at 4°C, we maintained the cell activity. Using this model, we ascertained two phenotypically distinct subsets of PP-DC (both OX62+) that were distinguished by the existence or nonexistence of CD4 and SIRP coexpression. We also explored whether age-related development of DC ontogeny and morphological change occurred simultaneously. Our results demonstrate that morphological change is associated with DC ontogeny, suggesting that the intestinal mucosal immune system continuously changes as young rats mature.
SD rats were purchased from the Shanghai Laboratory Animal Center, Chinese Academy of Sciences, Shanghai, China. Rats were held in plastic cages in temperature- and humidity-controlled animal quarters under a 12 h light/dark cycle and were fed a standard diet (rodent rat chow) ad libitum with free access to tap water. All procedures were approved by the Institutional Animal Care Committee.
Abs mouse anti-rat OX62:RPE, mouse anti-rat CD172a:FITC (OX41), and mouse anti-rat CD4:APC (Serotec) were purchased from Shanghai Jingmei Corporation.
SD rats were bred and maintained under specific pathogen-free conditions at Xinhua Hospital Affiliated to the Medical College, Shanghai Jiaotong University, Shanghai, China. Animals, aged 3 wk and weighing 52-58 g, were divided into 5 groups of equal size and approximately equal mean body weight (54 g), namely groups of 3, 5, 7, 9 and 11 wk. Rats were killed after a postpartum period of 21, 35, 49, 63 and 77 d. Abdominal cavities were opened by horizontal incision along the midsection and guts were excised. Central ileum tissue samples (0.5 cm) were taken. All PP tissue samples were taken from the small bowel.
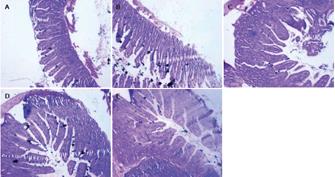
Immediately after collection, ileum tissue samples were washed 3 times in cold phosphate buffered saline (PBS) and fixed for 48 h in 4% formalin solution. After fixation, specimens were dehydrated and embedded in paraffin. Sections from each sample were cut at a thickness of 4 &mgr;m and stained with hematoxylin and eosin (HE).
Sections were examined under a light microscope. Villous height, villous width, and crypt depth in all tissues were determined using the image analysis system. Villous height was measured from the top of the villi to the Lamina muscularis mucosae. Villous width was defined as the distance from one crypt-villi junction to the next. The villous area (height × width) was calculated out of these 2 parameters. PP areas in the ileum were imaged and measured in triplicate using a digital camera and software. PP outlines were performed by hand.
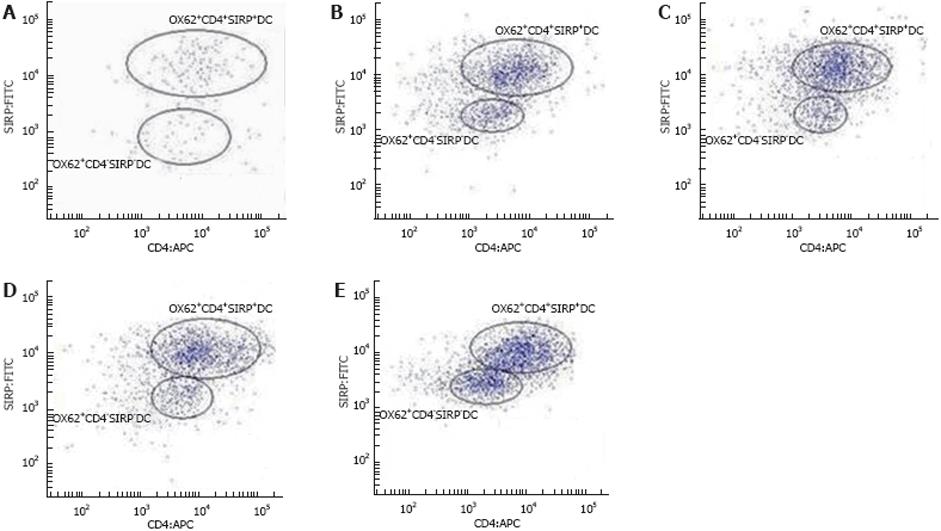
PP tissue samples were washed extensively 3 times in cold PBS. They were then cut into small segments and placed in cold PBS (4°C). After centrifugation at 800 rpm for 5 min at 4°C, the supernatant was removed and the remaining tissues were digested with 0.75 mg/mL collagenase A for 45 min at 37°C with periodic agitation. Undigested stromal material was removed by passing over membrane filtration. Single-cell suspension was prepared and cells were incubated with mouse anti-rat OX62:RPE, mouse anti-rat CD172a:FITC (OX41) and mouse anti-rat CD4:APC for 30 min.
