Published online Nov 14, 2008. doi: 10.3748/wjg.14.6551
Revised: August 11, 2008
Accepted: August 18, 2008
Published online: November 14, 2008
AIM: To investigate the protective effects and mechanisms of Baicalin and Octreotide on hepatic injury in rats with severe acute pancreatitis (SAP).
METHODS: The SAP rat models were prepared and randomly assigned to the model control group, Baicalin treated group, and Octreotide treated group while other healthy rats were assigned to the sham-operated group. Rat mortality, levels of ALT, AST, liver and pancreas pathological changes in all groups were observed at 3, 6 and 12 h after operation. Tissue microarray (TMA) sections of hepatic tissue were prepared to observe expression levels of Bax, Bcl-2 protein and Caspase-3, and changes of apoptotic indexes.
RESULTS: Rat survival at 12 h, expression levels of Bax, Caspase-3 protein and apoptotic indexes of liver were all significantly higher in treated groups than in model control group. While the liver and pancreas pathological scores, contents of ALT, AST, and expression levels of Bcl-2 protein were all lower in treated groups than in the model control group.
CONCLUSION: Both Baicalin and Octreotide can protect rats with SAP by decreasing the contents of ALT, AST and expression levels of Bcl-2 protein, and improving the expression levels of Bax protein, Caspase-3 protein, and inducing apoptosis.
- Citation: Zhang XP, Zhang J, Ren Z, Feng GH, Zhu W, Cai Y, Yang QJ, Ju TF, Xie Q, Yuan WQ. Study on protecting effects of Baicalin and Octreotide on hepatic injury in rats with severe acute pancreatitis. World J Gastroenterol 2008; 14(42): 6551-6559
- URL: https://www.wjgnet.com/1007-9327/full/v14/i42/6551.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6551
| Indexes | Sham-operated group | Model control group | Baicalin treated group | Octreotide treated group | ||||||||
| 3 h | 6 h | 12 h | 3 h | 6 h | 12 h | 3 h | 6 h | 12 h | 3 h | 6 h | 12 h | |
| Pathological score of liver | 0.0 | 0.0 | 0.0 | 2.01 | 3.01 | 3.01 | 1.01 | 2.01 | 2.01c | 2.01 | 2.01 | 2.01c |
| (0.0) | (0.0) | (0.0) | (1.0) | (1.0) | (0.25) | (2.0) | (2.0) | (2.0) | (2.0) | (2.0) | (1.0) | |
| Pathological score of pancreas | 0.0 | 0.0 | 0.0 | 8.01 | 9.01 | 10.51 | 7.01 | 7.01 | 9.01d | 7.01 | 6.01d | 8.01d |
| (1.0) | (1.0) | (1.0) | (2.0) | (3.0) | (1.5) | (1.5) | (3.0) | (4.0) | (2.0) | (2.0) | (2.0) | |
| Bax | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0ac | 0.0 | 0.0 | 0.0a | 0.0b |
| (0.0) | (0.0) | (0.0) | (1.0) | (0.0) | (0.0) | (0.5) | (1.0) | (0.0) | (1.0) | (1.0) | (1.0) | |
| Bcl-2 | 0.0 | 0.0 | 0.0 | 0.0a | 3.0a | 0.0a | 0.0ae | 0.0c | 0.0c | 0.0c | 0.0c | 0.0c |
| (0.0) | (0.0) | (0.0) | (1.0) | (2.5) | (2.0) | (1.0) | (0.0) | (0.0) | (0.0) | (0.0) | (0.0) | |
| Caspase-3 | 0.0 | 0.0 | 0.0 | 0.0a | 0.0a | 0.0a | 0.0b | 0.0b | 1.0bc | 1.0a | 0.0a | 0.0a |
| (0.0) | (0.0) | (0.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | (1.0) | |
| Apoptosis indexes | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02ad | 0.00 | 0.00 | 0.02ac | 0.00ac |
| (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.00) | (0.10) | (0.00) | (0.04) | (0.04) | (0.06) | |
| Indexes | Sham-operated group | Model control group | Baicalin treated group | Octreotide treated group | ||||||||
| 3 h | 6 h | 12 h | 3 h | 6 h | 12 h | 3 h | 6 h | 12 h | 3 h | 6 h | 12 h | |
| ALT | 28 (12) | 29 (10) | 35 (9) | 3321 (140) | 6231 (193) | 6411 (163) | 10212d (99) | 15012 (69) | 17812c (111) | 20812 (128) | 15812 (57) | 22612 (50) |
| AST | 130 (37) | 142 (26) | 158 (29) | 5281 (270) | 9751 (242) | 9871 (230) | 221123 (205) | 30612c (225) | 47212 (114) | 4231a (229) | 42012 (163) | 51312 (258) |
Severe acute pancreatitis (SAP) can cause systemic inflammatory response syndromes (SIRS) such as effusion of blood vessel, shock and multiple organ functional disturbances or even multiple organ dysfunction syndrome (MODS)[1-3]. SAP with extremely hazardous onset process and quite high mortality clinically remains unclear till now[4-7]. The main cause of early death is multiple organ failure, which most frequently affects liver, etc. Studies prove the incidence of hepatic injury and its severity degree are positively correlated with the severity of pancreatitis and hepatic injury prolongs the course of pancreatitis[8,9].
