Published online Feb 14, 2008. doi: 10.3748/wjg.14.892
Revised: November 22, 2007
Published online: February 14, 2008
AIM: To evaluate and characterize the patterns of disease progression of metastatic or unresectable gastrointestinal stromal tumor (GIST) treated with imatinib mesylate, and to determine the prognostic significance associated with disease progression.
METHODS: Clinical data and computed tomography (CT) images were retrospectively reviewed in 17 GIST patients who were treated with imatinib mesylate from October 2002 to October 2006. Apart from using size measurement for evaluation of tumor response [Response Evaluation Criteria in Solid Tumors (RECIST) criteria], patterns of CT changes during treatment were evaluated and correlated with clinical data.
RESULTS: There were eight non-responders and nine responders. Five patterns of CT change during treatment were found: focal progression (FP), generalized progression (GP), generalized cystic change (GC), new cystic lesion (NC) and new solid lesion (NS). At the end of study, all non-responders showed GP, whereas responders showed cystic change (GC and NC) and response according to RECIST criteria. Overall survival was significantly better in patients with cystic change or response within the RECIST criteria compared with GP patients (P = 0.0271).
CONCLUSION: Various patterns of CT change in patients with GIST who responded to imatinib mesylate were demonstrated, and might determine the prognosis of the disease. A combination of RECIST criteria and pattern of CT change are proposed for response evaluation in GIST.
- Citation: Phongkitkarun S, Phaisanphrukkun C, Jatchavala J, Sirachainan E. Assessment of gastrointestinal stromal tumors with computed tomography following treatment with imatinib mesylate. World J Gastroenterol 2008; 14(6): 892-898
- URL: https://www.wjgnet.com/1007-9327/full/v14/i6/892.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.892
Gastrointestinal stromal tumors (GISTs) are uncommon neoplasms that arise from mesenchymal cells in the walls of the gastrointestinal tract and account for 0.1%-3.0% of all gastrointestinal neoplasms and 5.7% of sarcomas[12]. GISTs arise most often from the stomach (60%-70%), followed by small intestine (20%-25%), but rarely from the rectum (5%), esophagus, colon or appendix[3]. GISTs differ from the other mesenchymal neoplasms histopathologically by immunohistochemical expression of CD 117 (c-kit proto-oncogene)[4].
Surgical resection is the standard initial treatment for non-resectable GISTs. However, locally advanced and metastatic tumors of the peritoneum or liver can effectively be treated with imatinib mesylate (known as Gleevec in the United States, Glivec in the rest of the world, and previously referred to as STI 571; Novartis Pharmaceuticals, Basel, Switzerland), a selective-tyrosine kinase inhibitor[56]. The drug acts as a c-kit blocker and is highly effective in patients with advanced disease[7], with nearly half of the patients responding to treatment[8].
For optimal clinical outcome, the accurate assessment of tumor response to imatinib mesylate has become important. Computed tomography (CT), magnetic resonance imaging (MRI), and fluorodeoxyglucose positron emission tomography (FDG-PET) are acceptable imaging techniques to assess response to imatinib mesylate, with FDG-PET being superior[59]. CT is commonly used to assess therapeutic response in patients with GISTs. CT enables characterization of primary and recurrent tumors. In the past, the treatment response has been classically quantified using only a decrease in size as the main criterion for tumor response[10]. Recent studies have shown a cyst-like appearance with no further decrease in size is inconsistent with therapeutic response[11–13].
The purpose of this study was to evaluate and characterize the patterns of disease progression of metastatic or unresectable GIST treated with imatinib mesylate. In addition, the study sought to determine the prognostic significance associated with disease progression.
The study was conducted under the approval of the Institutional Review Board and Ethics Committee of Ramathibodi Hospital Faculty of Medicine, Bangkok, Thailand. Seventeen consecutive patients with metastatic and unresectable GISTs diagnosed histopathologically as positive for CD117 were included in this retrospective review. All patients had a period of imatinib treatment during October 2002 to October 2006. Patients received a starting dose of 400 mg/d imatinib mesylate orally. In the event of disease progression, an increase in daily dosage to 800 mg was allowed. In cases of side effects, the dose was decreased to 300 mg/d. In cases of clinical non-response or death, the treatment was terminated. Our patients included 13 men and four women, aged 36-69 (mean 53) years. The primary tumor sites were the stomach (eight patients), small bowel (six), rectum (two) and retroperitoneum (one patient). At enrollment, 10 patients had initial metastatic disease, three had recurrent disease, three had no free surgical margin, and one had unresectable advanced disease.
