Published online Nov 21, 2008. doi: 10.3748/wjg.14.6738
Revised: October 13, 2008
Accepted: October 20, 2008
Published online: November 21, 2008
AIM: To investigate the value of clinical manifestations and ultrasound examination in the differential diagnosis of pancreatic lymphoma and pancreatic cancer.
METHODS: The clinical and ultrasonic characteristics of 12 cases of pancreatic lymphoma and 30 cases of pancreatic cancer were retrospectively analyzed.
RESULTS: Statistically significant differences were found in the course of disease, back pain, jaundice, carcino-embryonic antigen (CEA) and CA19-9 increase, palpable abdominal lump, superficial lymph node enlargement, fever and night sweats, lesion size, bile duct expansion, pancreatic duct expansion, vascular involvement, retroperitoneal (below the renal vein level) lymph node enlargement, and intrahepatic metastasis between pancreatic lymphoma and pancreatic cancer. There were no significant differences in age of onset, gender ratio, weight loss, nausea and vomiting, lesion position, the echo of the lesion, and the blood flow of the lesion.
CONCLUSION: Pancreatic lymphoma should be considered for patients with long lasting symptoms, superficial lymph node enlargement, palpable abdominal lump, fever and night sweats, relatively large lesions, and retroperitoneal (below the level of the renal vein) lymph node enlargement. A diagnosis of pancreatic cancer should be considered more likely in the patients with relatively short disease course, jaundice, back pain, CEA and CA19-9 increase, relatively small lesions, bile duct expansion, obvious pancreatic duct expansion, peripheral vascular wrapping and involvement, or intrahepatic metastases.
- Citation: Qiu L, Luo Y, Peng YL. Value of ultrasound examination in differential diagnosis of pancreatic lymphoma and pancreatic cancer. World J Gastroenterol 2008; 14(43): 6738-6742
- URL: https://www.wjgnet.com/1007-9327/full/v14/i43/6738.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6738
| Pancreatic lymphoma | Pancreatic cancer | P | |
| Age of onset (yr) | 52.8 ± 3.4 | 53.8 ± 4.2 | NS |
| Gender ratio (male/female) | 10/2 | 24/6 | NS |
| Course of disease | 3 mo to 1 yr | 4 wk to 3 mo | < 0.05 |
| Weight loss | 10 | 27 | NS |
| Nausea and vomiting | 4 | 12 | NS |
| Fever and night sweats | 5 | 1 | < 0.05 |
| Back pain | 1 | 18 | < 0.05 |
| Jaundice | 3 | 20 | < 0.05 |
| Palpable abdominal lump | 6 | 0 | < 0.05 |
| Superficial lymph node enlargement | 5 | 1 | < 0.05 |
| CEA, CA19-9 increase | 0 | 18 | < 0.05 |
| Pancreatic lymphoma | Pancreatic cancer | P | |
| Lesion position | |||
| Pancreatic head | 7 | 19 | NS |
| Pancreatic body & tail | 3 | 7 | NS |
| Diffuse | 2 | 4 | NS |
| Lesion echo | All 12 cases had low echo | All 30 cases had low echo | NS |
| Lesion size | 79.6 ± 10.7 mm | 34.7 ± 5.5 mm | < 0.05 |
| Blood flow of the lesion | 11 cases did not show any blood flow signals, 1 case showed some blood flow signals | None of the 30 cases showed blood flow signals | NS |
| Bile duct expansion | 3 | 20 | < 0.05 |
| Pancreatic duct expansion | 2 | 18 | < 0.05 |
| Blood vessel involvement | 2 | 15 | < 0.05 |
| Retroperitoneal (below the renal vein) lymph node enlargement | 5 | 1 | < 0.05 |
| Intrahepatic metastasis | 0 | 6 | < 0.05 |
Among the pancreatic tumors, there are much similarities in clinical manifestations and imaging characteristics of pancreatic cancer and pancreatic lymphoma and thus they are easy to be misdiagnosed[1-4]. However, the treatment and prognosis of pancreatic cancer and pancreatic lymphoma are quite different, and the differential diagnosis of these two diseases is therefore very important[5-7]. We retrospectively analyzed clinical and ultrasonic data of 12 cases of pancreatic lymphoma and 30 cases of pancreatic cancer to study the differentiation between the two diseases.
