INTRODUCTION
In non-cytolytic viral infections, the immune system (and mainly T-lymphocytes) is necessary to clear the infected cells. The most specialized and effective homeostatic control is provided by cytotoxic T lymphocytes (CTLs) (CD8+ T-lymphocytes). For clonal expansion, CTLs need activating “help” from CD4+ T-lymphocytes, which, in turn, require recognition of viral antigens on professional antigen-presenting cells (APC), including dendritic cells (DC), macrophages and B-lymphocytes.
Recognition of antigens by T-lymphocytes depends upon the expression of the major histocompatibility complex (MHC) on the surface of APC. MHC molecules involved in antigen presentation include MHC class I, MHC class II and MHC-like CD1 molecules[1]. MHC class I molecules are expressed on the nuclear cells and load protease-generated peptides to CD8+ T-lymphocytes. MHC class II molecules are expressed on the surface of specialized APC and load peptides generated in the endocytic compartment to CD4+ T cells. CD1 molecules bind acyl chains, therefore allowing T cells to recognize fatty acids, glycolipids and lipopeptide antigens (self or foreign)[2].
Hepatitis C virus (HCV) is an example of an intracellularly persistent virus, which targets liver cells and is eliminated by immune cells[34]. In acute HCV infection, antigen presentation plays a pivotal role in the activation of immune response, while HCV protein-induced defects in antigen presentation lead to insufficient activation of the immune system, which controls the killing of infected hepatocytes[5]. Accumulation and persistence of virally infected cells provide a basis for the chronic course of HCV infection. The reduced clearance of virus and increased viral load usually observed in immunodeficient and in alcohol-consuming patients are associated with a high risk of hepatocarcinoma development[67].
LIVER AS AN IMMUNE PRIVILEGE ORGAN
Liver is considered an immunological organ[8]. Liver cells are involved in the clearance of foreign antigens, which come from the gastrointestinal tract. The physiological role of the liver is to face antigenic flow from the gastrointestinal tract and to respond to this intervention by activating the immune response. To escape immune activation, liver cells use a mechanism of immune tolerance, which prevents unnecessary immune-mediated damage of hepatocytes.
Some liver cells, such as liver dendritic, Kupffer, endothelial and stellate cells serve as antigen presenting cells[9]. Kupffer cells (KC) are the resident macrophage population, which act as incompetent antigen-presenters[10]. They produce IL-10, which down-regulates CD4+ T-cell activation[1112]. Reactive oxygen species (ROS) production is an essential trigger of antigen presentation in KC, accompanied by induction of MHC class II and co-stimulatory molecule expression[13]. In contrast to KC, liver endothelial cells (LSEC) are known as potent APC that present antigens in a MHC class II-restricted manner to CD4+ T-cells[9]. The efficacy of their antigen presentation is comparable to DC[8]; however, IL-10 and TGFβ secreted by KC and LSEC as well as lipopolysaccharide (LPS), which comes with blood from the gastrointestinal tract, can down-regulate their antigen-presenting properties[1415]. Both KC and LSEC express MHC antigens, costimulatory and adhesion molecules and produce IL-1 and IFNγ, suggesting that these are relatively mature cells[16]. In contrast, liver DC are immature cells, which express MHC antigens on their surface, but express a relatively small amount of co-stimulatory molecules, which does not allow them to efficiently stimulate naïve T-cells[17]. Hepatic DC play an important role in the induction and regulation of immune responses, by interacting with CD4+, CD8+ lymphocytes and natural killers (NK) cells. These cells typically reside only around portal triads and like other DC, they capture, process and transport antigens to regional lymphoid tissues[16]. Two subpopulations of DC, myeloid and plasmocitoid cells are found in liver at a low density. Exposure of progenitor DC to IL-10 and TGFβ generates suppressive and tolerogenic effects[18]. In the liver, they are resistant to DC maturation stimuli, such as IFNγ and TNFα, while matrix proteins, such as collagen type 1, induces maturation[19]. However, even being tolerogenic in the liver, hepatic DC are the main professional liver APC, which can further migrate to lymphoid tissue to undergo maturation[20]. In addition to other liver cells, stellate cells have also recently been characterized as having antigen presentation properties[21].
