Published online Oct 14, 2008. doi: 10.3748/wjg.14.5827
Revised: August 12, 2008
Accepted: August 19, 2008
Published online: October 14, 2008
AIM: To explore the antitumor bioactivity of adenovirus-mediated mutant type p27kip1 gene in a colorectal cancer cell line SW480.
METHODS: We constructed recombinant adenovirus vector expressing a mutant type p27kip1 gene (ad-p27mt), with mutation of Thr-187/Pro-188 (ACGCCC) to Met-187/Ile-188 (ATGATC), and transduced into SW480 cells. Then we detected expression of p27, Bcl-2 and Bax protein in the transductants by Western blotting, cell cycle of transductants by a digital flow cytometric system, migrating potential with Boyden Chamber and SW480 tumor cell growth inhibition in vitro and in vivo.
RESULTS: We found that a recombinant adenovirus vector of expressing ad-p27mt, with mutation of Thr-187/Pro-188 (ACGCCC) to Met-187/Ile-188 (ATGATC) has potent inhibition of SW480 tumor cell growth in vitro and in vivo. Furthermore, ad-p27mt induced cell apoptosis via regulating bax and bcl-2 expressions, and G1/S arrest in SW480 cells and inhibited cell migration.
CONCLUSION: ad-p27mt has a strong anti-tumor bioactivity and has the potential to develop into new therapeutic agents for colorectal cancer.
- Citation: Sun ZQ, Deng CS, Xu SY, Du Y. Antitumor bioactivity of adenovirus-mediated p27mt in colorectal cancer cell line SW480. World J Gastroenterol 2008; 14(38): 5827-5833
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5827.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5827
| Sub-G1 (n = 3) | G0/G1 (n = 3) | S (n = 3) | G2/M (%) (n = 3) | |
| SW480 | 3.88 ± 1.85 | 73.17± 5.10 | 11.42 ± 2.93 | 11.21 ± 4.70 |
| SW480-ad-β-gal | 4.30 ± 1.02 | 68.27 ± 3.15 | 11.1 ± 0.96 | 15.43 ± 1.98 |
| SW480-ad-p27mt | 39.13 ± 1.841 | 49.40 ± 2.70 | 7.31 ± 0.70 | 3.92 ± 0.66 |
p27kip1, a member of the Kip/Cip family of cyclin-dependent kinases inhibitors (CKIs), is a putative tumor suppressor gene[1], and promoter of apoptosis[2] that has been demonstrated in cancer cells as well as in normal cells[3]. Simultaneously, p27kip1 acts as a safeguard inflammatory injury[4] and plays a role in cell differentiation[5]. Furthermore, p27kip1 was identified as an inhibitor of cyclin E/CDK2 in cells arrested in the G1 phase by lovastatin, transforming growth factor-beta (TGF-β), serum deprivation and contact inhibition[1,6].
Over the past years, p27kip1 protein has attracted our attention as an important prognostic factor in various malignancies. In short, lately, it has been reported that expression of p27kip1 protein is associated with poor prognosis in several types of malignancies, including breast, lung, gastric carcinoma[7-10] and colorectal adenocarcinoma[11-13].
The reduced expression of p27kip1 in cancer cells due to an increase in the rate of its degradation[14]. It is thought that the amount of p27kip1 protein is regulated by a posttranscriptional mechanism rather than p27kip1 gene aberrations because p27kip1 gene mutation seems to be uncommon in human malignancies[15]. It has been demonstrated that p27kip1 is poly-ubiquitinated both in vivo and in vitro, and p27kip1 ubiquitination requires its phosphorylation on threonine residue 187 (T187) both in vivo and in vitro[16,17].
Gene therapy is a promising approach to restore p27 expression using adenoviral vectors[11,18-20]. These agents have induced cell-cycle arrest and loss of cyclinE-CDK2 activity in cell lines and xenograft models and have triggered apoptosis in cancer cells[2,19,21-24]. The concentration of p27 is thought to be regulated predominantly by the ubiquitin-dependent proteolytic pathway[25]. Degradation of p27 triggered by its phosphorylation on Thr187 is required for the binding of p27 to Skp2, the F-box protein component of an SCF ubiquitin ligase (E3) complex, and such interaction in turn results in the poly-ubiquitylation and degradation of p27[16,25]. Reduction of p27 levels in various types of malignant tumors results from accelerated proteolytic degradation by this pathway[26].