Forty-two pancreatic lesions were retrospectively analyzed. Patients with lymphoma (n = 12) and patients with pancreatic cancer (n = 30) whose diagnoses were confirmed by surgical pathology at our hospital from April 2001 to December 2007 were studied. All patients received ultrasound examinations before treatment.
Instruments: GE Logiq 7, Philips HDI-5000, HD-11 color Doppler ultrasound diagnostic instrument were used. The probe frequency was 8-12 MHz; speed range, blood flow filtering and color gain were adjusted to achieve blood flow maps with the best display status.
Ultrasound examination: Patients were placed in the supine position, the gray-scale ultrasound was used to observe the size of the pancreatic lesion, lesion position, lesion echo, the main pancreatic duct, bile duct, peripancreatic vessels, and retroperitoneal lymph nodes. Then the color hemodromogram of the lesion area was displayed and divided into 3 grades: no blood flow signal, some blood flow signals, and abundant blood flow signals.
SAS V8.1 statistical software was used for data analysis. Statistical analyses were done for patient age, course of disease and lesion size using Wilcoxon’s rank sum test. Fisher’s exact probability and the χ2 test were carried out for analyses of sex, back pain, jaundice, palpable abdominal lump, weight loss, nausea and vomiting, fever and night sweats, carcino-embryonic antigen (CEA) or CA19-9 increases, superficial lymph node enlargement, lesion position, lesion echo, bile duct expansion, pancreatic duct expansion, vascular invasion, retroperitoneal (below the renal vein level) lymph node enlargement, intrahepatic metastasis, and blood flow. P < 0.05 was taken as an indicator of a significant difference between the two groups.
The clinical characteristics of pancreatic lymphoma and pancreatic cancer were compared. There was no significant difference in age of onset, gender ratio, weight loss, or nausea and vomiting between the two groups. The course of disease, back pain, jaundice, CEA and CA19-9 increase, palpable abdominal lump, superficial lymph node enlargement, fever and night sweats were significantly different between the two groups. This means that the disease course of the pancreatic lymphoma is longer, and superficial lymph node enlargement, palpable lump, and fever and night sweats occur more frequently in pancreatic lymphoma. In pancreatic cancer, back pain, jaundice, and CEA and CA19-9 increases occur more frequently (Table 1).
The ultrasound characteristics of pancreatic lymphoma and of pancreatic cancer were compared. There were no significant differences in lesion position, lesion echo, or blood flow of the lesion between the two groups. The lesions size, bile duct expansion, pancreatic duct expansion, vascular involvement, retroperitoneal (below the renal vein) lymph node enlargement, and intrahepatic metastasis were significantly different (Table 2). In other words, the lesion size of pancreatic lymphoma is relatively larger, which is prone to causing enlargement of the lymph nodes below the renal vein level. Pancreatic cancer tends to cause bile duct and pancreatic duct expansion more frequently and is more likely to affect the blood vessels and lead to intrahepatic metastasis.
The incidence of pancreatic cancer has grown substantially in developed countries and in some developing countries. Currently, it is one of the top ten malignant tumors, and its prognosis is very poor. The 1-year survival rate is below 20% and the 5-year survival rate is only 3%[1,5,8]. Pancreatic lymphoma is a rare pancreatic tumor that accounts for only 1% of all pancreatic tumors. Its main pathological type is B-cell non-Hodgkin’s lymphoma and the overwhelming majority of cases involve pancreatic infiltration by systemic lymphoma. It can also originate from the pancreas and show primary pancreatic manifestations, which are called primary pancreatic lymphoma (PPL). The average survival time of pancreatic lymphoma is 2-6.5 years, which is better than the prognosis for pancreatic cancer[9-11]. There are much similarities in the clinical and imaging examination of the two diseases so that they are easily misdiagnosed. However, the treatment and the prognosis of the two diseases are quite different. Therefore, the differential diagnosis of these two diseases is important.