Presently, the main medications for SAP in clinical practices are Somatostatin and its analogue Octreotide. Their mechanism of action is maily to inhibit pancreatin secretion, decreasing the generation of endotoxin, inhibiting the release of inflammatory mediators, and inhibiting platelet aggregation and other steps[10-12]. However, their high price, short half life and inconvenient administration have made it difficult to popularize their clinical application in economically poor and remote areas, resulting in the necessity of finding other cheap and effective alternatives that should be able to protect multiple organs[13-16]. There is a great prospect for developing and utilizing traditional Chinese medicine to treat SAP[17,18]. Its main advantages include cheap price, extensive pharmacological actions and fewer side effects. In this study, Baicalin, the main effective ingredient of baikal skullcap root has been chosen to treat rats with SAP. Antibacterial and anti-inflammatory Baicalin can inhibit platelet aggregation, eliminate oxygen free radicals and reduce the generation of endotoxin. Baicalein, the metabolite of Baicalin in body, is also potent at inhibiting pancreatin. Baicalin can block multiple phases during SAP onset. Baicalin also has many effects similar to those of Somatostatin and its analogues. Therefore, Baicalin can be used more extensively[13-16].
In this experiment, tissue microarray (TMA) was adopted to study the protective effects of Baicalin on the liver of rats with SAP. The therapeutic effects of Baicalin and Octreotide were compared to prove the therapeutic effects of Baicalin on SAP.
Experimental animals: Clean grade healthy male Sprague-Dawley (SD) rat in 250-300 g of body weight were purchased from the Experimental Animal Center of Medical School, Zhejiang University (China).
Experimental medicine and reagents: Sodium taurocholate and sodium pentobarbital purchased from USA Sigma Company, Octreotide purchased from Swiss pharmaceutical company Novartis, 5% Baicalin injection (China national invention patent number ZL200310122673.6) prepared by the first author with 305 mmol/L osmotic pressure. Bax and Bcl-2 antibody purchased from Santa Cruz Company. The main reagent Takara in situ Apoptosis detection Kit purchased from TaKaRa Biotechnology Co., Ltd, PK (protease K) purchased from Sigma Company, DAB (biphenyldiamine) purchased from China Huamei Company. The above determinations were all operated according to the instructions of the kits.
Preparation methods of animal models: Prepared 135 SAP rat models via retrograde injection of 3.5% sodium taurocholate to the pancreatic duct through epidural catheter and duodenal papilla.
Rats grouping: The 135 SAP rat models were randomly assigned to the model control group, Baicalin treated group and Octreotide treated group, 45 rats in each group while other 45 rats were assigned to the sham-operated group. In sham-operated group, only exploratory laparotomy was performed, namely after entering abdominal cavity, checking pancreas and duodenum and then closing abdomen. After that, the above-mentioned groups were randomly divided into 3 h group, 6 h group and 12 h group, 15 rats in each group[13-16].
Baicalin treated group: The animal experiments of 5% Baicalin injection have been completed including the acute toxicity test and SAP rat treated by small, middle and large dose. The large dose can achieve the best therapeutic effect (dose is 10 mg/h per 100 g) and the dosage referred to the result of the previous preliminary experiment. Ten min after successful modeling, Baicalin treated group was first injected 5% Baicalin injection 10 mg/100 g via external jugular vein passage followed by continuous intravenous administration (10 mg/h per 100 g) by microinfusion pump[13-16].
Octreotide treated group: The octreotide treated group was first injected Octreotide 0.2 μg/100g via external jugular vein passage followed by continuous intravenous transfusion by a microinfusion pump at a transfusion speed of 0.2 μg/h per 100 g. All above dosages have been proved as effective dosages in the previous preliminary experiment.
Sham-operated group and model control group: Both groups were injected saline of equivalent volume at the corresponding time points after operation.
Observations were made at 2, 6 and 12 h after the operation. Mortalities of rats in all groups followed by batch execution of rats with observation of gross liver pathological changes.
Hepatic tissue samples were collected and fixed in accordance with relevant requirements and the pathological score changes of liver and pancreas under HE staining observed.
Changes of ALT (GPT) and AST (GOT) in serum via blood sampling from heart were determined.
Bax, Bcl-2 and Caspase-3 protein expression: TMA was applied to prepare the hepatic TMA sections (2 mm in diameter) and the SP method for immunohistochemical staining. After Bax, Bcl-2 and Caspase-3 protein expression of lung tissue was observed under light microscope, the comprehensive judgment was carried out basing on the percentage of positive cells: (-) in case positive cell count < 10%; (+) in case positive cell count 10%-20%; (++) in case positive cell count 20%-50%; (+++) in case positive cell count > 50%.
TUNEL staining technique was performed to observe the changes of hepatic apoptotic cells and the apoptotic indexes were calculated. Apoptotic index = apoptotic cell count/total cell count × 100%.
Values were presented as mean and standard deviation for nomal distribution variables or median and quartile range for highly skewed variables. The significance of differences among the four groups was tested using the Kruskal-Wallis test for highly skewed data and analysis of variance (ANOVA) for normal distribution data. Mutiple comparisons were subjected to Bonfferoni correction test. The Chi-square test was used to evalute equality of frequencies for discrete variables. Correlations were tested using the Spearman rank correlation coefficients. P < 0.05 was considered statistical significant and all statistical analyses were conducted using SPSS version 11.5 for windows.
The mortalities of the model control group were respectively 0% (0/15), 13.33% (2/15) and 33.33% (5/15) at 3, 6 and 12 h, all the mortalities of Baicalin treated group and Octreotide treated group were 0% at different time points. The entire sham-operated group survived at the different time points. The survival of model control group was 66.67% (10/15) at 12 h while the survivals of both Baicalin treated group and Octreotide treated group were 100% at 12 h, indicating marked difference (P < 0.05)[13-16].