SPSS 11.0 statistical software was used for data analysis. Results were expressed as mean values with standard deviation (SD). Statistical analysis was completed using one-way ANOVA. Tukey’s test was used to determine the significance of the difference between groups when the ANOVA test indicated a significant effect. P values of less than 0.05 were considered statistically significant.
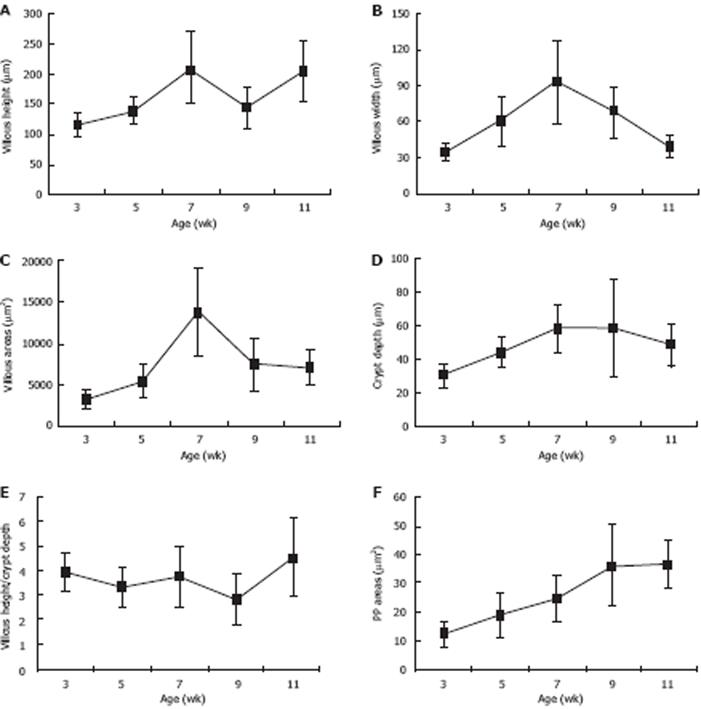
The morphological parameters of the villous-crypt axis in the small intestine included villous height, villous width, villous areas, crypt depth, and the ratio of villous height to crypt depth. As shown in Table 1, Figure 1 and Figure 2A-E, all morphometric parameters changed significantly with the development of pups in the different age groups (F = 10.751, 12.374, 16.527, 5.291, 3.486; P < 0.05). Villous height was unstable and increased from 115.24 ± 21.82 &mgr;m to 140.43 ± 22.30 &mgr;m as early as 3 wk postpartum, decreased from 210.71 ± 59.47 &mgr;m to 145.43 ± 34.21 &mgr;m at 7-9 wk, and then increased again after 9 wk. Villous width increased from 3 wk postpartum, peaked at 7 wk up to 93.06 ± 35.03 &mgr;m, and decreased thereafter. Villous areas increased significantly between 5 wk and 7 wk postpartum from 5451.92 ± 2029.10 &mgr;m2 to 13817.60 ± 5236.52 &mgr;m2, peeked at 7 wk, and decreased from then on, whereas few differences were found between 9 and 11 wk postpartum. Crypt depth increased from 3 wk to 7 wk postpartum, and decreased slightly at 9 wk. Few age-related differences were evident between 7 wk and 9 wk postpartum. Villous height and crypt depth ratio was relatively stable and increased significantly from 2.8 ± 1.01 to 4.54 ± 1.56 at 9-11 wk postpartum.