Almost 60% of liver cells are hepatocytes. Due to specific liver architecture, hepatocytes are exposed to a mixture of portal venous and hepatic arterial blood. Liver parenchyma is actively involved in immune response by expressing a variety of receptors. Because liver is a site of apoptotic CD8+ T-lymphocyte accumulation[22], it raises the question whether liver cells, including hepatocytes, induce apoptosis in activated CD8+ T-lymphocytes, thereby promoting a tolerogenic response or they specifically attract apoptotic T-lymphocytes without causing T-lymphocyte death. Resting hepatocytes act as APC for MHC class I-restricted T-cells, while MHC class II and CD1 are not constitutively expressed on hepatocytes[23]. However, the role of hepatocytes as potent APC activating intrahepatic lymphocytes is contradictory. While some studies indicate that activation of naïve CD8+ T-lymphocytes does not happen in case the primary activation occurs within the liver, other studies suggest that the presentation of antigens in the liver showed no immunosuppressive effect on the activation of CD8+ T-cells and that hepatocytes are an excellent priming site for naïve CD8+ T-cells[24–26].
ANTIGEN PROCESSING AND PRESENTATION IN THE CONTEXT OF MHC CLASS I
Proteolysis is the first step in the antigen processing and presentation pathway. Peptide products are transported by transporters associated with antigen processing (TAP) to endoplasmic reticulum (ER), where they assemble in a trimolecular complex with β2-microglobulin and the heavy chain of MHC. Assembly is facilitated by TAP and a number of chaperones and allows class I molecule to bind the peptide to achieve optimal MHC class I loading. These complexes are transported to the plasma membrane, where they interact with the T-cell receptor (TCR) of a T-lymphocyte. TAP has sequence preference and peptide-size limitation, which is matched to MHC class I. To be presented in a context of MHC class I, antigenic proteins undergo the processing to peptides and then the peptides are further cleaved, to fit into the MHC class I groove. Certain proteases participate in the cleavage of peptides for antigen presentation, and the major protease is proteasome.
For activation, CD8+ cells require presentation of MHC class I-peptide complexes on professional APC cells. Peptides can be generated from viral proteins sensitized by infected APC or from proteins originally synthesized by other infected “donor” cells (cross-priming, or cross presentation). Cross-priming can be carried by molecular chaperones and come from apoptotic cells[27]. In addition, it is suggested that these peptides are captured by the lysosomal compartment, with further trafficking to cytosol followed by proteasomal processing for MHC class I loading[28]. However, other authors question the role of proteasomal processing in the cross-presentation mechanism[29].
PROTEASOME, AMINOPEPTIDASES AND INTERFERON GAMMA-INDUCED GENERATION OF PEPTIDES FOR ANTIGEN PRESENTATION
Degradation of intracellular proteins into oligopeptides for antigen presentation is catalyzed by the proteasome[30]. This multicatalytic enzyme exists as a 26S particle, which is ATP-dependent and recognizes ubiquitylated polypeptides. Another form, the 20S proteasome, can degrade substrate proteins in an ubiquitin-independent manner. Both the 26S and 20S proteasome particles degrade antigenic proteins that may later be presented as peptides, depending on the properties of the protein. The chymotrypsin-like and the trypsin-like activities of the proteasome are related to antigen presentation due to their ability to cleave peptide bonds after hydrophobic and basic amino acids, respectively[30]. In cells treated with IFNγ, proteasome particles acquire two novel MHC-encoded low molecular weight subunits, known as LMP-2 and LMP-7, and a third subunit, MECL-1, encoded by genes outside the MHC locus[31]. The acquisition of these three subunits converts the constitutive proteasome to the immunoproteasome and alters its peptidase activities for the generation of uniform sized 8-10-mer peptides[32]. The importance of immunoproteasome induction for antigen presentation has been confirmed using LMP-2 and LMP-7-knockout mice, which demonstrated impaired antigen presentation, reduced expression of MHC class I and poor CTL response to viral epitopes[3334]. The immunoproteasome is responsible for cleavage of peptides at their C-termini[35]. However, some antigenic peptides have N-terminal extensions that are unable to bind to the MHC class I groove. Aminopeptidases, namely, cytosolic leucine aminopeptidase (LAP), finally trims the epitopes to 8-9 amino acid residues necessary to form the complexes with MHC class I[36]. This enzyme is also IFNγ-inducible[37]. Trimmed peptides are transported to the endoplasmic reticulum (ER) by TAP. In the ER lumen, the peptide can be further trimmed by downstream endoplasmic reticulum aminopeptidase I (ERAP1)[38] to generate a stable complex with MHC class I heavy and light chains (β2-microglobulin). These complexes, with the help of molecular chaperones, traffic to the cell surface to be recognized by CD8+ T-cells[3940]. The immune system relies heavily on intracellular protein degradation to recognize a complex of surface MHC class I molecules with short peptide fragments of eight to nine amino acids[41]. Induction of CTLs is programmed by the spectrum of presented MHC class I-peptide complexes on the cell surface[42].