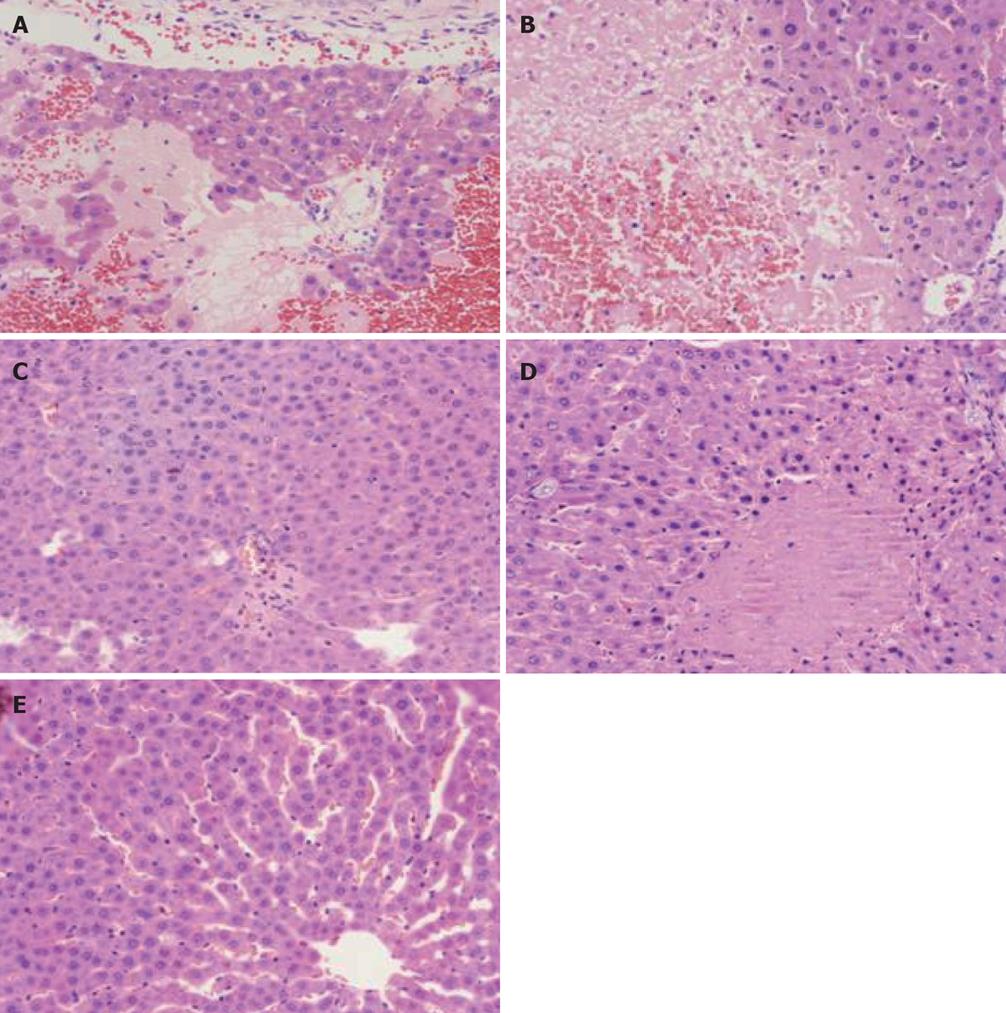
Model control group: (1) Gross changes. 3 h group: Mild swelling of liver, local grey plaques in liver of individual rat, obscure boundary; at 6 h and 12 h pale and muddy liver color or hemostasis change, part with scattered grey plaques or necrosis manifestation in irregular shapes, especially obvious at edge of liver; (2) Changes under light microscope. 3 h group: Swelling and apomorphosis of liver cells, inflammatory cell infiltration in portal area, dilation and hyperemia of sinus hepaticus, scattered focal or punctate necrosis in hepatic lobules; 6 h group: Obvious swelling of liver cells, collapse of liver cell cord within hepatic lobules caused by integrality damage due to relatively large area of focal necrosis of liver cells, local sinus hepaticus narrowing or vanishing, increased range and area of liver cell necrosis, visible focal or large lamellar necrosis, mainly hemorrhage necrosis and some coagulation necrosis, inflammatory cell infiltration within necrosis focus, obvious congestion in partial sinus hepaticus (Figure 1A); 12 h group: Obviously damaged hepatic lobule structure, further increased range and area of cell necrosis, residual metamorphic liver cells only at periphery of partial hepatic lobules; relatively large area of inflammatory cell infiltration within lobules or portal area, obvious congestion in sinus hepaticus (Figure 1B).
Baicalin and Octreotide treated group: (1) Gross changes. The gross liver pathological changes of the Baicalin and Octreotide treated group were milder than those of the model control group; (2) Changes under light microscope. Baicalin treated group at all time section: mild swelling of liver cells, mild dilation and hyperemia change of sinus hepaticus, scattered inflammatory cell infiltration in portal area; 6 h and 12 h group: Punctate necrosis and/or mild focal necrosis of liver cells, no obvious lamellar necrosis, inflammatory cell infiltration in portal area. The gross pathological changes in all groups were milder than those of the model control group; there was no marked difference between the Baicalin and Octreotide treated group, but Baicalin treated group had milder pathological manifestations (Figure 1C and D).
Sham-operated group: (1) Gross changes. No obvious swelling of liver, normal color; (2) Changes under light microscope. Complete structure of hepatic lobules, occasional inflammatory cell infiltration in portal area. Most liver cells have normal morphous, some local swelling of liver cells, cholestasis and stenosis of sinus hepaticus (Figure 1E).
Comparison of liver pathological scores in all groups: The pathohistological severity score standard made by first author[19]. The scores of the model control group, Baicalin treated group and Octreotide treated group were significantly exceeded the sham-operated group at the different time points (P < 0.001). The scores of the Baicalin treated group and Octreotide treated group were significantly lower than that of the model control group at 12 h (P < 0.05). There was no marked difference between the Baicalin treated group and Octreotide treated group at the different time points (P > 0.05) (Table 1).