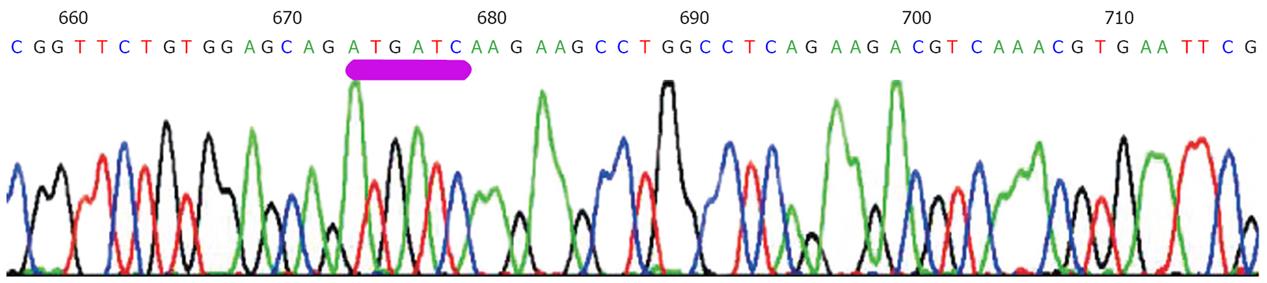
However, so far no report has been published on the effects of adenovirus-mediated mutant type p27kip1 gene on colorectal cancer cell. Thus, we constructed recombinant adenovirus vector expressing a mutant type p27kip1 gene (ad-p27mt), with mutation of Thr-187/Pro-188 (ACGCCC) to Met-187/Ile-188 (ATGATC), which will inhibit degradation of p27 protein[11,16,24,25,27], and explored its antitumor bioactivity in a colorectal cancer cell line SW480.
Human colorectal cancer cell line SW480 was purchased from Shanghai Cell Lines Bank (Shanghai, China). Cells were maintained in RPMI 1640 (Beyotime, China) supplemented with 10% fetal bovine serum (FBS; Beyotime, China).
Ad-p27mt was constructed in the Institute of Clinical Medicine of Yunyang Medical College (Hubei, China). Briefly, the cDNA of human p27mt gene was digested from plasmid of pORF9-hp27mt (Invitrogen) and subcloned into the plasmid of pBluescript II SK (+) (Stratagene) and formed plasmid of pBluescript-hp27mt. Then human p27mt gene was digested from pBluescript-hp27mt and subcloned into shuttle vector pBluescript-CMV (Stratagene) and gained shuttle plasmid of pShuttle-CMV-hp27mt. Adenovirus genomic DNA plasmid of pAdeasy-1 (Stratagene) was transformed into BJ5183 bacterium (Stratagene) and prepared competent BJ5183 bacterium containing pAdeasy-1. PShuttle-CMV-hp27mt was linearized with Pmel (New England Bio labs) and transformed into competent BJ5183 bacterium containing pAdeasy-1 and positive clone of homologous recombination was selected. Identified recombinant adenovirus plasmid of pAd-p27mt was digested with Pacl and transfected into HEK293 cells (Stratagene) with liposome polyFect (Qiagen) to package adenovirus particles. HEK293 cells were maintained in DMEM with 10% FBS until the onset of the cytopathic effect. PCR technique was used to detect target gene and the titer of the recombinant adenovirus was determined by measuring the absorbance at 260 nm and 280 nm. Ad-p27mt was propagated in HEK293 cells, purified by two cesium chloride density centrifugations, titered and stored at -70°C. Recombinant adenovirus expressing β-galactosidase (ad-β-gal) without any therapeutic gene was used as the control virus in all experiments.
Exponential growing of SW480 cells were transduced with ad-p27mt or ad-β-gal at 20 multiplicity of infection for 1 h with gentle frequent shaking and then incubated with complete media for the experiment.
Briefly, transductants were schizolysed and DNA was extracted using phenol/chloroform, and then dehydrated alcohol precipitated DNA. Sequence of DNA was assayed by Shanghai Sangon Company.
