Published online Oct 14, 2008. doi: 10.3748/wjg.14.5797
Revised: September 23, 2008
Accepted: September 30, 2008
Published online: October 14, 2008
AIM: To elucidate the distribution of CD4+CD25+ regulatory T cells (Tregs) in different lymphoid tissues and its local enhancement on tumor growth before and after depletion of CD4+CD25+ Tregs.
METHODS: Female ICR mice were gavaged with benzo[a]pyrene (BaP) to induce forestomach carcinoma. CD4+CD25+ Tregs were intraperitoneally depleted with monoclonal antibody PC61. These mice were divided into BaP-only, BaP + IgG, BaP + PC61, and control groups. The forestomach of mice was dissected for histological analysis, and tunnel test was performed for apoptosis of tumor cells. CD4+CD25+ Tregs were sorted from different lymphoid tissues and expression of Foxp3, IL-10, and chemokine receptors was analyzed by flow cytometry, semi-quantitative and real-time polymerase chain reaction.
RESULTS: The mice gavaged with only BaP showed increased forestomach papilloma and carcinoma at wk 16 and 32. The proportion of CD4+CD25+ Tregs was significantly higher in peri-stomach regional lymph nodes than in other lymphoid tissues. These CD4+CD25+ Tregs in regional lymph nodes expressed higher levels of Foxp3 and IL-10, enriched in the CD62L-subset, and CCR1 and CCR5 chemokine receptors. In mice gavaged with BaP + PC61, the number of tumor nodules and tumor volume decreased significantly with massive infiltrating cells and apoptosis of tumor cells. In the draining regional lymph nodes, the number of CD4+CD25+ Tregs also decreased significantly.
CONCLUSION: Inducible and activated CD4+CD25+ Tregs in the draining regional lymph nodes suppress host local immunity during tumor growth. Depletion of CD4+CD25+ Tregs can promote host local immunity to suppress tumor growth.
- Citation: Chen YL, Fang JH, Lai MD, Shan YS. Depletion of CD4+CD25+ regulatory T cells can promote local immunity to suppress tumor growth in benzo[a]pyrene-induced forestomach carcinoma. World J Gastroenterol 2008; 14(38): 5797-5809
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5797.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5797
| Group | Body wt. (g)/mouse | Stomach wt. (g)/mouse | % of mice with tumors | Tumors/mouse | Tumor vol. (mm3)/tumor | Total tumor vol. (mm3)/mouse |
| 16 wk | ||||||
| Control | 34.7 ± 3.1 | 0.30 ± 0.02 | 0 (0/3) | 0 | 0 | 0 |
| BaP-only | 33.5 ± 2.7 | 0.34 ± 0.02 | 100 (3/3) | 5.3 ± 1.2 | 2.8 ± 0.5 | 15.0 ± 5.0 |
| BaP + IgG | 35.0 ± 2.9 | 0.32 ± 0.04 | 100 (6/6) | 6.2 ± 1.3 | 2.5 ± 0.8 | 15.2 ± 4.2 |
| BaP + PC61 | 33.5 ± 2.7 | 0.30 ± 0.03 | 100 (3/3) | 3.7 ± 1.5a | 0.8 ± 0.5b | 3.1 ± 2.4b |
| BaP + PC61/5 wk1 | 32.3 ± 8.2 | 0.31 ± 0.05 | 100 (4/4) | 4.3 ± 1.5 | 1.0 ± 0.5b | 4.6 ± 2.8b |
| 24 wk | ||||||
| Control | 36.0 ± 3.3 | 0.33 ± 0.01 | 0 (0/5) | 0 | 0 | 0 |
| BaP + IgG | 35.6 ± 2.9 | 0.44 ± 0.08 | 100 (5/5) | ND | ND | ND |
| BaP + PC61 | 36.4 ± 4.1 | 0.32 ± 0.02b | 100 (5/5) | ND | ND | ND |
| 32 wk | ||||||
| Control | 36.0 ± 4.5 | 0.35 ± 0.03 | 0 (0/3) | 0 | 0 | 0 |
| BaP-only | 36.7 ± 2.1 | ND | 100 (3/3) | ND | ND | ND |
| BaP + IgG | 36.0 ± 2.5 | 0.53 ± 0.12 | 100 (4/4) | 15.5 ± 4.2 | 21.1 ± 16.4 | 276.8 ± 134.5 |
| BaP + PC61 | 39.7 ± 8.5 | 0.44 ± 0.03 | 100 (4/4) | 8.3 ± 2.2a | 6.8 ± 3.2 | 57.1 ± 33.6a |
| Time | BaP+IgG | BaP+PC61 | |
| Infiltrating cells | 16 wk | -/+ | + |
| 32 wk | -/+ | +++ | |
| Apoptotic cells | 16 wk | - | + |
| 32 wk | + | +++ | |
| Apoptosis occurred in tumor nodules | 16 wk | - | + |
| 32 wk | - | +++ |
CD4+CD25+ regulatory T cells (Tregs) constitute 5%-10% of peripheral CD4+ T cells in humans[1]. The immune regulatory function of these CD4+CD25+ Tregs has been attributed to their ability to secrete immuno-suppressive cytokines, including IL-10 and TGF-β1[2]. Although beneficial to protecting the host from autoimmune disease, CD4+CD25+ Tregs may also dampen anti-tumor response. Evidence from various cancers demonstrates that the proportion of CD4+CD25+ Tregs increases in tumor-infiltrating lymphocytes (TILs) and peripheral circulation of gastric cancers[3-5], and in the peripheral blood of patients with ovarian and lung cancers, hepatocellular carcinoma, and pancreas/breast adenocarcinoma[6-10]. Local accumulation of CD4+CD25+ Tregs in tumors is associated with a high death rate and reduced survival of ovarian carcinoma patients[9]. These reports suggest that CD4+CD25+ Tregs participate in cancer immunopathogenesis and are responsible for impairment of host immune surveillance to cancer. Viguier et al[11] reported that accumulation of CD4+CD25+ Tregs is higher in metastatic melanoma lymph nodes of humans and further demonstrated that these cells inhibit proliferation and cytokine production of infiltrating CD4+CD25- and CD8+ T cells in vitro. In contrast, Curiel et al[9] reported that CD4+CD25+ Tregs accumulate in ovarian tumors and malignant ascites but not in draining lymph nodes in later cancer stages. Thus, it is necessary to elucidate the potentially differential distributions and effects of CD4+CD25+ Tregs in local lymphoid tissues during cancer progression.