Pathological severity score of pancreas: A modified Schmidt’s severity score standard of pancreas made by us was referred[19]. The pancreatic severity score in model control group, Baicalin treated group and Octreotide treated group significantly exceeded than that in sham-operated group (P3, 6, 12 h < 0.001). The pancreatic severity score of Baicalin treated group was significantly lower than that in model control group at 12 h (P12 h < 0.01), and Octreotide treated group was significantly lower than those in model control group at 6 h and 12 h (P6, 12 h < 0.01). There was no marked difference in pancreatic severity score between Baicalin treated group and Octreotide treated group at 3, 6 and 12 h (P3, 6, 12 h > 0.05) (Table 1).
Comparison of serum ALT (GPT) contents in all groups: The model control group and treated groups were significantly higher than the sham-operated group at all time points (P < 0.001). The Baicalin treated group was significantly lower than the model control group at all time points (P < 0.001), the Octreotide treated group were significantly lower than the model control group (P < 0.001). The Baicalin treated group was significantly lower than the Octreotide treated group at 3 h (P < 0.01), no marked difference between the Baicalin treated group and Octreotide treated group at 6 h (P > 0.05), the Baicalin treated group was significantly lower than the Octreotide treated group at 12 h (P < 0.05) (Table 2).
Comparison of serum AST (GOT) contents in all groups: The model control group and treated groups were significantly higher than the sham-operated group at all time points (P < 0.001). The Baicalin treated group was significantly lower than the model control group (P < 0.001), the Octreotide treated group significantly lower than the model control group (P < 0.05), and the Baicalin treated group significantly lower than the Octreotide treated group (P = 0.001) at 3 h. The Baicalin treated group was significantly lower than the model control group (P < 0.001), the Octreotide treated group significantly lower than the model control group (P < 0.001) at 6 h and 12 h. The Baicalin treated group was significantly lower than the Octreotide treated group at 6 h (P < 0.05), no marked difference between the Baicalin treated group and Octreotide treated group at 12 h (P > 0.05) (Table 2).
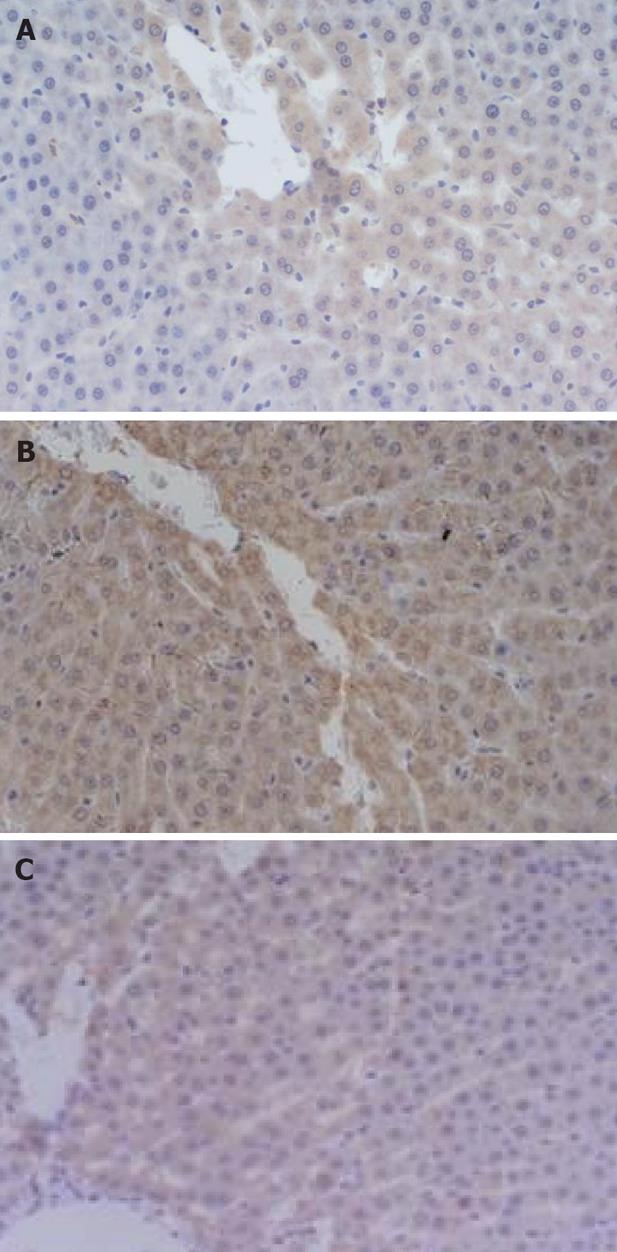
Comparison of the expression level of Bax protein in liver: The positive Bax protein staining position was in cytoplasm of liver cell. There was no marked difference in all groups at 3 h (P > 0.05). The Octreotide treated group significantly exceeded the sham-operated group at 12 h (P < 0.01). Both the Baicalin treated group and Octreotide treated group significantly exceeded the sham-operated group (P < 0.05) and the Baicalin treated group significantly exceeded the model control group (P < 0.05) at 6 h (Table 1 and Figure 2A).
Comparison of the expression level of Bcl-2 protein in liver: The positive Bcl-2 protein staining position was in cytoplasm of liver cell. There was no marked difference between the Octreotide treated group and sham-operated group, the model control group significantly exceeded the sham-operated group (P < 0.05), the Octreotide treated group significantly lower than the model control group (P < 0.05) at different time points. The Baicalin treated group significantly exceeded the sham-operated group at 3 h (P < 0.05), the Baicalin treated group significantly lower than the model control group at 6 h (P < 0.05), the Octreotide treated group significantly lower than the Baicalin treated group at 3 h (P < 0.05) (Table 1 and Figure 2B).