Sixty-two lesions (38 in the liver, nine in the mesentery, six in the pararectal region, three in the stomach, two in the peritoneum, two in the lymph nodes, one in the retroperitoneum, and one in bone) were evaluated on the basis of the Response Evaluation Criteria in Solid Tumors (RECIST) criteria for each organ and body compartment invaded by the tumor. Inclusion criteria were as follows: (1) all lesions > 1.0 cm in the longest diameter; and (2) lesions ranging from 0.5 to 1.0 cm in the longest diameter, with no more than five lesions. Exclusion criteria were: (1) lesions < 0.5 cm in the longest diameter; and (2) lesions which were difficult to follow up due to changing location (small bowel and mesentery), or having partial volume.
We retrospectively reviewed the medical records and radiological features of each patient.
Each patient underwent baseline CT before treatment (with the exception of patients who underwent scans at another hospital before treatment). Follow-up studies consisted of CT at 1-mo intervals for up to 6 mo after imatinib mesylate treatment. The routine CT at our institution was performed on a spiral CT unit (LightSpeed Plus; General Electric Medical Systems, Milwaukee, WI, USA; Somatom Sensation Cardiac 64; Siemens, USA). CT scans of the upper, lower or whole abdomen were performed (5- or 3-mm slice thickness, 1- or 1.5-pitch, and 5.0- or 7.5-mm collimination) from the level of the diaphragm to the end of the kidney, from the top of the kidney to the pubic symphysis, and from the level of the diaphragm to the pubic symphysis, in the upper, lower and whole abdomen, respectively. Oral contrast, 1000-1500 mL 2% diatrizoate meglumine was given about 2 h prior to scanning, and an intravenous bolus of 100-120 mL 60% non-ionic contrast agent (Omnipaque 300 (GE Healthcare, USA), Ultravist 300 (Schering AG, Germany) or Xentrix 300 (Guerbet, France)) was administered at the rate of 3 mL/s.
A triphasic scanning technique was used after intravenous injection of the contrast agent, with scanning pre-contrast, and delays of 20-30 s and 70-80 s for the arterial and porto-venous phase, respectively.
Images were viewed using the eFilm Workstation software. The CT findings at presentation and during therapy were viewed by one experienced radiologist, who determined the change in size, CT attenuation coefficients, characteristics of tumor images, and overall tumor response. Using eFilm Workstation, tumor size at the longest cross-sectional dimension of each lesion was measured by the same techniques as for baseline measurement. The sum of the longest diameters of lesions in each patient was calculated. The percentage change in the sum of the longest dimensions from the pretreatment evaluation to that at each visit was computed for each patient. The percentage change graded was determined using the RECIST criteria[14].
The CT attenuation co-efficient (density) of the tumor in Hounsfield units (HU) was measured by drawing a region of interest (ROI) that circumscribed the margin of the tumor. The portovenous phase images were used for the tumor-density measurement compared with the pre-contrast phase. The average tumor density was then computed for each patient.
The follow up encompassed all available clinical data, including physical examinations, performance status, laboratory tests, histopathological examinations, and radiology imaging procedures (CT, MRI). For definition of the standard of reference, all patients were rated as responders or non-responders by a medical oncologist at the end of the follow-up. Responders were defined as: (1) improvement or disease-free status that was confirmed by a medical oncologist; and (2) tumor response according to the RECIST criteria (CR, PR and SD). Non-responders were defined as: (1) progression, recurrence, or death from GIST that was confirmed by a medical oncologist; and (2) PD according to the RECIST criteria.