Preserved, smoked, and salted foods are highly associated with the development of gastric cancer. Polycyclic aromatic hydrocarbons (PAHs) are carcinogens that have been detected in cigarette smoke and in broiled and smoked foods[12]. Among PAHs, benzo[a]pyrene (BaP) is the most extensively studied compound and induces many carcinogen-specific effects[13]. Gavage-delivered BaP induces forestomach tumor in mice, providing a commonly used model for studying the chemopreventive effect of substances on gastric cancer[14-17]. Depletion of CD4+CD25+ Tregs has been shown to induce immune responses to a variety of different tumors in mice, including myeloma, leukemia, melanoma, and fibrosarcoma[18-20]. Lymphoid metastasis is also the determinative for gastric cancer. In this study, to understand the immunosurveillance effect of CD4+CD25+ Tregs in local lymphoid tissues during cancer progression, we used the BaP-induced forestomach tumor model to evaluate the differential effect of CD4+CD25+ Tregs by intervention at the initiation, promotion, or progression stages of multistage carcinogenesis. We first used tumor progression of BaP-induced autochthonous forestomach tumors in female mice to analyze the distribution and characteristics of CD4+CD25+ Tregs in different lymphoid tissues over time. Second, we used a CD4+CD25+ Treg-specific antibody to determine whether CD4+CD25+ Tregs are required for controlling tumor formation.
Benzo[a]pyrene and glycerol gelatin were purchased from Sigma-Aldrich (St. Louis, MO). An aminoethyl carbazole substrate kit was purchased from Zymed (San Francisco, CA). The following antibodies (Abs) were purchased from BD PharMingen (San Diego, CA) and used in this study: mouse anti-CD4 PE (H129.19), anti-CD8a PE (53-6.7), anti-CD3e PE (145-2C11), anti-CD25 FITC (7D4), anti-CD62L FITC (MEL-14), and anti-CD62L PE (MEL-14). FITC-anti-Foxp3 (FJK-16s) mAb and FITC rat IgG2a isotype control Ab were purchased from eBioscience.
BaP-induced forestomach tumors were generated in female mice, as previously reported[21]. Eight-week-old ICR mice were purchased from the Laboratory Animal Center of National Cheng Kung University. All animal experiments were carried out with the approval of the ethical committee of our institution. BaP was dissolved in corn oil. Corn oil control mice (n = 14) and BaP- only treated mice (n = 9) received 200 μL of corn oil with or without 3 mg of BaP, respectively, by po gavage twice weekly for 4 wk. The mice were then sacrificed at week 7 (n = 3), 16 (n = 3), and 32 (n = 3) after the first administration of BaP.
Buffered formalin-phosphate (10%) was immediately injected into the stomach by oral intubation to distend and fix the stomach. Each stomach was removed and placed on a plastic sheet. The tumor incidence, number, and size in the forestomach were measured under a dissection microscope. Tumor volume was calculated using the following formula: Volume = 4πR3/3[22]. Stomach samples were excised and immersed in a buffered 10% formalin solution for paraffin block preparation. Four-micron sections were dehydrated, embedded, and stained with hematoxylin and eosin.
CD25+ T cell-specific antibody, hybridoma PC61, was a gift from the Laboratory of Dr. Lai, MD. To deplete the CD25+ T cells, BaP-treated mice (n = 19) were injected intraperitoneally with 200 μL PC61 mAb fluid at a concentration of 3 mg protein per mL[23], twice a week beginning 5 wk after the initial BaP treatment until the time of sacrifice. The mice in the BaP + PC61 group were then sacrificed at weeks 7 (n = 3), 16 (n = 7), 24 (n = 5), and 32 (n = 4) respectively, after the first administration of BaP. BaP-treated mice were injected with purified rat IgG antibody as a control (ICN, Pharmaceuticals Inc. Cappel, OH, USA). The mice in the BaP + IgG group were then sacrificed at weeks 7 (n = 4), 16 (n = 6), 24 (n = 5), and 32 (n = 4) respectively, after the first administration of BaP.
To confirm the effectiveness of the depletion, splenocytes, thymocytes, or lymphocytes from the peripheral lymph nodes were harvested on the third and seventh days every week after BaP treatment and incubated with anti-CD4 PE, anti-CD8 PE, and anti-CD25-FITC respectively, at 4°C for 40 min in the dark. The mixture was washed twice with ice-cold HBSS, and then re-suspended in HBSS containing 2% FCS/0.1% NaN3. Stained splenocytes, thymocytes, or lymphocytes were analyzed by flow cytometry (FACSCalibur; BD Biosciences, San Jose, CA).