| 3 wk | 5 wk | 7 wk | 9 wk | 11 wk | F | P | |
| Villous height (&mgr;m) | 115.24 ± 21.82 | 140.43 ± 22.30 | 210.71 ± 59.47 | 145.43 ± 34.21 | 205.14 ± 51.31 | 10.751 | 0.000 |
| Villous width (&mgr;m) | 34.41 ± 8.67 | 60.57 ± 20.61 | 93.06 ± 35.03 | 66.91 ± 21.28 | 38.82 ± 8.96 | 12.374 | 0.000 |
| Villous areas (&mgr;m2) | 3334.46 ± 1134.11 | 5451.92 ± 2029.10 | 13817.60 ± 5236.52 | 7495.52 ± 3059.18 | 7209.91 ± 2087.77 | 16.527 | 0.000 |
| Crypt depth (&mgr;m) | 30.06 ± 6.61 | 44.12 ± 9.25 | 58.76 ± 14.16 | 58.70 ± 29.04 | 48.29 ± 12.94 | 5.291 | 0.001 |
| Villous height/crypt depth | 3.92 ± 0.76 | 3.30 ± 0.79 | 3.74 ± 1.22 | 2.80 ± 1.01 | 4.54 ± 1.56 | 3.486 | 0.015 |
PPs located in the small intestine showed a highly significant increase in size and number with age (P < 0.05), and the degree of increase in different age groups varied from a few to a moderate number (from 12.25 ± 4.69 &mgr;m2 to 37.12 ± 8.20 &mgr;m2 at 3-11 wk postpartum) (Table 2, Figure 2F).
| 3 wk | 5 wk | 7 wk | 9 wk | 11 wk | F | P | |
| PP areas (&mgr;m2) | 12.25 ± 4.69 | 18.91 ± 7.73 | 24.68 ± 7.73 | 36.13 ± 14.59 | 37.12 ± 8.20 | 7.474 | 0.000 |
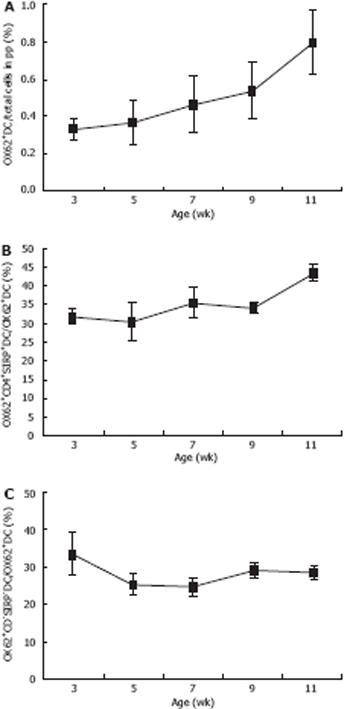
Single-cell suspensions of the total PP-DCs in rats were identified by immnolabelling for OX62, which contained two populations distinguished by co-expression of CD4 and SIRPα or not. As shown in Table 3, Figures 3 and 4, the expression of OX62+DC (i.e. the total PP-DC) increased from 33.3% ± 5.8% to 80% ± 17.3% at 3-11 wk postpartum. Moreover, age-related changes in total PP-DCs occurred as early as 3-5 wk postpartum and became more pronounced 9-11 wk postpartum. OX62+CD4+SIRP+DC subset levels detected in single-cell suspensions of rat total PP-DCs increased significantly from 30.73% ± 5.16% to 35.5% ± 4.08% at 3-5 wk postpartum and from 34.2% ± 1.35% to 43.6% ± 2.07% at 7-9 wk postpartum, whereas few age-related differences were found 9-11 wk postpartum. OX62+CD4-SIRP-DC levels varied in different age groups, whose trends were downward on the whole from 33.27% ± 5.71% to 28.43% ± 1.49% at 3-11 wk, although small fluctuations were observed 7-9 wk postpartum.