Cells lysates were prepared from transductants of ad-p27mt (SW480-ad-p27mt), transductants of ad-β-gal (SW480-ad-β-gal) and parental cells (SW480) as follows: cells were cultured for 96 h, washed three times with 100 mmol/L phosphate-buffered-saline (PBS, Beyotime, China), lysed with RIPA buffer [50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1% NP-40, 0.1% SDS; Beyotime, China]. The protein concentration of the samples was determined by Bio-Rad protein assay (Bio-Rad, Amersham). Fifty micrograms of protein samples were electrophoresed on SDS-polyacrylamide gel. Proteins from gels were transferred to PVDF membrane (Sigma), and membrane was incubated with a 1:1000 dilution of the monoclonal anti-p27 antibody (Beyotime, China), anti-Bax antibody (Beyotime, China), and anti-Bcl-2 antibody (Beyotime, China), respectively. The blots were developed using the enhanced chemoluminescence (ECL) Western blotting system and protocol (Amersham). In all immunoblotting experiments, blots were reprobed with an anti-β-actin antibody (Sigma) for internal control.
Cells were seeded on 96-well plates (Beyotime, China) at 4 × 103 cells per well in RPMI 1640 supplemented with 10% FBS. After 2 d, 4 d and 6 d, the number of cells was quantitated by an assay in which MTT; 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (Sigma) was used.
Cells were seeded in 75 cm2 culture flask (Beyotime, China) in RPMI 1640 supplemented with 10% FBS. After 96 h, the stuck and floating cells were collected in conical tubes (Beyotime, China). Then, the cells were fixed with 70% cold ethanol and washed with PBS. After treatment with 0.1 mg/mL RNaseA (Sigma), the cells were stained with 40 μg/mL propidium iodine (Sigma), and the cell cycle was analyzed by a digital flow cytometric system (Beckman Coulter EPICS-XL).
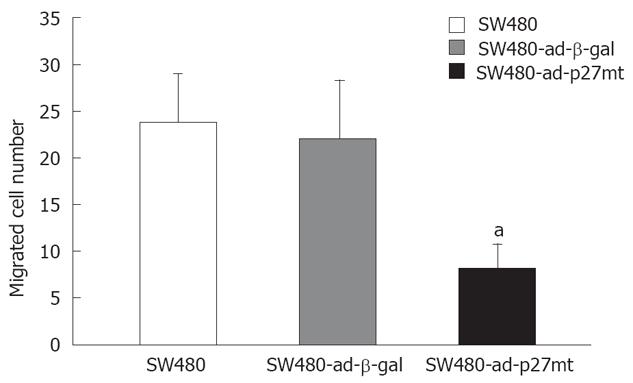
Migrative potential was evaluated in the Boyden chamber apparatus (Kylin-bell, China). This assay was developed to facilitate analysis of aspects of cancer invasion and metastasis. Briefly, subconfluence cells were starved for 24 h and harvested with 0.05% trypsin (Sigma) containing 0.02% EDTA (Sigma), washed twice with PBS, and resuspended to a final concentration of 5 × 105/mL in serum-free medium with 0.1% fraction V bovine serum albumin. PVP filters (Kylin-bell, China) of 8 μm pore size were precoated with gelatin, rinsed in sterile water, and were used for assay. Bottom wells of the chamber were filled with 25 μL of RPMI 1640 supplemented with 10% FBS per well and covered with a gelatin coated membrane, and then 50 μL of cells suspension was added to the top wells. After 24 h of incubation, the membranes were stained with Giemsa solution. Cells on the upper surface of the filter were carefully removed with a cotton swab, and the cells that had migrated through the membrane to the lower surface were counted in six different fields under a light microscope at 400 magnification. Each experiment was performed in triplicate wells and repeated 3 times.