Lymph nodes were collected from the mice at weeks 7 (n = 10), 16 (n = 12), and 32 (n = 11) respectively, after the initial BaP administration. Lymph nodes around the stomach and in the mesentery were collected and defined as regional lymph nodes (RLNs). Lymph nodes in the axillary, inguinal, brachial, and popliteal areas were collected and defined as PLNs. Cell suspensions from the lymph nodes, spleen, or thymus were isolated by flow sorting after sterile dissociation, filtration, and washing. Cells were stained using PE-conjugated anti-CD4 and FITC-conjugated anti-CD25 and then purified on a FACSAria-sorting flow cytometer (BD Biosciences, San Jose, CA). The purity of these CD4+CD25+ and CD4+CD25- T cells was > 90%. To determine the expression of cell surface markers in T cells, the cells were incubated with antibodies appropriately diluted for staining and further stained with anti-Foxp3 (Foxp3 regulates Treg development and function) or isotype control Ab according to the manufacturer’s protocol. Stained lymphocytes were analyzed by flow cytometry.
Cytotoxic T-cells are able to directly induce apoptosis in cells, which is the one of the host immune surveillances. To identify a host anti-tumor response after depletion of CD4+CD25+ Tregs, apoptotic cells in tumor nodules were detected by TUNEL labeling detection of free 3′-OH groups in fragmented DNA in situ (ApopTag® peroxidase in situ apoptosis detection kit, Chemicon). Paraffin-embedded, slide-mounted tissue sections were deparaffinized and treated with proteinase K for 15 min followed by 3% H2O2 for 5 min at room temperature. After nick-end labeling with digoxigenin-deoxyuridine triphosphate by terminal deoxynucleotidyl transferase, immunostaining was performed using peroxidase-conjugated anti-digoxigenin Ab. Apoptotic cells were visualized with diaminobenzidine substrate, becoming a dark-brown color. Specimens were then counterstained with hematoxylin.
We used PCR to assess expression levels of several CD4+CD25+ Treg-related genes. Total RNA was extracted from 5 × 105 sorted cells and purified using the RNeasy kit according to the manufacturer’s instructions (Qiagen, Valencia, CA) and converted to cDNA by moloney murine leukemia virus (M-MLV) reverse transcriptase with oligo(dT) primer in the presence of RNAsin (Promega, Madison, WI). Semi-quantitative PCR for Foxp3, TGF-β1, IL-10, CCR1, CCR5, CCR6, CCR7, and β-actin was performed as described previously[24-28]. The cDNA generated was subjected to 30-35 cycles of PCR amplification on a DNA thermal cycler (Perkin Elmer, GeneAmp PCR System). PCR was performed with specific primers for CCR4 and CCR8: CCR4 (forward, AGAGGCTCAAGTCCATGACG; reverse, AGTGTTGCAGAGTCCTAATG), and CCR8 (forward, CGGCATGCTACAATGTTTCC; reverse, TCTCTCCTATGAACGCGTAG). The β-actin served as a quantitative control for PCR. PCR products were fractionated by agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light.
Real-time PCR was performed on a LightCycler detection system (Roche Applied Science). Analyses were performed using primers, an internal fluorescent TaqMan probe specific to Foxp3 or HPRT, and the LightCycler TaqMan Master kit (Roche Applied Science, Penzberg, Germany). The primer and TaqMan probe sequences used as previously described[26] are as follows: Foxp3 primers (5'-CCCAGGAAAGACAGCAACCTT-3' and 5'-TTCTCACAACCAGGCCACTTG-3'), Foxp3 probe (5'-FAM-ATCCTACCCACTGCTGGCAAATGG AGTC-3'), HPRT primers (5'-TGAAGAGCTACTGTA ATGATCAGTCAAC-3' and 5'-AGCAAGCTTGCA ACCTTAACCA-3'), HPRT probe (5'-FAM-TGCTTT CCCTGGTTAAGCAGTACAGCCC-3'). Standard curves of cDNAs from ICR mice CD4+CD25+ T cells were used as previously described[27]. The normalized values for Foxp3 mRNA were calculated as the relative quantity of Foxp3 mRNA levels divided by the relative quantity of HPRT mRNA levels. All samples were run in triplicate.
Results are expressed as mean ± SE. Because of time constraints involving the large number of antibodies and RNA analyses in this study, values were compared using the Mann-Whitney test for independent experiments. P≤ 0.05 was considered statistically significant.