Comparison of the expression level of Caspase-3 protein in liver: The positive staining signal of Caspase-3 protein was localized in the cytoplasm of hepatic cells. At all time points after operation, the staining intensity of Caspase-3 protein in liver in the model control group, the Baicalin treated group and the Octreotide treated group were significantly higher than that in the sham-operated group (P < 0.05, P < 0.01 and P < 0.05, respectively); at 12 h after operation, the staining intensity in the Baicalin treated group was obviously higher than that in the model control group (P < 0.05); at all time points after operation, no marked difference was noted between the Baicalin treated group and the Octreotide treated group (P > 0.05) (Table 1 and Figure 2C).
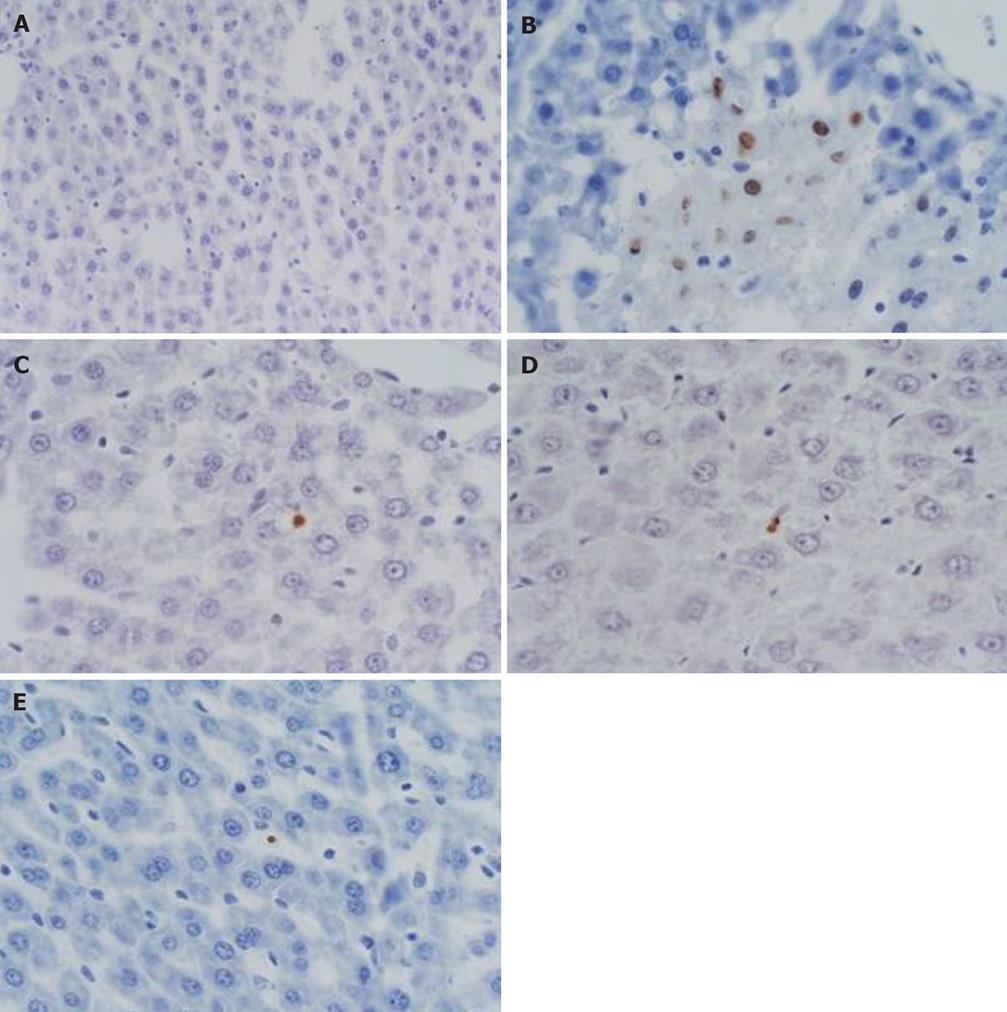
Comparison of apoptosis indexes in liver: Apoptosis occurred to liver Kupffer cells and liver cells. There was no marked difference in all groups at 3 h (P > 0.05); the Baicalin treated group significantly exceeded the sham-operated group (P < 0.05) and model control group (P < 0.01) at 6 h; Octreotide treated group significantly exceeded the sham-operated group and model control group at 6 h and 12 h (P < 0.05) (Table 1 and Figure 3).
Correlations between hepatic apoptosis indexes and Bax, Bcl-2: The 3 h apoptosis indexes of the Baicalin treated group was positively correlated with Bax (P < 0.001); the 12 h apoptosis indexes of the Baicalin treated group was positively correlated with Bax (P < 0.05); the 3 h apoptosis indexes of the Octreotide treated group was positively correlated with Bax (P < 0.01); There was no correlation between apoptosis indexes in all groups and Bcl-2 (P > 0.05).
Correlations between pathological score of hepatic injury and ALT, AST: The 6 h pathological score of the model control group was positively correlated with ALT (P < 0.01). The 3 h pathological score of the Baicalin treated group was positively correlated with AST (P < 0.01); the 12 h pathological score of the Baicalin treated group was positively correlated with ALT (P < 0.05). The 3 h pathological score of the Octreotide treated group was positively correlated with ALT (P < 0.05); the 6 h pathological score of the Octreotide treated group was positively correlated with ALT (P < 0.001) and meanwhile AST (P < 0.05); the 12 h pathological score of the Octreotide treated group was positively correlated with ALT (P < 0.01).
As the morbidity and mortality of SAP increases, improving the clinical therapeutic effects on SAP and finding cheap alternates with precise therapeutic effects and fewer side effects have been the hot spots of clinical research[20-23]. Our study results show the main mechanism of MODS onset during AP is the abnormal activation of mononuclear-macrophage system which generates excessive cytokines[24]. Kupffer cells of liver, which is the largest fixed macrophage population in body, account for 80%-90% of the total mononuclear macrophage system. They must affect the progression of SAP state. Therefore, it is necessary to find cheap alternates with precise therapeutic effects and fewer side effects to protect rats with SAP from hepatic injury.