There was no significant difference in the age of onset, gender ratio, weight loss, nausea and vomiting between the two diseases. Therefore, these characteristics cannot be used for the differential diagnosis.
The course of disease of the patients with pancreatic lymphoma is usually longer than that of the patients with pancreatic cancer. As the lesion is relatively large in pancreatic lymphoma, an abdominal lump is easily palpable. In our series, an abdominal lump was palpable in 6 (50%) patients in the pancreatic lymphoma group. In addition, as the majority of the pancreatic lymphoma cases originate from systemic lymphoma, characteristics of the lymphoma such as superficial lymph node enlargement and fever and night sweats are likely to occur. Those characteristics are relatively rare in patients with pancreatic cancer. Compared with pancreatic lymphoma, jaundice and back pain are more likely to occur in patients with pancreatic cancer. This is because pancreatic cancer has perivascular and perineural growth[12-15]. It is easy for pancreatic cancer to cause jaundice by bile duct infiltration or oppression or to cause back pain by erosion of the retroperitoneal nerve, which is more obvious at night. Regarding laboratory tests, since the pancreatic cancer is a tumor with duct infiltration, most patients show increases in two tumor markers, CEA and CA19-9.Those markers will usually not increase in patients with pancreatic lymphoma[16-18].
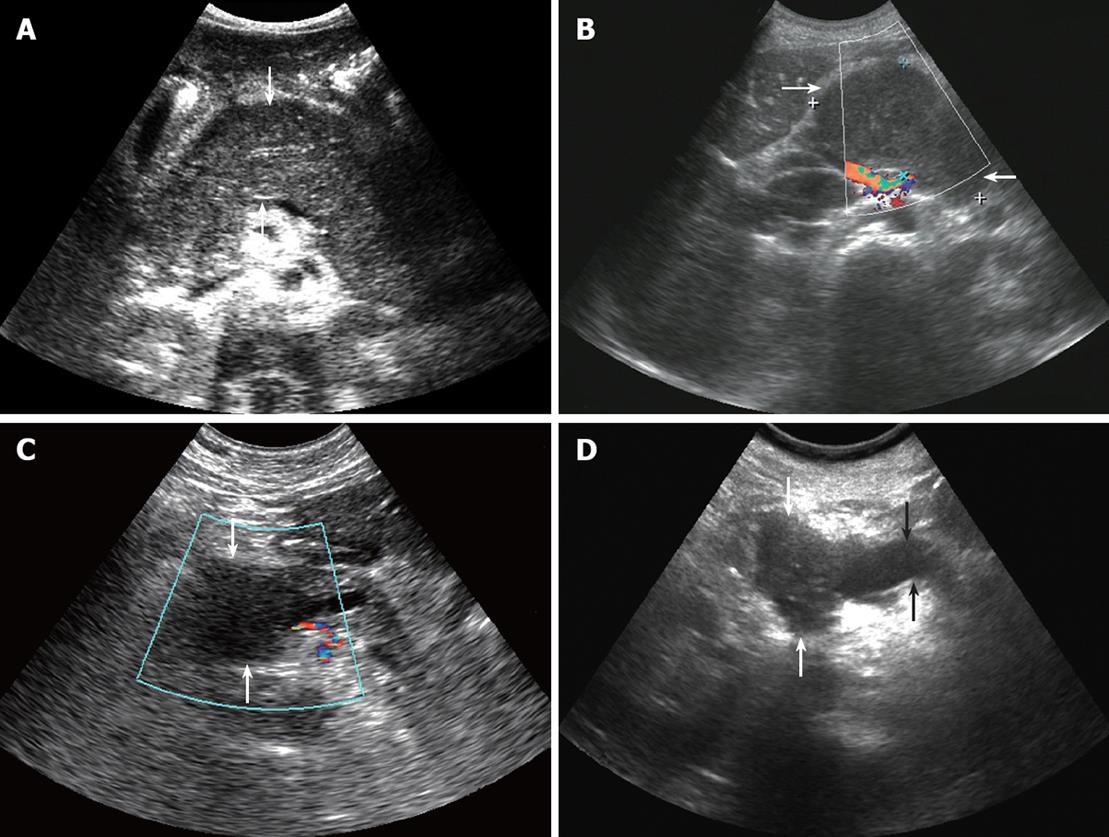
There were no significant differences in the lesion position, lesion echo, or the blood flow of the lesion between the two diseases. Pancreatic head lesions are commonly seen in both diseases. In this study, pancreatic head lesions accounted for 58.3% of cases in the pancreatic lymphoma group and 63.3% of cases in the pancreatic cancer group. In addition, some lesions located in the body or the tails of the pancreas were diffuse (Figure 1A). All the lesions had low echo, and most lesions did not have any blood flow signals (Figure 1B and C).