HCV, MHC CLASS I-RESTRICTED ANTIGEN PRESENTATION AND EFFECTS OF ETHANOL
The effects of HCV proteins on MHC class I-restricted antigen presentation are not widely studied and most of the findings are related to MHC class II-restricted antigen presentation. In HCV infected patients, associations between the human MHC and sustained virological response have been found, indicating that the various immunogenetic backgrounds of chronic hepatitis C patients are related to the differences in the course of the disease[43].
Processing of CTL epitopes in HCV infected cells may be altered due to mutations generated by HCV, which interfere with the ability of the peptide to be cleaved by the proteasome[44]. Nevertheless, interferon alpha-mediated induction of immunoproteasome or aminopeptidase expression has been shown as a necessary step for CD8+ T-cell activation in acute HCV infected chimpanzees[4546]. In addition to HCV-restricted mutations of viral proteins, proteasome activity is also modulated by HCV. Thus, proteasome activity in the nuclear compartment is up-regulated by PA28γ activator which forms a complex with HCV core protein[47], while non-structural HCV protein, NS3, interacts with LMP-7, an immunoproteasome subunit, and reduces its activity, potentially providing a negative effect on the generation of peptides for antigen presentation[48]. Our previous studies on HCV core protein expressing Huh7 cells, which also express CYP2E1, revealed that core protein slightly enhances 20S proteasome activity, by 20S proteasome-core protein direct interactions and by induction of low CYP2E1-dependent oxidative stress[49]. However, this proteasome activation is reversed after ethanol exposure, where ethanol treatment considerably reduces proteasome function due to induction of high oxidative stress[49]. Indeed, the dependence of proteasome on the level of oxidative stress has been previously demonstrated[5051], and ethanol is known to suppress proteasome function in liver cells[52–55]. Ethanol-elicited suppression of proteasome activity in the liver ultimately results in reduced generation of antigenic peptides[56] and reduced MHC class I-restricted antigen presentation on hepatocytes (Osna et al, Hepatology, in press). Presentation of peptide-MHC class I complexes on virally infected hepatocytes (HCV, HBV infections, etc) as target cells is crucial for CTLs because when clonal expansion of CTLs is established, the next important restriction for elimination of infected cell is the availability of peptide-MHC class I complexes on the surface of target cells (hepatocytes), which are recognized by CTLs. Thus, even if CD8+ T-cells are activated by the presentation of HCV peptides on professional APC, the recognition of the peptide-MHC class I complexes on hepatocytes (which are target cells for CTLs) may be limited under ethanol-induced oxidative stress.
MHC CLASS II-RESTRICTED ANTIGEN PRESENTATION
Loading of MHC class II molecules with antigenic peptides requires the involvement of specialized APC, such as DC, B-lymphocytes and macrophages. Phagocytosis is the main way to capture antigenic proteins by DC or macrophages, while B-lymphocytes use the antigen specific B-cell receptors[57]. Antigens are digested into peptides (which are longer than those required for MHC class I-restricted antigen presentation) by proteases at endosome compartments. Cathepsins, intracellular acidic proteases, play a pivotal role in the processing of internalized antigens into class II-presentable T cell epitopes[58]. These peptidases play a dual role by generating the peptides or by destroying them. The coupling of peptides with MHC class II is controlled by chaperones and takes place in late endocytic vesicules[44].
When pathogen-associated molecular patterns (PAMPs) on macrophages are recognized by innate immunity receptors (such as Toll-like (TLR)-, mannose-, Fc- and complement- receptors), they induce production of pro-inflammatory cytokines, namely, IFNγ and colony-stimulating factor (GM-CSF). These cytokines increase the expression of MHC class II and co-stimulatory molecules in the cells. Chronic TLR signaling induced by LPS may impair MHC class II-restricted antigen presentation[59]. A large number of class II-bound proteins are derived from cytosolic proteins. Because the autophagy inhibitor has been shown to block the presentation of cytosolic peptides by MHC class II molecules, some authors discuss the role of autophagy in delivery of these cytosolic peptides to the endocytic route[60].