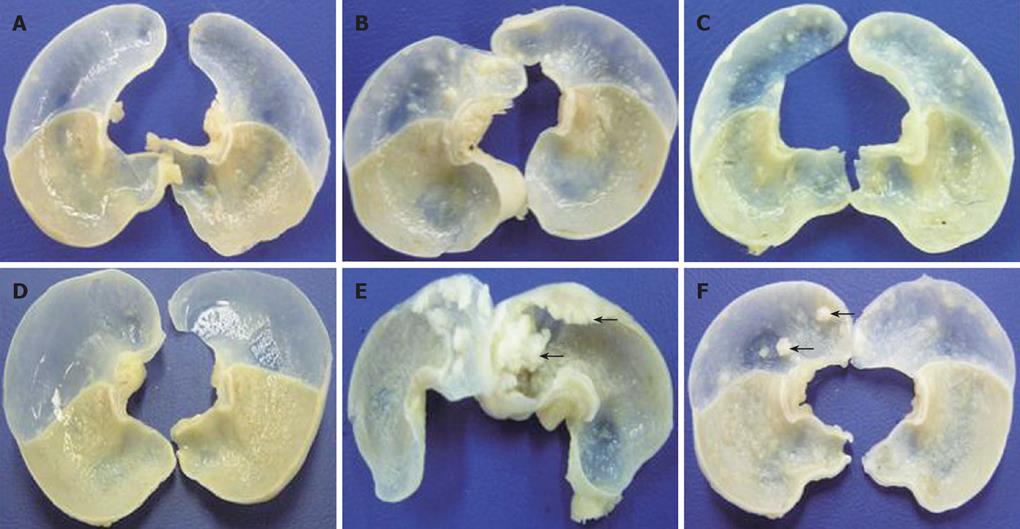
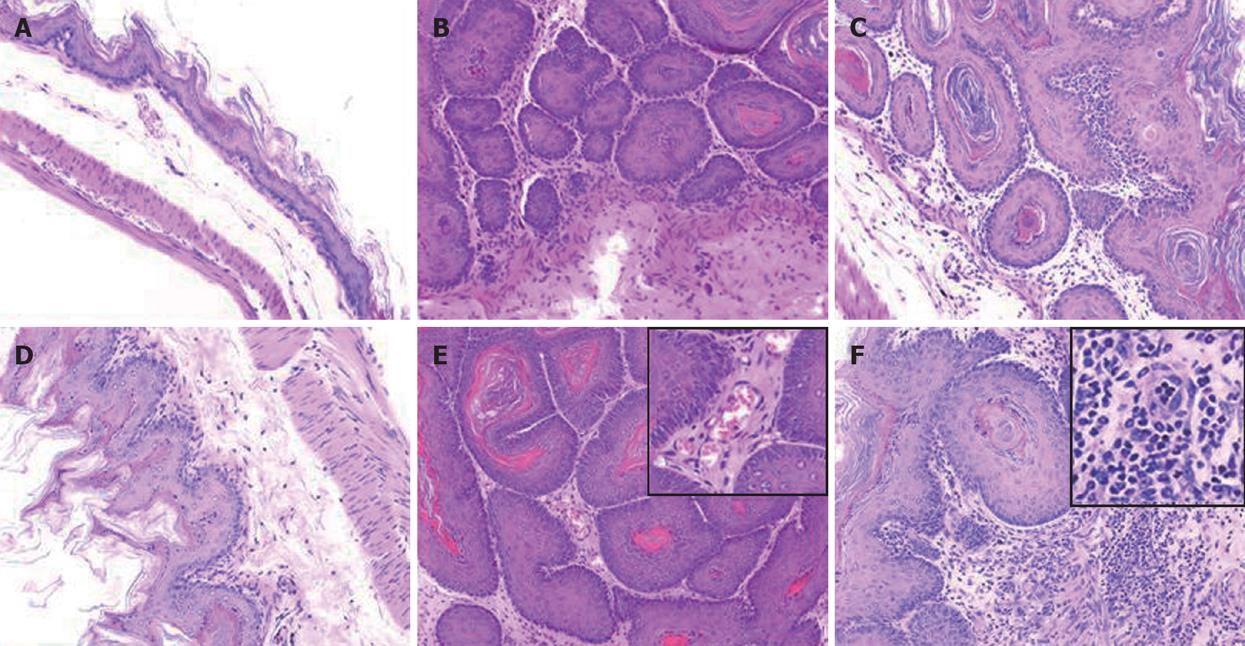
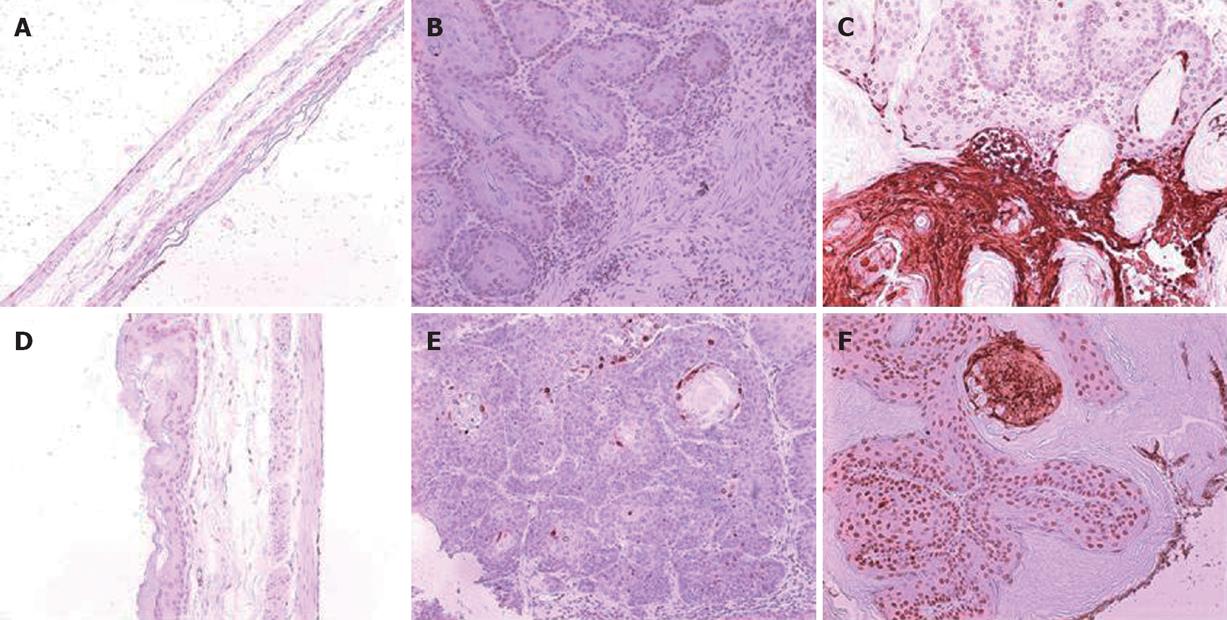
A total 65 mice used in this study were divided into corn oil control (n = 14), BaP-only (n = 9), BaP + IgG (n = 19), and BaP + PC61 (n = 19) groups. To prove the effect of a short course treatment with PC61, 4 mice in the BaP + PC61 only group received 5 wk of PC61 injection before sacrifice. The tumor incidence was 100% in the forestomach of BaP-treated mice at weeks 16, 24, and 32, respectively. There was no difference in tumor incidence, growth and pathological alternation between the BaP-only and BaP + IgG-treated mice. Formation of some small rash-like tumor nodules and more significant tumor nodules was observed in the forestomach at weeks 16 and 32, respectively (Figure 1B and E), but no tumor growth was detected in the forestomachs of mice in the corn oil control group (Figure 1A and D). Typical pathological alternations in tumor nodules, including papilloma and squamous cell carcinomas, were observed, but few lymphocytes and granulocytes accumulated in and around the tumor nodes (Figure 2B and E). The number of tumor nodules, both papilloma and squamous cell carcinoma, significantly increased in this period (Table 1).