The lesion sizes were significantly different between the two diseases. The lesion of the pancreatic lymphoma is usually greater than that of the pancreatic cancer. In this study, the lesion size of the pancreatic lymphoma was approximately 79.6 ± 10.7 mm, and the lesion size of the pancreatic cancer was approximately 34.7 ± 5.5 mm. Other literature reports showed similar results that the diameter of the pancreatic lymphoma lump is usually greater than 70 mm, and the diameter of the pancreatic cancer is usually less than 50 mm[19-22]. A possible reason is that the early symptoms of pancreatic lymphoma are not obvious and diagnosis is made relatively late; therefore the lesions are relatively big when they are found.
Bile duct expansion was significantly different between the two diseases. Pancreatic cancer readily infiltrates and oppresses the bile duct so that bile duct expansion occurs more frequently in pancreatic cancer than in pancreatic lymphoma. In this study, intrahepatic and extrahepatic bile duct expansion occurred in 20 (66.7%) cases of pancreatic cancer, but in only 3 (25%) cases of pancreatic lymphoma.
Pancreatic duct expansion was statistically significant between the two diseases. In this study, 18 (60%) cases of pancreatic cancer showed pancreatic duct expansion (Figure 1D), while only 2 (16.7%) cases of pancreatic lymphoma did so. Another study showed that the pancreatic duct in pancreatic lymphoma can be discontinuous or narrow, expansion is rare, and the ratio of the expanded pancreatic duct to the anteroposterior diameter of the gland is less than 0.5. However, this ratio in pancreatic cancer (in more than 89% of cases) is often greater than 0.5[23-26].
The two diseases were significantly different regarding blood vessel involvement. The pancreatic cancer frequently wraps and erodes the blood vessels. There were 15 (50%) cases of pancreatic cancer with vascular involvement in this study; there were only 2 (16.7%) cases of pancreatic lymphoma with vascular involvement. The pancreatic lymphoma mainly pushes the surrounding blood vessels.
The two diseases were significantly different in retroperitoneal (below the renal vein level) lymph node enlargement. Peri-pancreatic and retro-peritoneal lymph node enlargement can occur in both pancreatic cancer and pancreatic lymphoma, but pancreatic lymphoma is often part of a systemic lymphoma. Therefore, there are more sites of lymph node enlargement in pancreatic lymphoma than in pancreatic cancer. It has been reported in the literature that retroperitoneal lymph node enlargement below the level of the renal vein seldom appears in pancreatic cancer[27-29]. In this study, 5 (41.7%) patients with pancreatic lymphoma showed lymph node enlargement below the level of the renal vein, but only 1 (3.3%) case of pancreatic cancer showed similar lymph node enlargement.
The two diseases were significantly different in intrahepatic metastasis. Intrahepatic metastasis occurred more easily in patients with pancreatic cancer compared with pancreatic lymphoma[30]. There were 6 (20%) cases of pancreatic cancer with intrahepatic metastasis in this study while no intrahepatic metastasis occurred in pancreatic lymphoma.