After immature DC engulf pathogens, they undergo biochemical changes, such as secretion of TNFα, IL-6, IL-12, IL-10 and IFNα and expression of co-stimulatory molecules, including CD80, CD86 and CD40. These cells traffic to the nearest lymph node for presentation to T-lymphocytes. However, only mature DC can efficiently prime naïve T-lymphocytes, because only these cells generate MHC class II molecules, by redistribution of class II molecules and cathepsins to peptide-loading compartments and by enhancement of lysosomal acidification[61]. It is still unclear how the peptide-MHC class II complex is recruited to the cell surface from the endocytic compartment in DC. Human DC are subdivided into two big categories: myeloid DC and plasmocytoid DC. In addition to phenotypic differences, they express different TLRs and secrete a different spectrum of cytokines. Thus, myeloid DC express TLR3 and upon stimulation, produce IL-12p70, thereby promoting Th1 response, while plasmocytoid DC express TLR7 and TLR9 and secrete IFNα[62]. Therefore, myeloid DC cells are considered classic antigen presenters, while plasmocytoid DC have a limited ability to capture, process and load antigen onto MHC molecules[63]. Activation of DC is positively regulated by IFNγ and additional CD40 ligation; anti-inflammatory cytokines, such as IL-10, inhibit both IL-12 and IFNα expression, which results in pathogen survival[64].
MHC CLASS II-RESTRICTED ANTIGEN PRESENTATION, HCV AND ETHANOL
HCV infection affects DC function in many ways. Firstly, there is a decrease in the frequency of peripheral myeloid or plasmocytoid DC in chronically infected patients[65]. This may be, in part, related to an accumulation of intrahepatic DC in HCV infection, due to altered DC trafficking[66]. However, another study suggested that HCV directly targets mature cells because some HCV proteins (core, NS3 and NS5) induce apoptosis in DC[67]. Secondly, myeloid DC from chronic HCV patients have a decreased capacity to stimulate allogenic T-lymphocytes[68]. Furthermore, HCV core and E1 genes introduced in DC obtained from uninfected donors, lower their capacity to stimulate allogenic T-cell response[69]. In addition, core, NS3 and NS4 viral proteins were reported to influence the differentiation of DC from monocytes, due to IL-10 secretion[70]. The function of myeloid DC obtained from HCV patients is also decreased due to a reduced level of co-stimulatory molecules and down-regulated HLA-DR expression[568]. In addition, in chronic HCV patients, the production of IL-12 by DC is suppressed by HCV proteins, but can be restored by successful antiviral therapy[71]. Thirdly, the impairment of plasmocytoid DC function has been reported in HCV patients and reduced IFNα production upon DC stimulation with TLR ligands has also been observed[72]. This decrease in IFNα production can be attributed to monocyte-derived TNFα and IL-10[73]. Interestingly, the other studies did not demonstrate defective abilities in plasmocytoid DC to initiate immune response[74], thereby questioning the altered presentation of HCV proteins by these cells to CD4+ cells.
The proposed mechanisms of ethanol-HCV interactions were summarized elsewhere[75]. Ethanol drastically enhances the negative effects of HCV on DC function. A possible reason for this is that alcohol stimulates HCV expression at HCV RNA and proteins levels, which is, in part, related to up-regulation of the Cox2 pathway[76]. Chronic ethanol feeding suppresses CD4+ T cell proliferation in response to antigens as well as CTL activity after DNA-based vaccine immunization with NS5 and HCV-expressing plasmids[77–79]. This CD4+T-cell response was restored by co-administration of IL-2–expressing plasmid, while the restoration of CTL activity required administration of GM-CSF[7778]. Alcohol doubles the effects of HCV by decreasing expression of co-stimulatory molecule, B7, and IL-12 production, increasing IL-10 production and lowering allostimulatory capacity[80–82]. Furthermore, human studies demonstrated that ethanol affects the allostimulatory activity of resting and activated DC generated from peripheral blood mononuclear cells in the presence of cytokines[81], indicating that HCV infection impairs DC function, which is further exacerbated by ethanol. Recent studies performed on alcohol-fed mice, immunized with HCV DNA-based vaccine that induces immune response to NS5 HCV protein, demonstrated that ethanol exposure reduced the number of splenic DCs without altering endocytosis capacity. It also reduced the lymphoid DC population, as well as expression of co-stimulatory molecules, CD40 and CD86, altered cytokine profile (enhanced production of IL-1 and IL-10 and decreased secretion of IL-12, IFNγ and IL-6)[83]. Finally, CTL response to NS5 was shown to be impaired, but was corrected by syngeneic transfer of control DC[83].