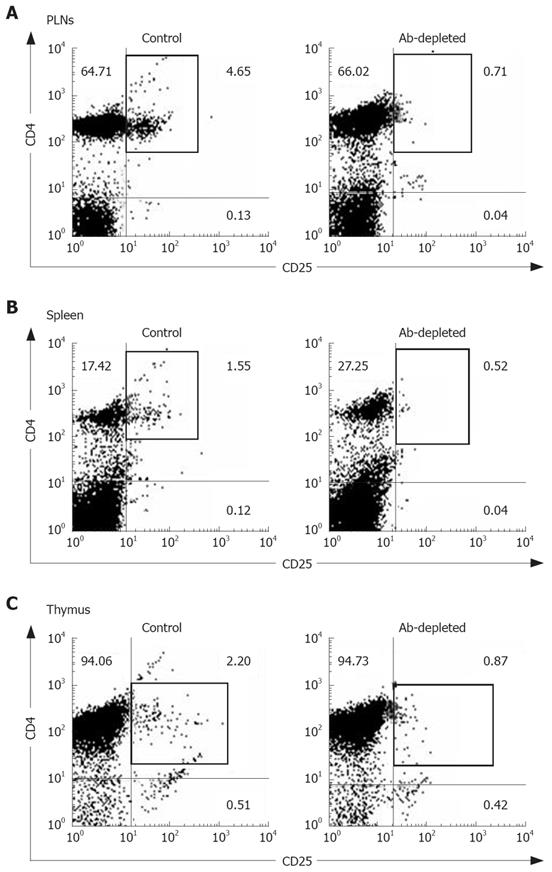
The constituted number of CD4+CD25+ T cells in PLNs, spleen, and thymus of naïve mice after depletion by PC61 was maintained at less than 1% (Figure 3A and C). These naïve mice treated with PC61 mAb did not develop histologically evident autoimmunity. After a short-course treatment with PC61 for 5 wk, the tumor volume decreased significantly by as much as 60% in individual mice of BaP + PC61 group at week 16 (Table 1). The effect of a long-course treatment, until sacrifice, was more significant. The forestomach tumor growth was more progressively reduced (Figure 1C and E), and the tumor volume decreased by as much as 80% per mouse in comparison to the mice in the BaP + IgG group at weeks 16 and 32, respectively (Table 1). A significant increase in infiltration of lymphocytes and granulocytes at the tumor sites in mice of the BaP + PC61 group was also observed upon histological examination (Figure 2C and F).