As one of the common clinical SAP medications at present, Octreotide can effectively reducing SAP complications and improve survival[12,25]. However, it is also expensive and inconvenient. It is still not sure whether Octreotide can protect liver, leading to the need of further study. However, Baicalin is cheap and convenient. Baicalin also can protect liver. At present, our hospital is still the only hospital that has submitted a study report on SAP treated by Baicalin injection[13-16]. We have been granted a Chinese invention patent number for our Baicalin injection prescription, which has paved the way for further developing and utilizing the new drug.
This study showed the pancreatic score of Baicalin treated group was significantly lower than that in model control group at 12 h, which mentioned that Baicalin can relieve the severity of pancreas in SAP procedure. The protective effect of Baicalin may be related to inhibit secretion of pancreatin and decrease the contents of endotoxin and TNF-α. From the prospect of hepatic pathological score, spotty necrosis and/or mild focal necrosis, visible inflammatory cell infiltration in portal area and no lamellar necrosis were observed in Baicalin treated group microscopically. The gross pathological changes were milder in Baicalin treated group than in model control group. And there was no difference between Baicalin treated group and Octreotide treated group in gross changes[15].
ALT is the abbreviation for alanine aminotransferase. It is found mainly in liver cells. AST is the abbreviation for aspartate aminotransferase, which is found mainly in cardiac muscle, followed by the liver. Both are non-specific intracellular functional enzymes and are normally found in the serum at very low levels. When hepatic cells are damaged, their membrane permeability increases. As a consequence, cytoplasmic ALT and AST are released into the blood, resulting in an increase in the activities and contents of serum ALT and AST. Therefore, serum ALT and AST levels are sensitive parameters for the evaluation of hepatic cell damage. The correlation analysis has demonstrated a positive correlation between 6 h and 12 h pathological scores and ALT (P < 0.01 and P < 0.05), and a positive correlation between 3 h score and AST (P < 0.01) in Baicalin treated group, which means Baicalin can alleviate the hepatic injury of rats with SAP, and protect rats from SAP hepatic injury. We believe these effects of Baicalin are related to its many capacities. For instance, Baicalin can lower AST and AST content in serum, and down regulate the expression level of hepatic Bcl-2 protein. According to our results from a series of experiments, we believe that the protective effect of Baicalin and Octreotide on the liver may be mediated by reducing the contents of inflammatory mediators such as NO, MDA, TNF-α, IL-6 and PLA2etc[14-16].
Bax and Bcl-2 are two important components of apoptosis regulating system. When Bax forms dimers, it will induce apoptosis. As Bcl-2 expression increases, the apoptosis promoting effect of Bax dimers are inhibited. In this experiment, it was found that the expression levels of both Bax protein and Bcl-2 protein rose during SAP. It is possible that both apoptosis induction and inhibition have been enhanced so equally that no marked difference occurred between model control group and sham-operated group in apoptotic index. In addition, according to the experimental results, the expression level of Bax protein at 6 h was higher in Baicalin treated group than in model control group (P < 0.05). The expression levels of Bcl-2 protein at 6 h and 12 h were lower in Baicalin treated group than in model control group (P < 0.05). These findings indicate that Baicalin can induce Bax and inhibit the expression of Bcl-2 protein to let apoptosis inducing factor prevail in treated group. Therefore, Baicalin can protect liver and alleviate pathological changes. The expression levels of Bcl-2 protein were lower in Octreotide treated group than in model control group at different time points. The apoptotic indexes at 6 h and 12 h were higher in Octreotide treated group than in model control group (P < 0.05). These findings indicate that Octreotide can induce apoptosis in hepatic cells. From the prospect of correlation analysis, the apoptotic indexes at 3 h and 12 h were positively correlated with Bax, and not correlated with Bcl-2 in treated group, which means that Bax gene participates in the regulation of apoptosis of hepatic cell while Bcl-2 gene may prevent the apoptosis of hepatic cell and promote its hyperplasia during hepatic injury of rats with SAP.
Caspase-3 is an interleukin-converting enzyme that belongs to a member of caspase family (cysteine protease)[26,27]. Some studies have demonstrated that cell apoptosis is negatively correlated with the severity of SAP[28,29]. Activated Caspase-3 is the main executor of cell apoptosis[30,31] and works as the final common signal molecule of various apoptotic mechanisms[32]. During apoptosis signal transduction, caspases function as downstream signal molecules. The binding of caspases to cytochrome c can induce the activation of Caspase-3 which enables cell apoptosis completed. Some characteristic markers for cell apoptosis, such as chromosome condensation and DNA fragmentation, are directly related to Caspase-3[33,34]. This study showed that, at 12 h after operation, the expression level of Caspase-3 protein in liver in the Baicalin treated group was significantly higher than that in the model control group, suggesting that Baicalin is able to effectively upregulate the expression of Caspase-3 protein and promote apoptosis. Although some studies have indicated that cell apoptosis is positively correlated with hepatic injury[35-37], the results of the present study showed that low proportion of hepatic apoptosis at the early stage was favorable to the body. Considering that apoptotic cells do not release inflammatory mediators and, therefore, reduce their contents and weaken the body’s inflammatory reaction[14], we believe that cell apoptosis which occurred at early stage of SAP has some protective effect on the liver.
The TMA we adopted has surpassed the traditional tissue pathological section technique that is inefficient only using a single sample[38]. There is a great potential for TMA in oncopathological research since it can achieve high-throughput, reliable results and so on. This field is also the focus of current study[39-41].