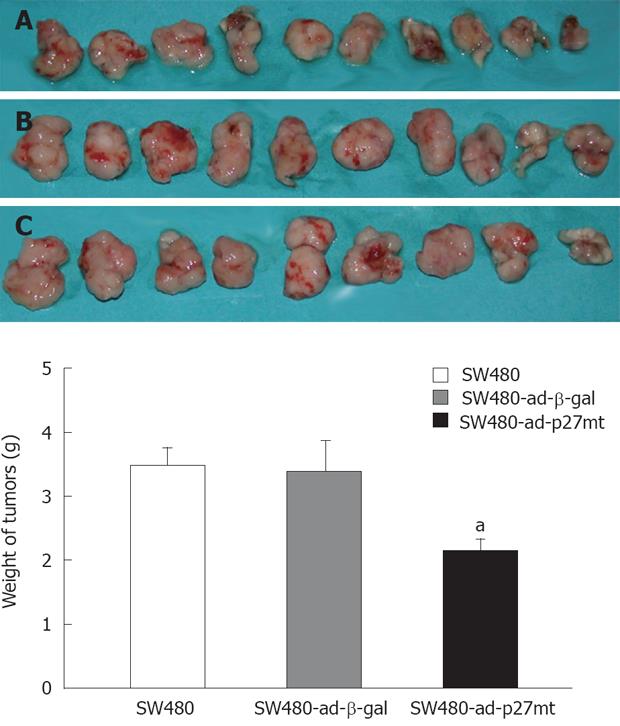
BALB/c nude mice were purchased from Animal experiment center of Hubei (Hubei, China; Qualification number: SCXK: Hubei 2003-0005). All animal experimental procedures were conducted and approved by the Institutional Animal Care and Use Committee of Yunyang Medical College (Approval number: SYXK: Hubei 2004-0021). Briefly, SW480 cells were cultured in RPMI 1640 supplemented with 10% FBS. Cells were harvested through two consecutive trypsinizations, centrifuged at 300 ×g for 5 min, washed twice, and resuspended in sterile PBS. Cells (1 × 107) in 0.2 mL were injected subcutaneously into a 6-week-old nude mouse between the scapulae of each nude mouse. After 14 days, tumors reached a mean size of 200 mm3 in the mice’s bodies. To test in vivo growth suppressive potential of ad-p27mt on nude mouse xenografts of SW480, intratumoral injections of ad-p27mt, ad-β-gal or PBS were made every other day for total 3 times, respectively. There were ten mice in each group. Twenty-eight days after inoculation, mice were sacrificed by cervical dislocation, and tumor specimens were taken, photographed and weighed.
Data are mean of at least 3 independent experiments ± SD. Results were compared by one-way analysis of variance (ANOVA). A two-tailed P < 0.05 was regarded as statistically significant. All calculations were performed using the SPSS for Windows version 13.0 statistical program on a personal computer.
As shown in Figure 1, sequencing result was consistent with p27mt sequence in gene bank.
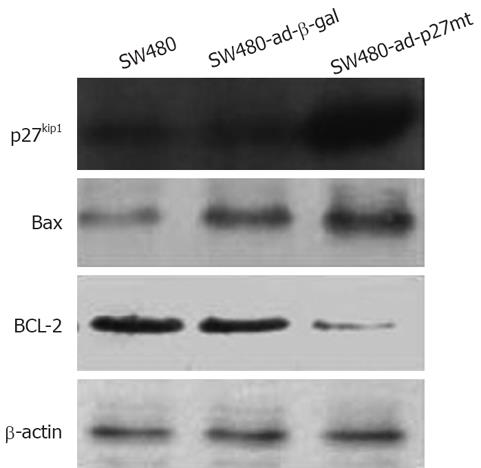
As shown in Figure 2, expression of p27 and Bax protein was increased significantly and Bcl-2 protein level was decreased in the SW480-ad-p27mt when compared with that in SW480-ad-β-gal and SW480. The expression of β-actin as an internal control was approximately the same in all of the cells.