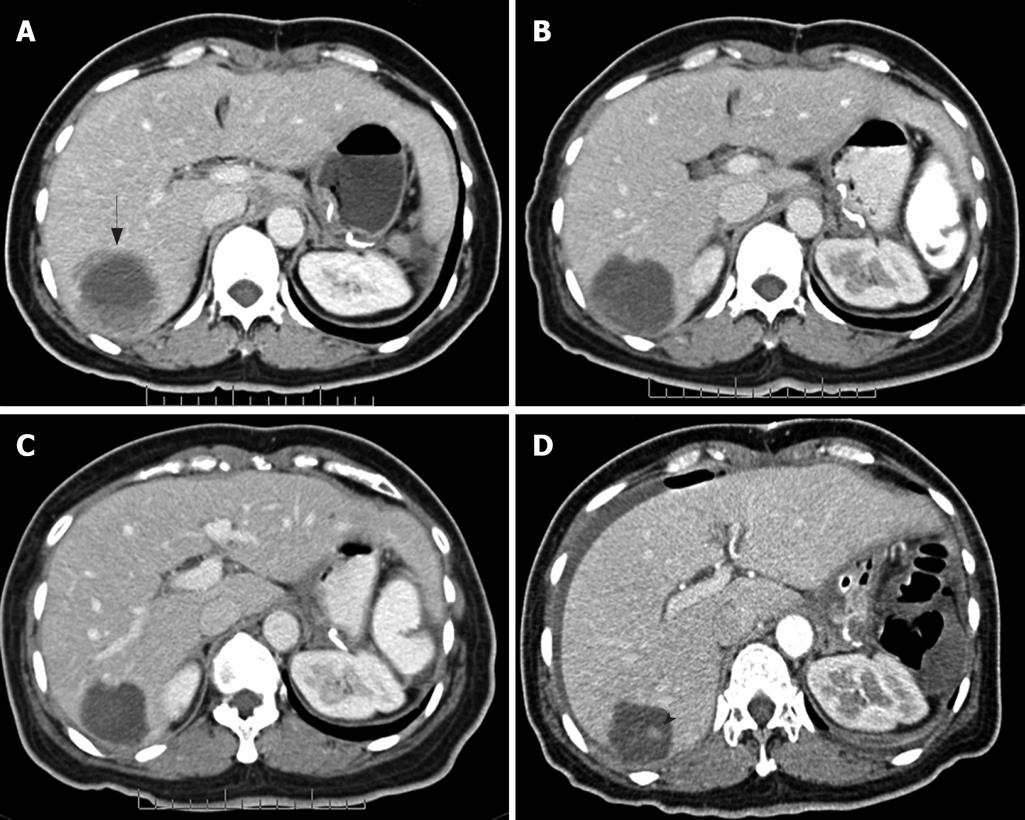
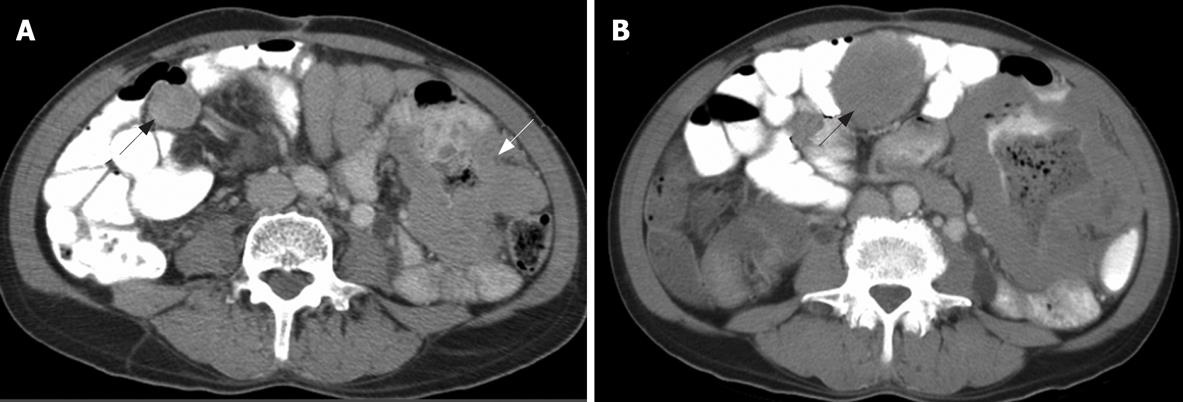
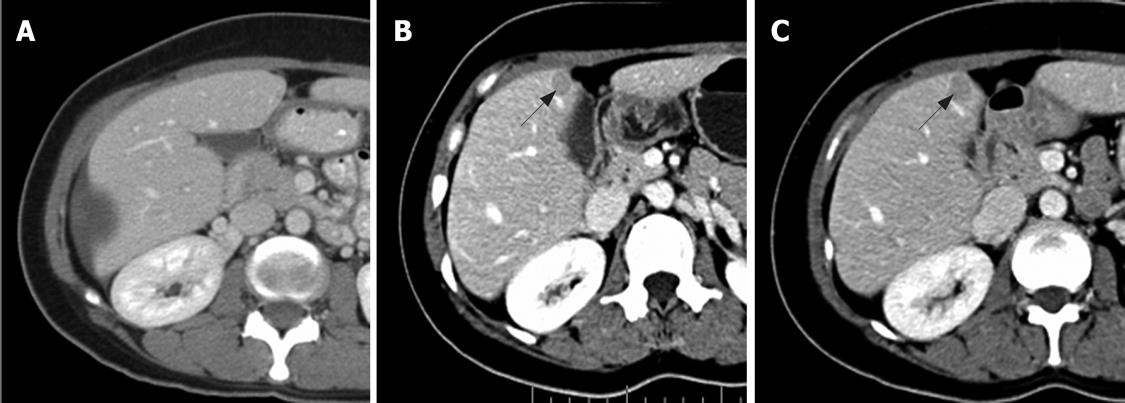
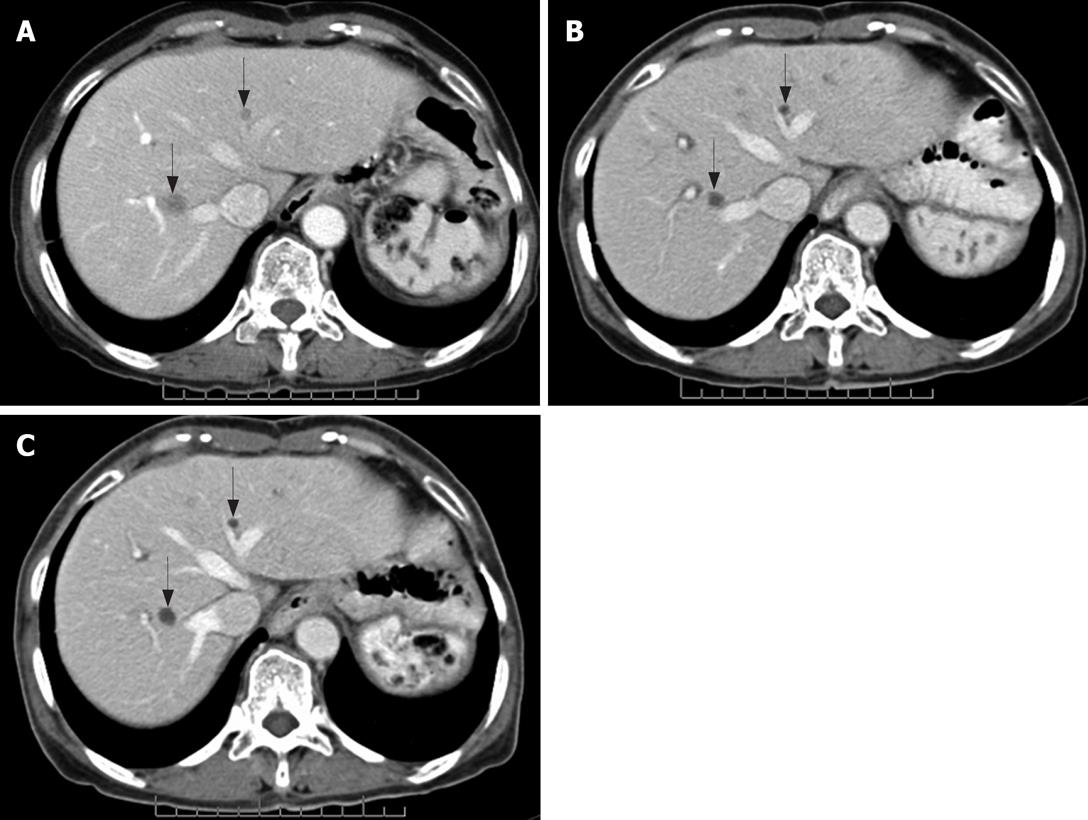
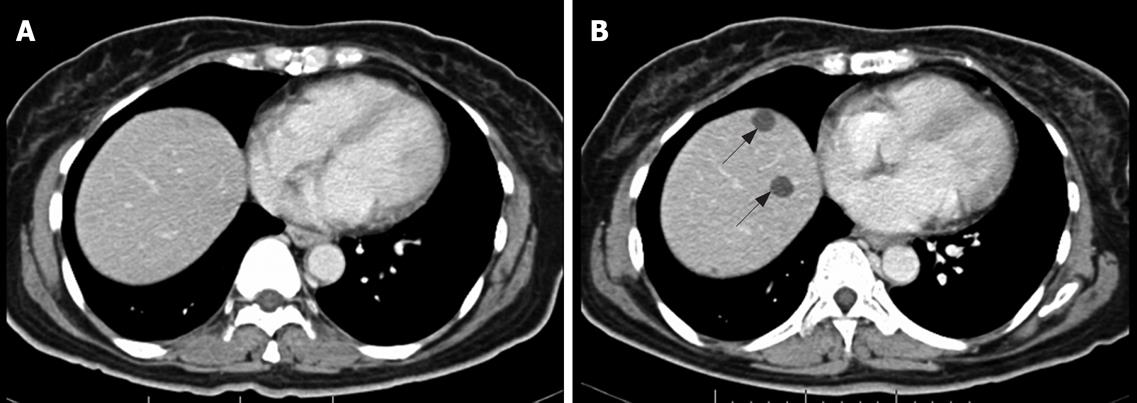
In addition to distinguishing changes in size using the RECIST criteria, the patterns of changes in CT findings were categorized into five groups. (1) Focal progression (FP) was defined as single-site progression, which was either an increase in size (PD in the RECIST criteria), an increase in the solid part of the tumor, or development of a new enhancing focus within the pre-existing tumor mass (or described as a nodule within the mass) (Figure 1). (2) Generalized progression (GP) was defined as an increase in tumor size (PD in RECIST criteria), with an enhancing pattern in two or more tumor masses (Figure 2). (3) New solid lesion (NS), with or without a cystic component, was defined as the appearance of one or more new lesions that had an enhancing lesion with or without a cystic component (Figure 3). (4) Generalized cystic change (GC) was defined as the appearance of cystic change in two or more tumor masses (Figure 4). (5) New cystic lesion (NC) was defined as the appearance of one or more new lesions that had relatively low density, without any enhancing component (Figure 5).