In conclusion, Baicalin and Octreotide can enhance the apoptosis promoting effect of Bax dimmers by lowering the expression level of Bcl-2 protein, leading to the apoptosis of hepatic Kupffer cells and hepatic cells, thus alleviating the pathological changes of liver. Baicalin and Octreotide also can lower the content of ALT, AST in serum to improve the survival of rats with SAP. Baicalin is more advantageous than Octreotide in many aspects. TMA is time and energy saving, highly efficient and representative for the pathological examination of pancreatitis. We believe that as a cheap new drug with precise therapeutic effects and fewer side effects, Baicalin can play certain role in future SAP treatment.
Severe acute pancreatitis (SAP), which is a systemic disease, seriously life-threatening with acute onset, severe state and multiple complications can cause multiple organ injuries, especially to liver. Octreotide mainly treats SAP by inhibiting trypsin secretion, lowering endotoxin generation, inhibiting the release of inflammatory mediators and platelet aggregation, etc. As the main effective ingredient of Scutellaria Baicalensis Georgi, the pharmacologic effects of Baicalin (monomer) can antagonize multiple phases during SAP onset, Baicalin is a possible medication for treating SAP. This article has observed the effects of Baicalin and Octreotide on SAP with hepatic injury at different time points, and compared their therapeutic effects and mechanisms, providing the theoretical basis for application of Baicalin to treat SAP with hepatic injury.
Tissue microarray (TMA) has become a new member of the chip family recently. This technique has been extensively applied to the technological fields. It surpasses the concept of traditional histopathologic slide which can afford the relevant biological function study at the three levels including gene, genetic transcription and related expression product. Presently, there are some reports on Baicalin treatment of SAP made by the authors. This experiment is one of series studies about Baicalin treatment to SAP. The authors applied TMA technique to prepare the sections of liver samples, and conducted pathological examinations of different indexes.
Firstly, the authors applied TMA technique to study the pathological changes in SAP complicated with hepatic injury; Secondly, they found Baicalin injectin has good effects to treat SAP rats, and has a similar function with Octreotide.
The application of hepatic TMA in the study of SAP is economical and efficient, and it is worthy to be further popularized. This article will make us realize the value of applying Baicalin in SAP treatment.
Caspase-3 is an interleukin-converting enzyme that belongs to a member of caspase family (cysteine protease). Activated Caspase-3 is the main executor of cell apoptosis and works as the final common signal molecule of various apoptotic mechanisms.
This article showed that the Baicalin and Octreotide can protect rats with SAP by improving the expression levels of Bax protein, Caspase-3 protein, etc. This is a well-written paper.
Peer reviewers: Ke-Ji Chen, Professor, Xiyuan Hospital, Chinese Tranditional Medicine University, Beijing 100091, China; Yoshiharu Motoo, MD, PhD, FACP, FACG, Professor and Chairman, Department of Medical Oncology, Kanazawa Medical University, 1-1 Daigaku, Uchinada, Ishikawa 920-0293, Japan
S- Editor Li DL E- Editor Yin DH
| 1. | Zhang Q, Ni Q, Cai D, Zhang Y, Zhang N, Hou L. Mechanisms of multiple organ damages in acute necrotizing pancreatitis. Chin Med J (Engl). 2001;114:738-742. |
| 2. | Zhang XP, Ye Q, Jiang XG, Ma ML, Zhu FB, Zhang RP, Cheng QH. Preparation method of an ideal model of multiple organ injury of rat with severe acute pancreatitis. World J Gastroenterol. 2007;13:4566-4573. |
| 3. | Rau BM, Bothe A, Kron M, Beger HG. Role of early multisystem organ failure as major risk factor for pancreatic infections and death in severe acute pancreatitis. Clin Gastroenterol Hepatol. 2006;4:1053-1061. |
| 4. | Garcea G, Jackson B, Pattenden CJ, Sutton CD, Neal CP, Dennison AR, Berry DP. Early warning scores predict outcome in acute pancreatitis. J Gastrointest Surg. 2006;10:1008-1015. |
| 5. | Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24 Suppl 1:45-51. |
| 6. | Lytras D, Manes K, Triantopoulou C, Paraskeva C, Delis S, Avgerinos C, Dervenis C. Persistent early organ failure: defining the high-risk group of patients with severe acute pancreatitis? Pancreas. 2008;36:249-254. |
| 7. | Yousaf M, McCallion K, Diamond T. Management of severe acute pancreatitis. Br J Surg. 2003;90:407-420. |
| 8. | Zhang HY, Xia Q. [Clinical study on severe acute pancreatitis complicated by hepatic insufficiency]. Zhongxiyi Jiehe Xuebao. 2006;4:17-19. |
| 9. | Wang G, Sun B, Gao Y, Meng QH, Jiang HC. The effect of emodin-assisted early enteral nutrition on severe acute pancreatitis and secondary hepatic injury. Mediators Inflamm. 2007;2007:29638. |
| 10. | Shor NA, Levina VP, Ioffe IV, Andreeva IV, Chumak IuF, Zhadanov VI, Zelenyi II. [Application of octreotide in patients with acute pancreatitis]. Klin Khir. 2004;15-17. |
| 11. | Suzuki M, Shimizu T, Kudo T, Shoji H, Ohtsuka Y, Yamashiro Y. Octreotide prevents L-asparaginase-induced pancreatic injury in rats. Exp Hematol. 2008;36:172-180. |