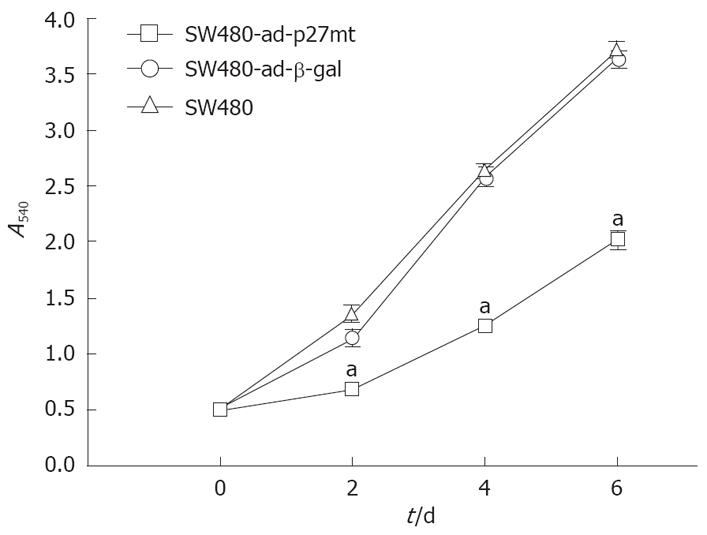
Relative cell number was evaluated by comparing the absorbance in each cell at day 2, day 4 and day 6. As shown in Figure 3, the growth of SW480-ad-p27mt was markedly inhibited compared with SW480-ad-β-gal and SW480.
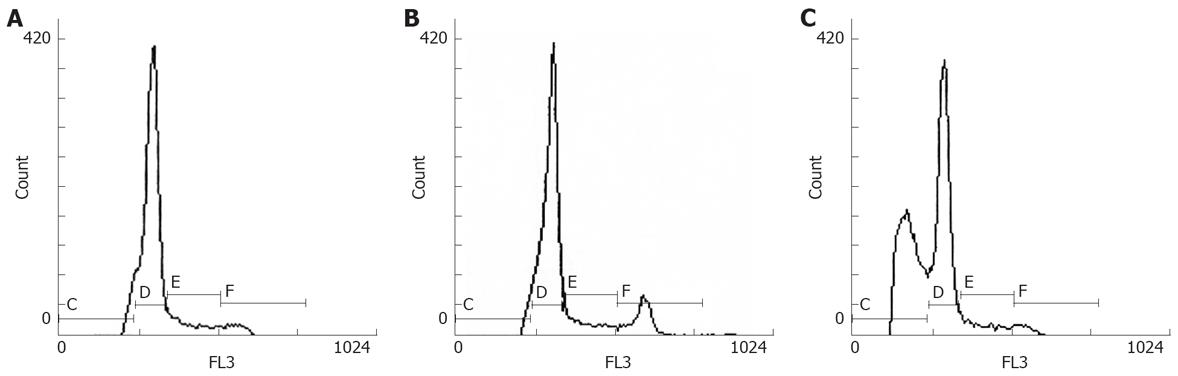
To further determine whether up-regulation of p27 protein can induce apoptosis and or cell cycle arrest, flow cytometric analysis was performed on each transductant. It is suggested that cells containing a sub-G1 content of DNA reflect the extent to which apoptosis is occurring. Flow cytometric analysis demonstrated that p27mt gene transduced into SW480 induced G1/S arrest and apoptosis. A marked sub-G1 peak and decreased percentage of cells in S phase were detected in SW480-ad-p27mt. Decreased percentage of cells in S phase suggested G1/S arrest (Figure 4 and Table 1).
SW480-ad-p27mt significantly showed the poor ability of migration when compared with that of SW480-ad-β-gal and SW480 ( Figure 5).
Twenty-eight days after inoculation, ten mice in each group all survived. Intratumoral injection of ad-p27mt into established tumors induced marked growth suppression. As shown in Figure 6, the weight of tumors receiving ad-p27mt(SW480-ad-p27mt) was significantly lighter than that of ad-β-gal treated(SW480-ad-β-gal) or PBS treated(SW480).
In this study, we found that transduction of ad-p27mt into SW480 cells resulted in induction of overexpression of p27 protein, which suggests that the approach to restore p27 expressing using adenoviral vectors is available and p27mt protein may be resistant to degradation by ubiquitin and more stable. Other studies also have shown that the p27 (T187A) mutant is not ubiquitinated[11,16,24,25,27], and the control of p27 protein levels is affected by ubiquitin-dependent degradation[17,25,26,28,29], in a ubiquitin-independent and Skp2-independent manner at G1[30-32], and by Jab1-dependent degradation[33].