| Age | 3 wk | 5 wk | 7 wk | 9 wk | 11 wk | F | P |
| OX62+DC/total cells in PP (%) | 0.333 ± 0.058 | 0.367 ± 0.115 | 0.467 ± 0.153 | 0.533 ± 0.153 | 0.8 ± 0.173 | 5.536 | 0.013 |
| Ox62+CD4+SIRP+DC/OX62+DC (%) | 31.87 ± 1.99 | 30.73 ± 5.16 | 35.50 ± 4.08 | 34.20 ± 1.35 | 43.60 ± 2.07 | 7.216 | 0.005 |
| Ox62+CD4-SIRP-DC/OX62+DC (%) | 33.27 ± 5.71 | 25.33 ± 2.82 | 24.53 ± 2.64 | 28.97 ± 1.89 | 28.43 ± 1.94 | 3.242 | 0.060 |
PPs[21] are typical gut-associated lymphoid tissues located along the small intestine wall and serve as the major sites for generation of immunity to intestinal antigens. PPs contain large amounts of immunocytes, of which DCs are the most potent antigen-presenting cell for activation of further immune response. Moreover, intestinal DC results in immune response to the intestinal mucosa, and therefore will not stimulate the immune response system. Thus, intestinal DC[22], one of the best characters representing the maturation and activation of intestinal mucosal immunity, can exist in different levels of maturation and activation that are reflected in different ways, including antigen capture and process, effector cell activation, and cytokine networking.
It is well known that rats at 21-28 d, 28-60 d, and > 60 d postpartum approximate to the human weaning stage, childhood period, and adult period, respectively. Therefore, in our study we selected rats at the ages of 3, 5, 7, 9 and 11 wk postpartum to simulate the different human development periods. We found that DCs isolated from single-cell suspensions of rat PP revealed OX62 expression and were subdivided based on the differential expression of CD4 and a member of the signal inhibitory regulatory protein (SIRP) family of molecules (detected by the OX41). These results concur with a previous study[1720]. Trinite et al[23] have proposed that CD4 and SIRP expression levels may be related to DC developmental stages or reflect differential induction in response to the nature of the microenvironment and their expression is entirely tissue-specific and related to the function of DC in these tissues. DCs that coexpress CD4 and OX41, a member of the SIRP family, have functional properties typical of mature DCs. In contrast, CD4-OX41-DCs are weak APCs for specific Ag, and in the allogeneic MLR survive poorly in cultures, contain large cytoplasmic inclusions, and are very strongly nonspecific esterase (NSE).