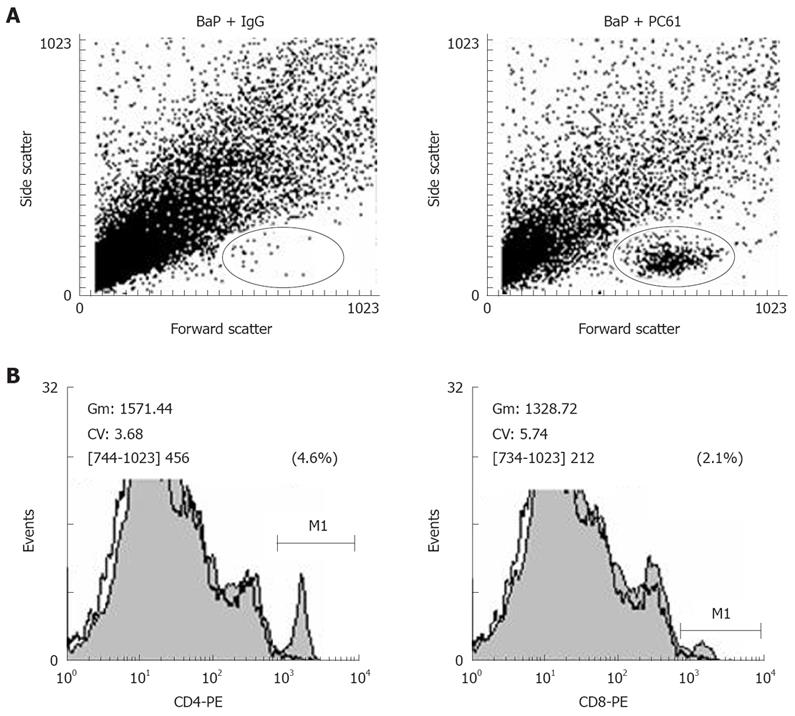
We further investigated whether depletion of CD4+CD25+ Tregs increases host anti-tumor immunity, using apoptosis as a marker. After depletion using PC61, the portion of CD4+CD25+ Tregs in CD4+ and CD8+CD25+ in CD8+ was significantly decreased, the tumor infiltrating lymphocytes (TILs) were significantly increased in the nodules (Figure 4A), and the fraction of CD4+ (3.90% ± 0.99%) and CD8+ (2.25% ± 0.21%) cells in TILs was also observed in mice of the BaP + PC61 group at week 32 (Figure 4B). The tumor size and volume were decreased, and massive apoptotic cells were observed in the forestomach tumors of mice in the BaP + PC61 group (Figure 5C and F), but rare apoptotic cells were found in the tumor mass of mice in the BaP + IgG group at weeks 16 and 32 (Figure 5B and E). The effect of depletion of CD4+ CD25+ Tregs is summarized in Table 2.
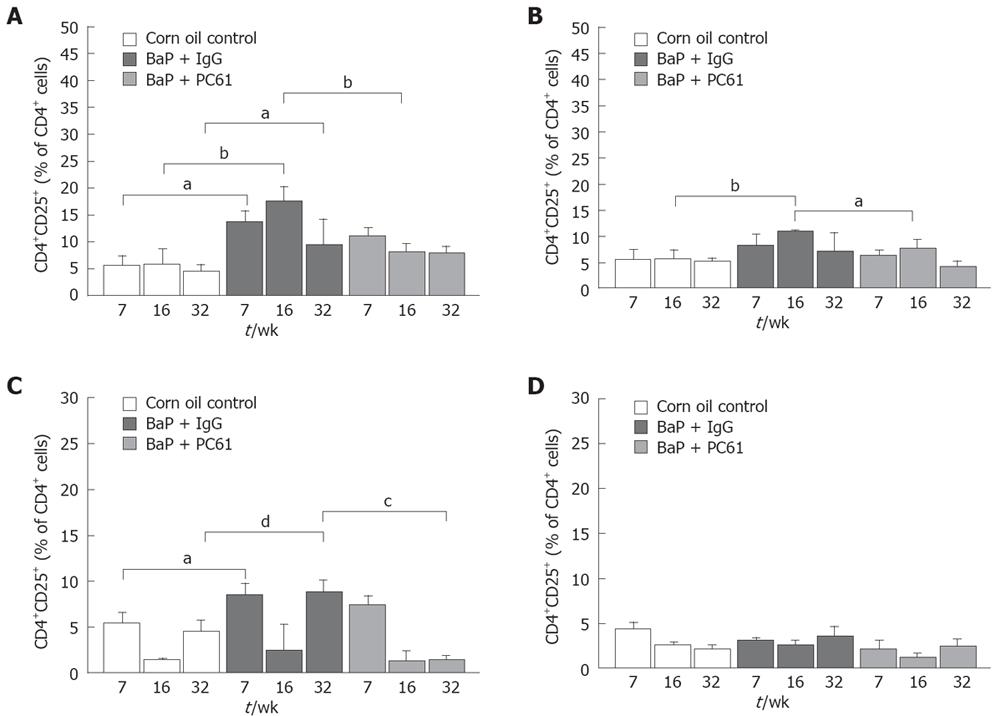
To elucidate whether CD4+CD25+ Tregs are responsible for local immune surveillance during tumor growth, the population of CD4+CD25+ Tregs in different lymphoid organs was analyzed. The RLNs in mice of the BaP-only group had a significantly higher proportion of CD4+CD25+ Tregs at weeks 7, 16, and 32, respectively (9.26% ± 1.56%, 9.61% ± 0.52%, 5.09% ± 2.03%) than in mice of the control group (3.72% ± 0.83%, 3.07% ± 1.52%, 2.45% ± 0.70%). The population of total CD4+ Tregs at weeks 7, 16 and 32 (13.51% ± 2.21%, 17.63% ± 2.62%, 9.36% ± 4.75%) was also significantly higher than that in mice of the control group (5.57% ± 1.79%, 5.73% ± 2.95%, 4.51% ± 1.15%) (Figure 6A). The proportion of total CD4+ Tregs (6.42% ± 0.59% and 10.96% ± 0.28%, respectively) in the PLNs of mice of the BaP-only group was significantly higher than that in mice of the control group (3.50% ± 1.11%; 5.52% ± 1.78%, respectively) at week 16 (Figure 6B). In spleen, the proportion of Tregs in total CD4+ T cells was significantly higher at weeks 7 and 32 (Figure 6C). However, a difference in Treg distribution was not observed in thymus (Figure 6D).