Statistic analysis was performed with STATA software (StataCorp LP, College Station, TX, USA). Patient age, tumor location, overall response, and pattern of tumor changes were analyzed. Survival was calculated from the day of imatinib treatment until death or the final day of the patient’s visit to the outpatient clinic. Kaplan–Meier analysis with a log rank test was used to compare patterns of CT changes and survival distribution. P < 0.05 was considered to indicate a significant difference between groups.
There were 17 patients included in this study (13 men and four women) with a median age of 52 years (range 36-69 years). Primary tumor sites were in the stomach in eight patients (47%), small bowel in six (35%), rectum in two (12%), and retroperitoneum in one (6%). The sites of active disease were in the liver in 10 patients, mesentery in three, pararectal region in three, stomach in three, and one each in the peritoneum, lymph nodes, retroperitoneum and bone. Ten patients (59%) had initial metastatic disease, three (17.5%) had recurrent disease, three (17.5%) had no free surgical margin, and one (6%) had unresectable advanced disease.
At the end of study, there were eight non-responders and nine responders. Non-responders consisted of two live and six dead patients. The median time of follow up was 11 mo (range 3-29 mo). The majority of active disease was found to be located in the liver, with a minority in the mesentery, retroperitoneum and bone. All patients developed the GP pattern at the end of follow up. Two patients developed FP, whereas one developed NS. One of the FP patients showed initial GC and NC. For one patient who had active sites in the liver and mesentery, GP was observed in the mesentery, while the liver lesions showed a GC pattern. The remainder of GP and NS had the same pattern throughout till the end. The median time to GP was 5.5 mo (range 2-23 mo). However, one patient showed GP after a prolonged 23 mo of SD and had early initial NC.
There were nine responders. They showed GC, NC, and response according to RECIST criteria in five, one and three patients at the end of study, respectively. The active sites of GC were in the liver (one patient), pararectum (two), and one each in the stomach and peritoneum/intra-abdominal nodes. One patient showed NC in the liver. The median time to GC and NC was 3.5 mo (range 2-8 mo). Of the three patients with no change in pattern, two patients had a change in size only (PR and CR within the RECIST criteria), while the third patient showed a slight decrease in size (SD). The patient with CR had an active site in the stomach (no free margin in surgery) without metastasis, and the time to CR was 4 mo. The SD patient had an active site in the mesentery. Half of the responders showed PR in the initial follow up.