The current study revealed that development of the rat intestinal mucosa varies among different age groups, and the morphological parameters of the villous-crypt axis in the small intestine reflected proliferation and differentiation with age. Additionally, the total number of PP-DCs as well as the DC subpopulation trends varied significantly. Zaph et al[24] have also proposed that immune cells could promote the development of intestinal mucosa, and the mature immune system could promote the development of intestinal morphology. Our results therefore indicate that the development changes consisting of the proliferation and differentiation of intestinal morphology and the activation and maturity of intestinal immune cells (particularly DC) occurred simultaneously and not accidentally. The small intestine morphometric parameters and the total PP-DC increased with age and were more pronounced 9-11 wk postpartum, which indicates that the function of the intestinal mucosal barrier and mucosal immune system of rats gradually matured. The trend of CD4+SIRP+ DC subpopulations was upward at the same time that CD4-SIRP- DC subpopulations were downward, which indicates that the immune system matured with age. The number of PP-DC subpopulations changed notably in the early post-weaning period (3-5 wk postpartum), which may be related to the change in diet from milk to solid feeding as well as intestinal adaptive adjustment induced by an increase in the number of antigens. All morphometric parameters detected in the small intestine increased promptly in rats aged 5-7 wk postpartum compared with rats at other age periods. It has been reported that the phenomenon of villous atrophy in mammals occurs after diet changes; this mechanism is related to a reduction in the rate of cell update and an increase in the rate of cell loss. Moreover, villous atrophy can also result in the intestinal immune function decline; therefore, we speculate that the phenomenon of delayed development of villous parameters may correspond to the diet change in the early post-weaning period (3-5 wk postpartum), which further impacted intestinal mucosal immune function in the late post-weaning period (5-11 wk postpartum). Finally, in the late post-weaning period, the development rate of PP-DCs decreased and the PP-DCs were in a relatively stable state. Additionally, the villous height/crypt depth ratio is a useful criterion for estimating digestive capacity in the small intestine[25]. Although a reduction in villous height occurred 7-11 wk postpartum, maintenance of the villous height/crypt depth ratio 7-11 wk postpartum suggested that a reduction in villous height was less deleterious when it was not accompanied by increased crypt depth. These results coincide with those of Montagne et al[25].
In conclusion, results from the current study indicate that the intestinal mucosal immune system is continuously changing as young rats mature. This study confirmed the age-related changes in villous-crypt axis proliferation and differentiation in the small intestine. Simultaneously, there are also developments and maturations in rat PP-DCs phenotypic expression. Furthermore, the morphological changes of intestinal mucosa and the development of immune cells (especially DC) peaked at 9-11 wk postpartum, indicating that the intestinal mucosae reached a relatively mature state at 11 wk postpartum. In the early weaning period, the number and the phenotypic expression of PP-DCs as well as the morphology changed rapidly due to ingestion of solid food, however, its mechanism requires further studies.
Recent evidences indicated that the different development period plays a role in controlling the development of gastrointestinal mucosal immunity. Peyer’s patches (PPs) were the primary sites for the induction of mucosal immune responses. Dendritic cell (DC) populations play a major role in regulating gastrointestinal mucosal immune responses. The morphometry of the villous-crypt axis in the small intestine reflects the function and adaptation of intestinal mucosal barriers. It is therefore of interest to investigate the potentially disparate phenotypic expression of DC and the morphology of intestinal mucosa found in different periods.
PPs are typical gut-associated lymphoid tissues, which contain large amounts of immunocytes. The PP-DC can result in immune response to the intestinal mucosa, and therefore will not stimulate the immune response system. However, the study on DC was most thorough in vitro or in serum, because the techniques for DC isolation from mucosal lymphoid issue such as PP are relatively rare. Therefore, this research investigated the PP-DCs and the morphology of intestinal mucosa at different periods as a basis for determining the mechanism by which it may initiate regulatory events that are apparently critical in intestinal mucosal immunity.
The authors evaluated, perhaps for the first time, the morphological and ontogeny of dendritic cells at different development periods. The analysis was carried out in 5 groups As a result, all morphometric parameters (including villous height, villous width, villous areas, crypt depth, and villous height and crypt depth ratios) changed significantly with the development of pups at the different age groups (F = 10.751, 12.374, 16.527, 5.291, 3.486; P < 0.05). The OX62+CD4+SIRP+DC subset detected in single-cell suspensions of the rat total PP-DCs increased significantly at 3-11 wk postpartum, while the OX62+CD4-SIRP-DC levels varied in different age groups.
This analysis of morphometric parameters and ontogeny of dendritic cells at different development periods may help further study the gastrointestinal mucosal immunity.