In summary, clinical and ultrasonic manifestations can aid in the differentiation between pancreatic lymphoma and pancreatic cancer. For the differential diagnosis of the two diseases, pancreatic lymphoma should be considered for patients with long lasting symptoms, superficial lymph node enlargement, palpable abdominal lump, fever and night sweats, relatively large lesions, and retroperitoneal (below the level of the renal vein) lymph node enlargement. A diagnosis of pancreatic cancer should be considered more likely for patients with relatively short disease course, jaundice, back pain, CEA and CA19-9 increase, relatively small lesions, bile duct expansion, obvious pancreatic duct expansion, peripheral vascular wrapping and involvement, or intrahepatic metastases. The diagnosis can be confirmed by biopsy or postoperative pathological results.
Differential diagnosis of pancreatic masses is a frequent clinical challenge. Differential diagnosis is difficult between pancreatic cancer and pancreatic lymphoma because there are a lot of similarities in clinical manifestations and imaging characteristics of pancreatic cancer and pancreatic lymphoma among pancreatic tumors. However, the treatment and prognosis of pancreatic cancer and pancreatic lymphoma are quite different, and the differential diagnosis of these two diseases is therefore, very important.
Further researches are needed in order to improve the diagnosis of pancreatic cancer and pancreatic lymphoma. Availability of ultrasound examination and clinical manifestations may provide more information for the diagnosis of pancreatic cancer and pancreatic lymphoma.
Because the morbidity of pancreatic lymphoma is low, in this study, 12 cases of pancreatic lymphoma were important which could provide much favorable information for diagnosis.
Clinical and ultrasonic manifestations can aid in the differentiation between pancreatic lymphoma and pancreatic cancer.
This is an interesting paper. The contents are important and significant because there is no much information about pancreatic lymphoma in the existing literature.
Peer reviewer: Marko Duvnjak, MD, Department of Gastroenterology and Hepatology, Sestre milosrdnice University Hospital, Vinogradska cesta 29, Zagreb 10000, Croatia
S- Editor Li DL L- Editor Ma JY E- Editor Yin DH
| 1. | Miura F, Takada T, Amano H, Yoshida M, Furui S, Takeshita K. Diagnosis of pancreatic cancer. HPB (Oxford). 2006;8:337-342. |
| 2. | Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol. 2006;12:3180-3185. |
| 3. | Leite NP, Kased N, Hanna RF, Brown MA, Pereira JM, Cunha R, Sirlin CB. Cross-sectional imaging of extranodal involvement in abdominopelvic lymphoproliferative malignancies. Radiographics. 2007;27:1613-1634. |
| 4. | Sheth S, Fishman EK. Imaging of uncommon tumors of the pancreas. Radiol Clin North Am. 2002;40:1273-1287, vi. |
| 5. | Mortenson MM, Katz MH, Tamm EP, Bhutani MS, Wang H, Evans DB, Fleming JB. Current diagnosis and management of unusual pancreatic tumors. Am J Surg. 2008;196:100-113. |
| 6. | Paissan A, Wachs A, Arias M, Abeldaño A, Frider B. [Obstructive jaundice associated Burkitt's lymphoma mimicking pancreatic carcinoma]. Acta Gastroenterol Latinoam. 2007;37:246-249. |
| 7. | Canto MI. Screening and surveillance approaches in familial pancreatic cancer. Gastrointest Endosc Clin N Am. 2008;18:535-553, x. |
| 8. | Helmstaedter L, Riemann JF. Pancreatic cancer-EUS and early diagnosis. Langenbecks Arch Surg. 2008;393:923-927. |