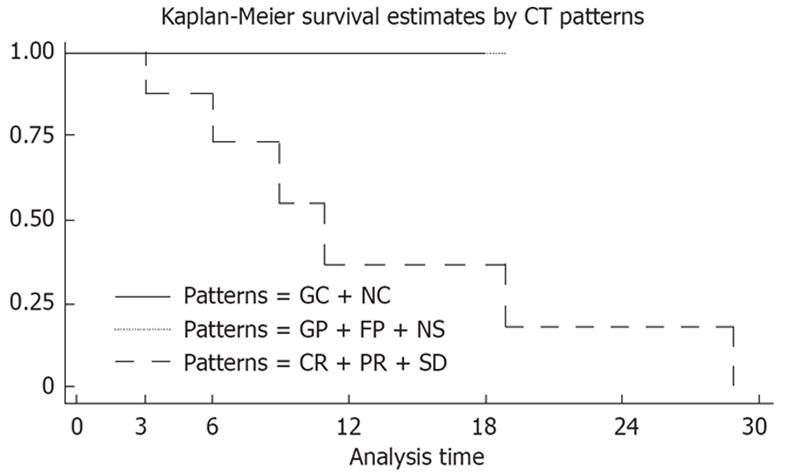
Complete information on the patients’ clinical course of imatinib treatment was obtained in 17 cases. Median follow-up time was 15 mo (range 3-29 mo). Survival status was alive in 11 (64.7%) and dead due to disease in six (35.3%). Median overall survival time of the patients after imatinib treatment was 19 mo (95% CI, 9.3-80.5). Overall survival was better in GC/NC and CR/PR/SD (response according to RECIST) compared with GP patients, and P was 0.0271 (Table 1, Figure 6).
| Patterns of CT change | No. of patients (%) | No. of deaths | Median survival time (mo) | P value |
| GC + NC | 6 (35) | 0 | - | 0.00271 |
| CR + PR + SD | 3 (18) | 0 | - | 0.00271 |
| GP | 8 (47) | 6 | 11 | 0.00271 |
GIST is an uncommon tumor with no effective therapy in advanced disease. This has been the case for inoperable tumors until the advent of imatinib mesylate, which is a molecular targeted therapy. Imatinib mesylate is the first effective systemic treatment for advanced GISTs, and yields a benefit of 50%-80%[15]. Although, the efficacy and safety of imatinib mesylate have been examined in large studies, few have focused on the pattern of tumor changes after treatment. Knowledge on these patterns of response to treatment are important to adequately manage patients and to interpret clinical trials employing new tyrosine kinase inhibitors known as sunitinib[16].
The percentage overall response with imatinib treatment in this study was nearly equal to that in a previous study, which showed an overall response rate of about 50%, with 5% of those with a CR demonstrating a clinical response by CT scan[8].
This study demonstrated different patterns of CT changes in responders and non-responders during treatment with imatinib mesylate. Apart from the RECIST criteria, cystic change (GC, NC) was used to evaluate the response to treatment. If the lesions showed cystic change, even though it was a new lesion or a cystic change in a previous lesion, it was considered to be disease improvement. Several studies have supported the finding that cystic change is a feature of tumors which have responded to treatment, due to tumor necrosis and cystic or myxoid degeneration[1017–19]. Many authors have suggested new cystic lesions are characteristic of a response in small solid hepatic lesions, which cannot be seen in the initial image due to iso-density compared to liver parenchyma[20–22]. FDG-PET in these patients has confirmed no glucose radiotracer uptake in the cystic lesions, whereas the tumor size remains unaltered[82324]. This suggested there were no metabolically active tumor cells.
The non-responder lesions showed three patterns of disease progression during treatment: GP, FP and NS. These patterns represented an increased solid component in terms of generalized or focal change. The FP pattern manifested as an increase in the solid component, such as increased wall thickness or a nodule within a mass[2526]. Several studies have demonstrated that a solid nodule appearing inside a residual cystic mass indicating early retrogression after partial response to imatinib mesylate that corresponded to depicting new foci of increased FDG uptake in PET scan[1123]. Few non-responders showed early FP or NS before developing GP, and finally, death. This may urge further treatment, such as surgery of the focal mass, or the new tyrosine kinase inhibitor sunitinib[1627–30]. Additionally, one of non-responders showed relapse of FP after cystic change of hepatic lesions. The authors speculated this event may have been a regrowth from a residual tumor cell. However, the patient had prolonged stable disease before developing GP.