The measurement of morphometric parameters and ontogeny of immunity cells in gastrointestinal mucosa is useful for studies of gastrointestinal mucosal immunity. Although further studies are required, this study indicates the novel possibility for investigating the mucosal immunity cells in gastrointestine.
| 1. | Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142-1162. |
| 2. | Steinman RM, Nussenzweig MC. Dendritic cells: features and functions. Immunol Rev. 1980;53:127-147. |
| 3. | Spalding DM, Koopman WJ, Eldridge JH, McGhee JR, Steinman RM. Accessory cells in murine Peyer’s patch. I. Identification and enrichment of a functional dendritic cell. J Exp Med. 1983;157:1646-1659. |
| 4. | Spalding DM, Griffin JA. Different pathways of differentiation of pre-B cell lines are induced by dendritic cells and T cells from different lymphoid tissues. Cell. 1986;44:507-515. |
| 5. | Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med. 1999;190:229-239. |
| 6. | Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur J Immunol. 2002;32:1445-1454. |
| 7. | Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88-93. |
| 8. | Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Márquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963-969. |
| 9. | Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157-1160. |
| 10. | Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978-2986. |
| 11. | Maldonado-López R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587-592. |
| 12. | den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(-) dendritic cells in vivo. J Exp Med. 2002;196:817-827. |
| 13. | Kelsall BL, Strober W. Distinct populations of dendritic cells are present in the subepithelial dome and T cell regions of the murine Peyer’s patch. J Exp Med. 1996;183:237-247. |
| 14. | Iwasaki A, Kelsall BL. Localization of distinct Peyer’s patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381-1394. |
| 15. | Salazar-Gonzalez RM, Niess JH, Zammit DJ, Ravindran R, Srinivasan A, Maxwell JR, Stoklasek T, Yadav R, Williams IR, Gu X. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623-632. |
| 16. | Brenan M, Puklavec M. The MRC OX-62 antigen: a useful marker in the purification of rat veiled cells with the biochemical properties of an integrin. J Exp Med. 1992;175:1457-1465. |
| 17. | Brenan M, Rees DJ. Sequence analysis of rat integrin alpha E1 and alpha E2 subunits: tissue expression reveals phenotypic similarities between intraepithelial lymphocytes and dendritic cells in lymph. Eur J Immunol. 1997;27:3070-3079. |
| 18. | Robinson AP, White TM, Mason DW. Macrophage heterogeneity in the rat as delineated by two monoclonal antibodies MRC OX-41 and MRC OX-42, the latter recognizing complement receptor type 3. Immunology. 1986;57:239-247. |
| 19. | Adams S, van der Laan LJ, Vernon-Wilson E, Renardel de Lavalette C, Döpp EA, Dijkstra CD, Simmons DL, van den Berg TK. Signal-regulatory protein is selectively expressed by myeloid and neuronal cells. J Immunol. 1998;161:1853-1859. |
| 20. | Liu L, Zhang M, Jenkins C, MacPherson GG. Dendritic cell heterogeneity in vivo: two functionally different dendritic cell populations in rat intestinal lymph can be distinguished by CD4 expression. J Immunol. 1998;161:1146-1155. |
| 21. | Shreedhar VK, Kelsall BL, Neutra MR. Cholera toxin induces migration of dendritic cells from the subepithelial dome region to T- and B-cell areas of Peyer’s patches. Infect Immun. 2003;71:504-509. |
| 22. | Uhlig HH, Powrie F. Dendritic cells and the intestinal bacterial flora: a role for localized mucosal immune responses. J Clin Invest. 2003;112:648-651. |
| 23. | Trinite B, Voisine C, Yagita H, Josien R. A subset of cytolytic dendritic cells in rat. J Immunol. 2000;165:4202-4208. |
| 24. | Zaph C, Troy AE, Taylor BC, Berman-Booty LD, Guild KJ, Du Y, Yost EA, Gruber AD, May MJ, Greten FR. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552-556. |
| 25. | Montagne L, Pluske JR, Hampson DJ. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003;108:95-117. |