| 10. | Basu A, Patil N, Mohindra P, Zade B, Gujral S, Muckaden MA, Laskar S. Isolated non-Hodgkin's lymphoma of the pancreas: case report and review of literature. J Cancer Res Ther. 2007;3:236-239. |
| 11. | Aloui-Kasbi N, Mbarek S, Bellagha I, Hammou A. [Primary T-cell lymphoma of the pancreas in children]. Tunis Med. 2005;83:114-116. |
| 12. | Gardner TB, Chari ST. Endoscopic ultrasonography and pancreatic cancer. Minerva Gastroenterol Dietol. 2008;54:161-176. |
| 13. | Rabinowitz CB, Prabhakar HB, Sahani DV. Recent advances in imaging of pancreatic neoplasms. Cancer Treat Res. 2008;143:229-254. |
| 14. | Battula N, Srinivasan P, Prachalias A, Rela M, Heaton N. Primary pancreatic lymphoma: diagnostic and therapeutic dilemma. Pancreas. 2006;33:192-194. |
| 15. | Dietrich CF, Braden B, Hocke M, Ott M, Ignee A. Improved characterisation of solitary solid pancreatic tumours using contrast enhanced transabdominal ultrasound. J Cancer Res Clin Oncol. 2008;134:635-643. |
| 16. | Choi EK, Byun JH, Lee SJ, Jung SE, Park MS, Park SH, Lee MG. Imaging findings of leukemic involvement of the pancreaticobiliary system in adults. AJR Am J Roentgenol. 2007;188:1589-1595. |
| 17. | McCauley AM, Gottlieb KT. Primary pancreatic lymphoma coexisting with chronic lymphocytic leukemia: EUS findings. Gastrointest Endosc. 2008;68:188-189. |
| 18. | Jayanthi V, Randhir J, Rajesh N. Problems in diagnosing lymphoma of the pancreas with computed tomography. A case report. J Gastrointestin Liver Dis. 2007;16:101-103. |
| 19. | Sata N, Kurogochi A, Endo K, Shimura K, Koizumi M, Nagai H. Follicular lymphoma of the pancreas: a case report and proposed new strategies for diagnosis and surgery of benign or low-grade malignant lesions of the head of the pancreas. JOP. 2007;8:44-49. |
| 20. | Leite NP, Kased N, Hanna RF, Brown MA, Pereira JM, Cunha R, Sirlin CB. Cross-sectional imaging of extranodal involvement in abdominopelvic lymphoproliferative malignancies. Radiographics. 2007;27:1613-1634. |
| 21. | Shah S, Mortele KJ. Uncommon solid pancreatic neoplasms: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR. 2007;28:357-370. |
| 22. | Ji Y, Kuang TT, Tan YS, Chen Y, Zeng HY, Jin DY. Pancreatic primary lymphoma: a case report and review of the literature. Hepatobiliary Pancreat Dis Int. 2005;4:622-626. |
| 23. | Rickes S, Malfertheiner P. Echo-enhanced ultrasound--a new imaging modality for the differentiation of pancreatic lesions. Int J Colorectal Dis. 2006;21:269-275. |
| 24. | Kalra MK, Maher MM, Mueller PR, Saini S. State-of-the-art imaging of pancreatic neoplasms. Br J Radiol. 2003;76:857-865. |
| 25. | Ishida H, Konno K, Ishida J, Naganuma H, Komatsuda T, Sato M, Watanabe S. Abdominal lymphoma: differentiation from pancreatic carcinoma with Doppler US. Abdom Imaging. 2002;27:461-464. |
| 26. | Lee JH, Yu JS, Kim H, Kim JK, Kim TH, Kim KW, Park MS, Kim JH, Kim YB, Park C. Solid pseudopapillary carcinoma of the pancreas: differentiation from benign solid pseudopapillary tumour using CT and MRI. Clin Radiol. 2008;63:1006-1014. |
| 27. | Keter D, Melzer E. Endoscopic ultrasound in clinical practice. Acta Gastroenterol Latinoam. 2008;38:146-151. |
| 28. | Malbora B, Avci Z, Alioglu B, Tutar NU, Ozbek N. A case with mature B-cell acute lymphoblastic leukemia and pancreatic involvement at the time of diagnosis. J Pediatr Hematol Oncol. 2008;30:87-89. |
| 29. | Stoopen ME. [Imaging of cancer of the pancreas]. Rev Gastroenterol Mex. 2007;72 Suppl 2:160-164. |