We also observed that ad-p27mt induced growth suppression, apoptosis, and G1/S arrest in SW480 cells in vitro as well as the transplanted tumor growth inhibition in vivo, as was expected from the nature of p27 as a CDKIs. These findings are consistent with those of another study in which ad-p27 (T187A) had a greater effect on cell cycle arrest and apoptosis induction because of its resistance to degradation, and suppressed the growth of established lung cancer xenografts[24]. In several studies, intratumoral injections of ad-p27 have been shown to partially suppress tumor formation in animal models[18,23,24,34]. In other experiments, ad-p27 was the most potent of several cyclin kinase inhibitors in terms of inducing cell-cycle arrest, apoptosis and inhibiting tumorigenesis[2,18,21-23,27,34]. The effect of p27 on the cell cycle is regulated mainly by its stability[35,36], but recent studies have shown that the function of p27 is also associated with its subcellular localization[37-39]. Besides Thr187, there are three phosphorylation sites Ser10, Thr157 and Thr198 that are involved in cellular localization[40,41]. Phosphorylation at Ser10 stabilizes p27 protein in G1[41]. Phosphorylation at Thr157 by protein kinase B/Akt impairs the nuclear import of p27 but does not affect its stability in breast cancer and other cells[37,38]. Chu et al[42] indicating that the oncogenic kinase Src regulates p27 stability through phosphorylation of p27 at tyrosine 74 and tyrosine 88. Src inhibitors increase cellular p27 stability, and Src overexpression accelerates p27 proteolysis.
The relationship between p27 and induction of apoptosis is still unclear. Katayose et al[2] and Naruse et al[19] have suggested that the growth-inhibitory effect and apoptosis induction by overexpression of p27 requires expression of pRb. The pRb-regulated checkpoint in G1 phase is an important apoptotic checkpoint. CyclinE-CDK2 is the primary complex that phosphorylates pRb, which prevents interactions of it with the E2F transcription factor. To further understand the mechanism of ad-p27mt induced apoptosis in SW480 cells, we examined the expression of apoptosis-related genes Bax and Bcl-2 in each transductant. Results showed that ad-p27mt resulted in a marked increase in protein expression of Bax, a pro-apoptotic factor, and a marked decrease in protein expression of Bcl-2, an anti-apoptotic factor that binds to Bax and antagonizes its function. These results suggest that ad-p27mt-induced apoptosis in SW480 cells involves in induction of Bax.
Cell migration is an essential process involved in tumor invasion and metastasis. In this study, we tested the ability of cell migration in each transductant. We found that ad-p27mt resulted in strong migration inhibition. Supriatno et al[11] have reported a similar result in oral cancer cell line. However, its mechanisms remain unclear and need further investigations. We speculate that it may be directly associated with decreased cell proliferation or/and alterations of structural proteins.
In conclusion, ad-p27mt shows a strong anti-tumor bioactivity in a colorectal cancer cell line SW480 and has the potential to develop into new therapeutic agents for colorectal cancer.
The incidence of colorectal cancer is increasing all over the world. However it is short of effective therapeutic approach. Gene therapy to restore p27 expressing has been promising, furthermore, a mutant type p27 gene, with mutant of Thr-187/Pro-188 to Met-187/Ile-188, can inhibit degradation of p27 protein by ubiquitin-mediated pathway.
p27, as a cyclin-dependent kinases inhibitor, tumor suppressor gene, and promoter of apoptosis, has been widely investigated. Furthermore, antitumor activity of p27 has been demonstrated in breast, lung, and oral cancer. But the antitumor bioactivity of p27 mt has not been studied on colorectal cancer.
The study indicates that ad-p27mt has a strong anti-tumor bioactivity in a colorectal cancer cell line SW480.
This will develop into new therapeutic agents for colorectal cancer.
This is an interesting manuscript on the antitumor activity of the adenovirus mediated mutant p27kip gene in a colorectal cancer (CRC) cell line. Major finding of the study was that the tumor cell growth was inhibited both in vitro and in vivo by the gene transfer.