HCV, IFN SIGNALING AND ETHANOL
Antigen presentation is so tightly regulated by IFNs that it should be analyzed in the context of IFN signaling. The reported effects of HCV proteins on IFN signaling are quite controversial. Most studies revealed down-regulation of STAT1 phosphorylation in response to IFNα[84–86] attributed to SOCS3 activation by HCV core or NS5A proteins. In addition to that, NS3 protein plays a role in dissociation of the adaptor molecule, MAVS that disrupts the early activation of IFNβ signaling by the virus[8788]. Subsequently, the disruption of STAT1 phosphorylation by the NS3/NS4 complex was documented[89]. In some studies, suppressed STAT1 phosphorylation was linked to either the ability of the core protein to specifically interact with the SH2 domain, preventing STAT1 hetero- or homodimerization[90] or to direct targeting of STAT1 with core protein and subjecting activated STAT1 to degradation by the proteasome[91]. The latter hypothesis is consistent with our recent findings that core protein activates proteasome function[49]. Core protein also inhibits the IFN-induced nuclear import of STAT1 and STAT2 and transcription of antiviral genes[9293]. HCV proteins interfere with IFNα signaling related to the attachment of activated STAT1 to DNA. Methylation on arginine residues, which prevents the formation in STAT1-PIAS1 complexes, is altered in chronic hepatitis C patients[9495]. Protective effects of the methylation regulators, S-adenosyl methionine and betaine, were shown to correct HCV-induced inhibition of IFNα signaling in vitro[96]. Interestingly, in other studies, core protein has been shown to activate interferon stimulated response element (ISRE) and gamma activated site (GAS) sequence promoters in the presence or absence of IFNα and γ and to stimulate INOS promoter, INOS protein induction as well as activation of IFN-inducible 2’5’-oligoadenylate synthetase (2’5’-OAS)[9798]. Less is known about the interference of HCV with IFNγ-induced signaling, which is up-regulated by core protein, due to enhanced IFNγR2 expression[99]. In a cell culture system, prolonged treatment with IFNγ attenuated IFNα-signaling[100]. Acute ethanol treatment of hepatoma cells inhibited anti-viral actions of IFN against HCV replicon, induced serine STAT1 phosphorylation, but blocked tyrosine phosphorylation[101]. Recently, it has been shown that alcohol metabolism induces increased replication of HCV and attenuates the antiviral effects of IFN[102]. However, the number of studies regarding the combined effects of ethanol and HCV proteins on IFN signaling is very limited.
CONCLUSION
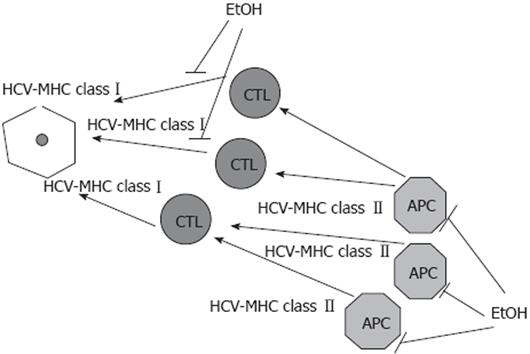
HCV alters antigen-presentation capacities of professional APC, thereby contributing to the persistence of virally infected cells. The most prominent effects were observed on MHC class II-restricted antigen presentation and DC functions. Ethanol potentiates the effects of HCV, providing further suppression of both MHC class I- and class II-restricted antigen presentation on DC, hepatocytes and others hepatic APC, which diminish CTL response. IFNs which support antigen presentation by activating APC and peptidases lose this property in the presence of HCV and alcohol, because HCV and alcohol, separately or in combination, reduce IFN signaling. This, in part, explains why alcohol consumption exacerbates the course, worsens the outcome and reduces the responsiveness to interferon alpha treatment in chronic HCV infection. The effects of alcohol (ethanol) on HCV-restricted antigen presentation are summarized in Figure 1.
Figure 1 Alcohol (ethanol) reduces antigen presentation on HCV-infected liver cells.
HCV interferes with the capacity of professional antigen presenting cells (APC) to prime the immune response. Ethanol (EtOH) further dysregulates antigen presentation, suppressing both MHC class I-restricted antigen presentation on infected hepatocytes and MHC class II-restricted antigen presentation on APC.
Supported by Development funds from Section of Gastroenterology/Hepatology, Internal Medicine, University of Nebraska Medical Center
Peer reviewer: Eva Herrmann, Professor, Department of Internal Medicine, Biomathematics Saarland University, Faculty of Medicine, Kirrberger Str., 66421 Homburg/Saar, Germany
S- Editor Li LF L- Editor Webster JR E- Editor Ma WH