The population of CD8+CD4+ Tregs in different lymphoid organs was also analyzed. The RLNs in mice of the BaP-only group had a significantly higher proportion of CD8+CD25+ Tregs at weeks 7, 16 and 32, respectively (1.68% ± 0.28%, 5.24% ± 1.90%, 1.14% ± 0.29%) than those in mice of the control group (1.93% ± 0.34%, 0.65% ± 0.36%, 0.46% ± 0.20%). The population of CD8+CD25+ in total CD8+ T cells at weeks 7, 16, and 32 (9.36% ± 0.52%, 18.54% ± 4.48%, 6.74% ± 2.24%) was also significantly higher in mice of the BaP-only group than in mice of the control group (11.98%± 0.13%, 4.49% ± 1.91%, 2.82% ± 1.32%). The proportion of CD8+CD25+ and CD8+CD25+ in total CD8+ T cells in the PLNs of mice of the BaP-only group at weeks 7, 16, and 32, respectively, was significantly higher in mice of the BaP-only group than in mice of the control group (data not shown). In spleen, the proportion of CD8+CD25+ and CD8+CD25+ in total CD8+ T cells was significantly higher in mice of the BaP-only group than in mice of the control group at wk 32 (0.75%± 0.14% and 12.99% ± 1.87% versus 0.20%± 0.07% and 3.74% ± 1.40%). However, no difference in CD8+CD25+ distribution was observed in thymus.
The population of CD4+T cells and the pattern of surface expression for CD3 in RLNs, PLNs, spleen, and thymus was consistent among the corn oil control, BaP + IgG, and BaP + PC61 groups at weeks 7, 16 and 32 (data not shown), revealing that the increased proportion of Tregs and CD8+CD25+ in the RLNs of BaP-treated mice may be responsible for local immune surveillance during progression of forestomach tumors.
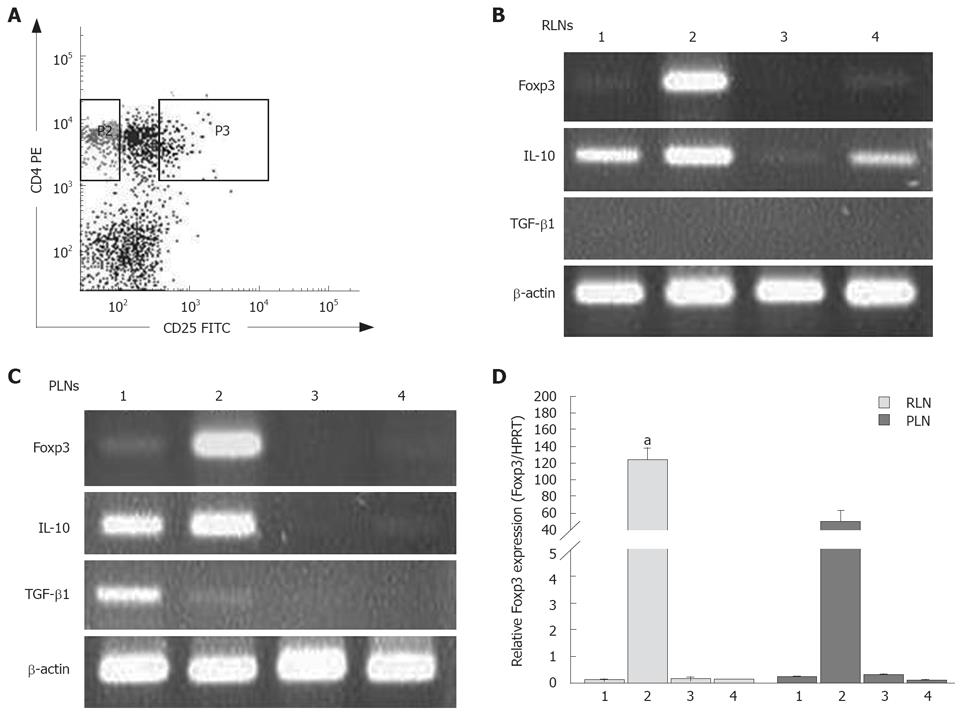
Foxp3 is a crucial transcription factor and the specific marker for inducible CD4+CD25+ Tregs. After enumeration of isolated CD4+CD25+ (combined CD25int and CD25high-expressing cells) and CD4+CD25- T cells within RLNs and PLNs (Figure 7A), the expression of Foxp3 in CD4+CD25+ Tregs and CD4+CD25- t cells in the RLNs and PLNs of mice in the BaP-only and corn oil control groups was examined. As expected, CD4+CD25+ Tregs in the RLNs and PLNs expressed high levels of Foxp3, while CD4+CD25- T cells expressed very low levels in mice of the BaP group (Figure 7B and C). Furthermore, the Foxp3 transcripts of CD4+CD25+ Tregs in RLNs of mice of the BaP group were significantly higher than those in PLNs of mice of the BaP group at wk 32 (Figure 7D).
We further analyzed whether the CD4+CD25+ Tregs or CD4+CD25- T cells in the RLNs and PLNs can produce immunosuppressive cytokines. The two types of T cells in the RLNs and PLNs of the BaP-only treated mice enhanced the expression of IL-10 at transcription levels at wk 32 (Figure 7B and C), but only the CD4+CD25+ Tregs in the RLNs of mice in the control group expressed low-level IL-10 transcripts at wk 32 . The Tregs in the RLNs and PLNs of mice in the BaP-only and control groups produced few or no IL-10 transcripts at weeks 7 and 16 (data not shown). Regarding the TGF-β1 transcripts, the RLNs and PLNs in mice of the BaP-only and control groups were negative at weeks 7, 16, and 32, and only the CD4+CD25- T cells from the PLNs in week of the BaP-only group produced low levels of TGF-β1 transcripts at week 32 (Figure 7B and C).