Interestingly, there was one non-responder, who had two active sites of disease and showed a mixed response. The patient had GC in hepatic lesions but GP in mesenteric lesions. Thus, evaluation of the tumor response should be analyzed on a lesion by lesion basis.
The present study had several limitations. First, a small number of patients were enrolled. Second, the follow-up duration after imatinib treatment was uneven (range 1-6 mo), due to lack of certain protocol criteria for imaging follow up. Third, there was no gold standard reference (such as surgical pathology after imatinib treatment or FDG-PET scan) for evaluation of tumor response.
In conclusion, various patterns of CT changes for evaluation the tumor response were presented which might determine disease prognosis. A combination of RECIST criteria and patterns of CT change have been proposed for better response evaluation in GISTs. The early detection of focal solid or new solid lesions after maximal dose of imatinib mesylate suggests disease progression and might be helpful in early intervention such as surgery or new tyrosine kinase inhibitors.
For optimal clinical outcome, the accurate assessment of tumor response to any treatments is important. RECIST criteria have commonly been used to assess response in solid tumors for decades. The criteria are based solely on changes in size of mass on cross-sectional imaging studies. However, there are circumstances in which the response according to RECIST criteria does not correspond to clinical outcome. This means other imaging criteria may be needed to improve assessment. There are observations that show changes in pattern enhancement can be different in tumors after treatment. Among solid tumors, metastatic GISTs are a good example to show how pattern of enhancement in CT can predict clinical outcome.
Many new anticancer drugs are being developed, particularly those which affect tumor blood vessels, so-called antiangiogenic drugs. Imatinib mesylate (also known as Gleevec, a selective-tyrosine kinase inhibitor) is one of these, and is highly effective in patients with metastatic GISTs. Due to its effect on tumor angiogenesis, the change may be able to be observed during dynamic contrast enhancement acquisition by using CT or MRI techniques.
This study showed evaluating response of metastatic GISTs to imatinib by size alone is not enough. Combined changes in density or pattern enhancement on CT are more reliable.
The combined use RECIST criteria and changes in the pattern of enhancement are useful in evaluating tumor response, particularly in patients treated with antiangiogenic drugs or other means that have an effect on tumor blood vessels.
This is an interesting study on the follow-up of non-resectable GIST treated with imatinib. Tumor response was assessed by the RECIST criteria complemented by other criteria on CT. Overall, the results show beautiful iconography.
| 1. | Horton KM, Fishman EK. Current role of CT in imaging of the stomach. Radiographics. 2003;23:75-87. |
| 2. | Burkill GJ, Badran M, Al-Muderis O, Meirion Thomas J, Judson IR, Fisher C, Moskovic EC. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology. 2003;226:527-532. |
| 3. | Lau S, Tam KF, Kam CK, Lui CY, Siu CW, Lam HS, Mak KL. Imaging of gastrointestinal stromal tumour (GIST). Clin Radiol. 2004;59:487-498. |
| 4. | Stroszczynski C, Jost D, Reichardt P, Chmelik P, Gaffke G, Kretzschmar A, Schneider U, Felix R, Hohenberger P. Follow-up of gastro-intestinal stromal tumours (GIST) during treatment with imatinib mesylate by abdominal MRI. Eur Radiol. 2005;15:2448-2456. |
| 5. | Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3:655-664. |
| 6. | Saund MS, Demetri GD, Ashley SW. Gastrointestinal stromal tumors (GISTs). Curr Opin Gastroenterol. 2004;20:89-94. |
| 7. | Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, Peake D. Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation. Health Technol Assess. 2005;9:1-142. |
| 8. | Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127-1134. |