Peer reviewer: Peter L Lakatos, MD, PhD, Assistant Professor, 1st Department of Medicine, Semmelweis University, Koranyi S 2A, Budapest H1083, Hungary
S- Editor Zhong XY L- Editor Ma JY E- Editor Lin YP
| 1. | St Croix B, Florenes VA, Rak JW, Flanagan M, Bhattacharya N, Slingerland JM, Kerbel RS. Impact of the cyclin-dependent kinase inhibitor p27Kip1 on resistance of tumor cells to anticancer agents. Nat Med. 1996;2:1204-1210. |
| 2. | Katayose Y, Kim M, Rakkar AN, Li Z, Cowan KH, Seth P. Promoting apoptosis: a novel activity associated with the cyclin-dependent kinase inhibitor p27. Cancer Res. 1997;57:5441-5445. |
| 3. | Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59-66. |
| 4. | Ophascharoensuk V, Fero ML, Hughes J, Roberts JM, Shankland SJ. The cyclin-dependent kinase inhibitor p27Kip1 safeguards against inflammatory injury. Nat Med. 1998;4:575-580. |
| 5. | Durand B, Gao FB, Raff M. Accumulation of the cyclin-dependent kinase inhibitor p27/Kip1 and the timing of oligodendrocyte differentiation. EMBO J. 1997;16:306-317. |
| 6. | Polyak K, Kato JY, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9-22. |
| 7. | Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997;3:227-230. |
| 8. | Esposito V, Baldi A, De Luca A, Groger AM, Loda M, Giordano GG, Caputi M, Baldi F, Pagano M, Giordano A. Prognostic role of the cyclin-dependent kinase inhibitor p27 in non-small cell lung cancer. Cancer Res. 1997;57:3381-3385. |
| 9. | Mori M, Mimori K, Shiraishi T, Tanaka S, Ueo H, Sugimachi K, Akiyoshi T. p27 expression and gastric carcinoma. Nat Med. 1997;3:593. |
| 10. | Mineta H, Miura K, Suzuki I, Takebayashi S, Amano H, Araki K, Harada H, Ichimura K, Wennerberg JP, Dictor MR. Low p27 expression correlates with poor prognosis for patients with oral tongue squamous cell carcinoma. Cancer. 1999;85:1011-1017. |
| 11. | Supriatno , Harada K, Kawaguchi S, Onoue T, Yoshida H, Sato M. Characteristics of antitumor activity of mutant type p27Kip1 gene in an oral cancer cell line. Oral Oncol. 2004;40:679-687. |
| 12. | Moore HG, Shia J, Klimstra DS, Ruo L, Mazumdar M, Schwartz GK, Minsky BD, Saltz L, Guillem JG. Expression of p27 in residual rectal cancer after preoperative chemoradiation predicts long-term outcome. Ann Surg Oncol. 2004;11:955-961. |
| 13. | Lin LC, Lee HH, Hwang WS, Li CF, Huang CT, Que J, Lin KL, Lin FC, Lu CL. p53 and p27 as predictors of clinical outcome for rectal-cancer patients receiving neoadjuvant therapy. Surg Oncol. 2006;15:211-216. |
| 14. | Loda M, Cukor B, Tam SW, Lavin P, Fiorentino M, Draetta GF, Jessup JM, Pagano M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat Med. 1997;3:231-234. |
| 15. | Kawamata N, Morosetti R, Miller CW, Park D, Spirin KS, Nakamaki T, Takeuchi S, Hatta Y, Simpson J, Wilcyznski S. Molecular analysis of the cyclin-dependent kinase inhibitor gene p27/Kip1 in human malignancies. Cancer Res. 1995;55:2266-2269. |
| 16. | Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181-1189. |
| 17. | Vlach J, Hennecke S, Amati B. Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J. 1997;16:5334-5344. |
| 18. | Katner AL, Hoang QB, Gootam P, Jaruga E, Ma Q, Gnarra J, Rayford W. Induction of cell cycle arrest and apoptosis in human prostate carcinoma cells by a recombinant adenovirus expressing p27(Kip1). Prostate. 2002;53:77-87. |
| 19. | Naruse I, Hoshino H, Dobashi K, Minato K, Saito R, Mori M. Over-expression of p27kip1 induces growth arrest and apoptosis mediated by changes of pRb expression in lung cancer cell lines. Int J Cancer. 2000;88:377-383. |