| 12. | Paran H, Mayo A, Paran D, Neufeld D, Shwartz I, Zissin R, Singer P, Kaplan O, Skornik Y, Freund U. Octreotide treatment in patients with severe acute pancreatitis. Dig Dis Sci. 2000;45:2247-2251. |
| 13. | Zhang XP, Zhang L, He JX, Zhang RP, Cheng QH, Zhou YF, Lu B. Experimental study of therapeutic efficacy of Baicalin in rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:717-724. |
| 14. | Zhang XP, Tian H, Lai YH, Chen L, Zhang L, Cheng QH, Yan W, Li Y, Li QY, He Q. Protective effects and mechanisms of Baicalin and octreotide on renal injury of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:5079-5089. |
| 15. | Zhang XP, Zhang L, Yang P, Zhang RP, Cheng QH. Protective effects of baicalin and octreotide on multiple organ injury in severe acute pancreatitis. Dig Dis Sci. 2008;53:581-591. |
| 16. | Zhang XP, Tian H, Chen HQ, Chen L, Wang ZW, Wang KY, Yan W, Li Y, Li QY, He Q. The protecting effects and mechanisms of Baicalin and Octreotide on heart injury in rats with SAP. Mediators Inflamm. 2007;2007:19469. |
| 17. | Gu XD, Zhang Q. Clinical progress in the treatment of severe acute pancreatitis with integrative Chinese and Western medicine. Chin J Integr Med. 2007;13:235-240. |
| 18. | Li YY, Sibaev A, Zhou MZ, Zhu GY, Yuce B, Storr M. The Chinese herbal preparation Qing Yi Tang (QYT) improves intestinal myoelectrical activity and increases intestinal transit during acute pancreatitis in rodents. Phytother Res. 2007;21:324-331. |
| 19. | Zhang XP, Zhang L, Wang Y, Cheng QH, Wang JM, Cai W, Shen HP, Cai J. Study of the protective effects of dexamethasone on multiple organ injury in rats with severe acute pancreatitis. JOP. 2007;8:400-412. |
| 20. | Lese M, Tamasan A, Stoicescu B, Branduse M, Puia I, Mare C, Lazar C. [Surgical treatment in severe acute pancreatitis. Last 15 years of experience in Emergency County Hospital of Baia Mare]. Chirurgia (Bucur). 2005;100:445-450. |
| 21. | Cappell MS. Acute pancreatitis: etiology, clinical presentation, diagnosis, and therapy. Med Clin North Am. 2008;92:889-923, ix-x. |
| 22. | Lankisch PG, Lerch MM. Pharmacological prevention and treatment of acute pancreatitis: where are we now? Dig Dis. 2006;24:148-159. |
| 23. | Hochman D, Louie B, Bailey R. Determination of patient quality of life following severe acute pancreatitis. Can J Surg. 2006;49:101-106. |
| 24. | Norman J. The role of cytokines in the pathogenesis of acute pancreatitis. Am J Surg. 1998;175:76-83. |
| 25. | Czakó L, Hegyi P, Takács T, Góg C, Farkas A, Mándy Y, Varga IS, Tiszlavicz L, Lonovics J. Effects of octreotide on acute necrotizing pancreatitis in rabbits. World J Gastroenterol. 2004;10:2082-2086. |
| 26. | Yang J, Fier A, Carter Y, Liu G, Epling-Burnette PK, Bai F, Loughran TP Jr, Mastorides S, Norman JG, Murr MM. Liver injury during acute pancreatitis: the role of pancreatitis-associated ascitic fluid (PAAF), p38-MAPK, and caspase-3 in inducing hepatocyte apoptosis. J Gastrointest Surg. 2003;7:200-207; discussion 208. |
| 27. | Meyerholz DK, Samuel I. Morphologic characterization of early ligation-induced acute pancreatitis in rats. Am J Surg. 2007;194:652-658. |
| 28. | Bhatia M. Apoptosis of pancreatic acinar cells in acute pancreatitis: is it good or bad? J Cell Mol Med. 2004;8:402-409. |
| 29. | Xu XF, Lou WH, Wang DS, Jin da Y, Ni XL, Wu ZH. Influence of glutamine on pancreatic blood flow and apoptosis of pancreatic acinar in rats with severe acute pancreatitis. Chin J Dig Dis. 2006;7:121-126. |
| 30. | Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479-489. |
| 31. | Yasuda T, Takeyama Y, Ueda T, Shinzeki M, Kishi S, Sawa H, Nakajima T, Kuroda Y. Protective effect of caspase inhibitor on intestinal integrity in experimental severe acute pancreatitis. J Surg Res. 2007;138:300-307. |
| 33. | Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99-104. |
| 34. | Peng Y, Sigua CA, Gallagher SF, Murr MM. Protein kinase C-zeta is critical in pancreatitis-induced apoptosis of Kupffer cells. J Gastrointest Surg. 2007;11:1253-1261. |
| 35. | Zhang XP, Wang L, Zhang J. Study progress on mechanism of severe acute pancreatitis complicated with hepatic injury. J Zhejiang Univ Sci B. 2007;8:228-236. |
| 36. | Takeyama Y. Significance of apoptotic cell death in systemic complications with severe acute pancreatitis. J Gastroenterol. 2005;40:1-10. |
| 37. | Takeyama Y, Hori Y, Takase K, Ueda T, Yamamoto M, Kuroda Y. Apoptotic cell death of hepatocytes in rat experimental severe acute pancreatitis. Surgery. 2000;127:55-64. |
| 38. | Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844-847. |
| 39. | Waddell SJ, Butcher PD. Microarray analysis of whole genome expression of intracellular Mycobacterium tuberculosis. Curr Mol Med. 2007;7:287-296. |
| 40. | Das K, Mohd Omar MF, Ong CW, Bin Abdul Rashid S, Peh BK, Putti TC, Tan PH, Chia KS, Teh M, Shah N. TRARESA: a tissue microarray-based hospital system for biomarker validation and discovery. Pathology. 2008;40:441-449. |
| 41. | Yu G, Wang J, Chen Y, Wang X, Pan J, Li Q, Xie K. Tissue microarray analysis reveals strong clinical evidence for a close association between loss of annexin A1 expression and nodal metastasis in gastric cancer. Clin Exp Metastasis. 2008;25:695-702. |