| 9. | Antoch G, Kanja J, Bauer S, Kuehl H, Renzing-Koehler K, Schuette J, Bockisch A, Debatin JF, Freudenberg LS. Comparison of PET, CT, and dual-modality PET/CT imaging for monitoring of imatinib (STI571) therapy in patients with gastrointestinal stromal tumors. J Nucl Med. 2004;45:357-365. |
| 10. | Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, Silberman S, Capdeville R, Dimitrijevic S, Druker B. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344:1052-1056. |
| 11. | Vanel D, Albiter M, Shapeero L, Le Cesne A, Bonvalot S, Le Pechoux C, Terrier P, Petrow P, Caillet H, Dromain C. Role of computed tomography in the follow-up of hepatic and peritoneal metastases of GIST under imatinib mesylate treatment: a prospective study of 54 patients. Eur J Radiol. 2005;54:118-123. |
| 12. | Kim HC, Lee JM, Choi SH, Han H, Kim SS, Lee SH, Han JK, Choi BI. Cystic changes in intraabdominal extrahepatic metastases from gastrointestinal stromal tumors treated with imatinib. Korean J Radiol. 2004;5:157-163. |
| 13. | Bechtold RE, Chen MY, Stanton CA, Savage PD, Levine EA. Cystic changes in hepatic and peritoneal metastases from gastrointestinal stromal tumors treated with Gleevec. Abdom Imaging. 2003;28:808-814. |
| 14. | Padhani AR, Ollivier L. The RECIST (Response Evaluation Criteria in Solid Tumors) criteria: implications for diagnostic radiologists. Br J Radiol. 2001;74:983-986. |
| 15. | Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472-480. |
| 16. | Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329-1338. |
| 17. | Chen MY, Bechtold RE, Savage PD. Cystic changes in hepatic metastases from gastrointestinal stromal tumors (GISTs) treated with Gleevec (imatinib mesylate). AJR Am J Roentgenol. 2002;179:1059-1062. |
| 18. | Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759. |
| 19. | Desai J, Shankar S, Heinrich MC, Fletcher JA, Fletcher CD, Manola J, Morgan JA, Corless CL, George S, Tuncali K. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13:5398-5405. |
| 20. | Linton KM, Taylor MB, Radford JA. Response evaluation in gastrointestinal stromal tumours treated with imatinib: misdiagnosis of disease progression on CT due to cystic change in liver metastases. Br J Radiol. 2006;79:e40-e44. |
| 21. | Ryu MH, Lee JL, Chang HM, Kim TW, Kang HJ, Sohn HJ, Lee JS, Kang YK. Patterns of progression in gastrointestinal stromal tumor treated with imatinib mesylate. Jpn J Clin Oncol. 2006;36:17-24. |
| 22. | Reichardt P, Schneider U, Stroszczynski C, Pink D, Hohenberger P. Molecular response of gastrointestinal stromal tumour after treatment with tyrosine kinase inhibitor imatinib mesylate. J Clin Pathol. 2004;57:215-217. |
| 23. | Hong X, Choi H, Loyer EM, Benjamin RS, Trent JC, Charnsangavej C. Gastrointestinal stromal tumor: role of CT in diagnosis and in response evaluation and surveillance after treatment with imatinib. Radiographics. 2006;26:481-495. |
| 24. | Gayed I, Vu T, Iyer R, Johnson M, Macapinlac H, Swanston N, Podoloff D. The role of 18F-FDG PET in staging and early prediction of response to therapy of recurrent gastrointestinal stromal tumors. J Nucl Med. 2004;45:17-21. |
| 25. | Shankar S, vanSonnenberg E, Desai J, Dipiro PJ, Van Den Abbeele A, Demetri GD. Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology. 2005;235:892-898. |
| 26. | Al-Batran SE, Hartmann JT, Heidel F, Stoehlmacher J, Wardelmann E, Dechow C, et al. Focal progression in patients with gastrointestinal stromal tumors after initial response to imatinib mesylate: a three-center-based study of 38 patients. Gastric Cancer. 2007;10:145-152. |
| 27. | Eisenberg BL, Judson I. Surgery and imatinib in the management of GIST: emerging approaches to adjuvant and neoadjuvant therapy. Ann Surg Oncol. 2004;11:465-475. |
| 28. | Scaife CL, Hunt KK, Patel SR, Benjamin RS, Burgess MA, Chen LL, Trent J, Raymond AK, Cormier JN, Pisters PW. Is there a role for surgery in patients with "unresectable" cKIT+ gastrointestinal stromal tumors treated with imatinib mesylate? Am J Surg. 2003;186:665-669. |
| 29. | Chiang KC, Chen TW, Yeh CN, Liu FY, Lee HL, Jan YY. Advanced gastrointestinal stromal tumor patients with complete response after treatment with imatinib mesylate. World J Gastroenterol. 2006;12:2060-2064. |