| 20. | Ishii T, Fujishiro M, Masuda M, Goshima Y, Kitamura H, Teramoto S, Matsuse T. Effects of p27Kip1 on cell cycle status and viability in A549 lung adenocarcinoma cells. Eur Respir J. 2004;23:665-670. |
| 21. | Wang X, Gorospe M, Huang Y, Holbrook NJ. p27Kip1 overexpression causes apoptotic death of mammalian cells. Oncogene. 1997;15:2991-2997. |
| 22. | Craig C, Wersto R, Kim M, Ohri E, Li Z, Katayose D, Lee SJ, Trepel J, Cowan K, Seth P. A recombinant adenovirus expressing p27Kip1 induces cell cycle arrest and loss of cyclin-Cdk activity in human breast cancer cells. Oncogene. 1997;14:2283-2289. |
| 23. | Schreiber M, Muller WJ, Singh G, Graham FL. Comparison of the effectiveness of adenovirus vectors expressing cyclin kinase inhibitors p16INK4A, p18INK4C, p19INK4D, p21(WAF1/CIP1) and p27KIP1 in inducing cell cycle arrest, apoptosis and inhibition of tumorigenicity. Oncogene. 1999;18:1663-1676. |
| 24. | Park KH, Seol JY, Kim TY, Yoo CG, Kim YW, Han SK, Shim YS, Lee CT. An adenovirus expressing mutant p27 showed more potent antitumor effects than adenovirus-p27 wild type. Cancer Res. 2001;61:6163-6169. |
| 25. | Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193-199. |
| 26. | Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682-685. |
| 27. | Zhang Q, Tian L, Mansouri A, Korapati AL, Johnson TJ, Claret FX. Inducible expression of a degradation-resistant form of p27Kip1 causes growth arrest and apoptosis in breast cancer cells. FEBS Lett. 2005;579:3932-3940. |
| 28. | Sheaff RJ, Groudine M, Gordon M, Roberts JM, Clurman BE. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 1997;11:1464-1478. |
| 29. | Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207-214. |
| 30. | Hara T, Kamura T, Nakayama K, Oshikawa K, Hatakeyama S, Nakayama K. Degradation of p27(Kip1) at the G(0)-G(1) transition mediated by a Skp2-independent ubiquitination pathway. J Biol Chem. 2001;276:48937-48943. |
| 31. | Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S, Yoshida M, Nakayama K, Nakayama KI. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol. 2004;6:1229-1235. |
| 32. | Shapira M, Ben-Izhak O, Linn S, Futerman B, Minkov I, Hershko DD. The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer. 2005;103:1336-1346. |
| 33. | Tomoda K, Kubota Y, Kato J. Degradation of the cyclin-dependent-kinase inhibitor p27Kip1 is instigated by Jab1. Nature. 1999;398:160-165. |
| 34. | Rakkar AN, Li Z, Katayose Y, Kim M, Cowan KH, Seth P. Adenoviral expression of the cyclin-dependent kinase inhibitor p27Kip1: a strategy for breast cancer gene therapy. J Natl Cancer Inst. 1998;90:1836-1838. |
| 35. | Boehm M, Yoshimoto T, Crook MF, Nallamshetty S, True A, Nabel GJ, Nabel EG. A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J. 2002;21:3390-3401. |
| 36. | Ishida N, Hara T, Kamura T, Yoshida M, Nakayama K, Nakayama KI. Phosphorylation of p27Kip1 on serine 10 is required for its binding to CRM1 and nuclear export. J Biol Chem. 2002;277:14355-14358. |
| 37. | Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153-1160. |
| 38. | Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145-1152. |
| 39. | Ogino S, Kawasaki T, Ogawa A, Kirkner GJ, Loda M, Fuchs CS. Cytoplasmic localization of p27 (cyclin-dependent kinase inhibitor 1B/KIP1) in colorectal cancer: inverse correlations with nuclear p27 loss, microsatellite instability, and CpG island methylator phenotype. Hum Pathol. 2007;38:585-592. |
| 40. | Fujita N, Sato S, Tsuruo T. Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic localization. J Biol Chem. 2003;278:49254-49260. |