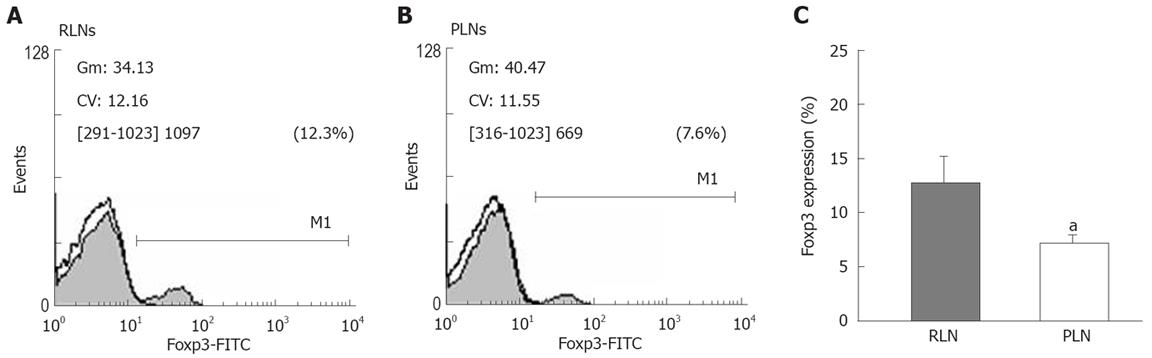
To confirm the difference in the proportion of Tregs between RLN and PLN, we analyzed the expression of Foxp3 by flow cytometry (Figure 8A and B). The expression of Foxp3 in lymphocytes of RLNs was significantly higher than that in lymphocytes of PLNs of BaP-only-treated mice at week 32 (Figure 8C), suggesting that activation of inducible CD4+CD25+ Tregs is associated with tumor formation and that CD4+CD25+ Tregs secrete IL-10 but not TGF-β1, as a mediator for local inhibition of host immune functions during tumor progression.
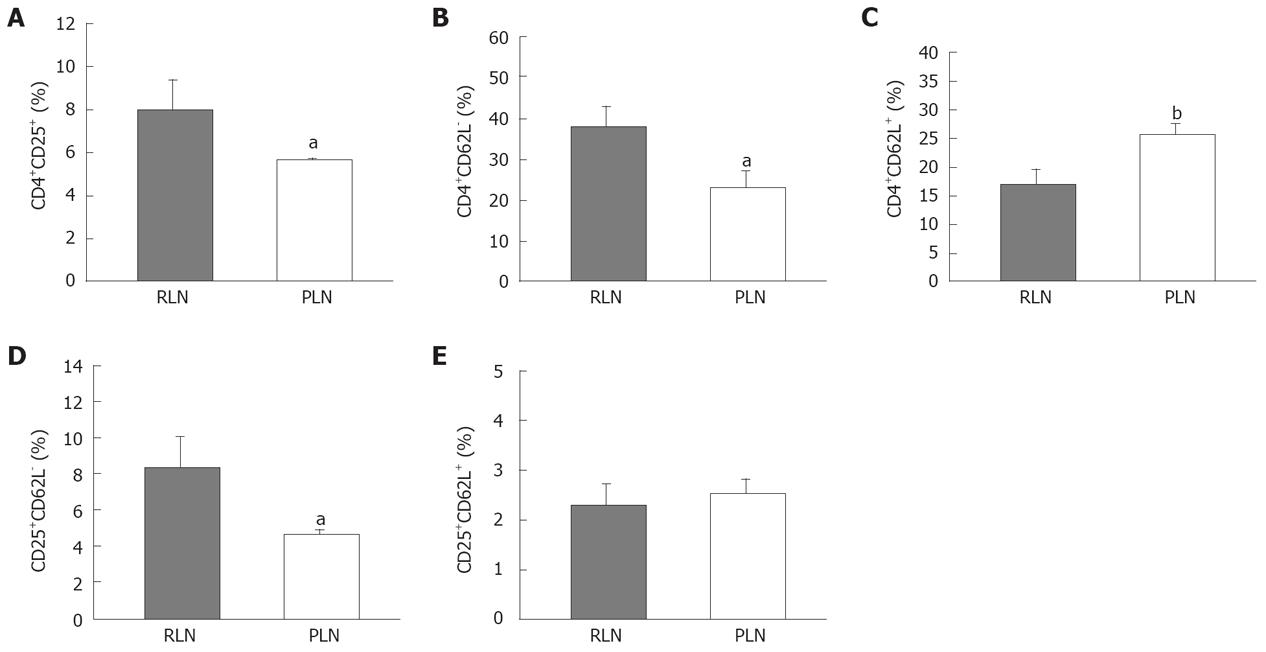
The down-regulation of expression of L-selectin (CD62L) on the cell surface depends on cell activation. Loss of CD62L expression is a useful indicator for T cell maturation or terminal differentiation. The RLNs had a significantly higher proportion of Tregs than the PLNs (Figure 9A). The percentage of CD4+CD62L- cells in RLNs was higher than that in PLNs of mice in the BaP-only group at week 32 (Figure 9B), and the percentage of CD4+ CD62L+ cells in PLNs, belonging to the naïve pool, was higher than that in RLNs (Figure 9C). Furthermore, the percentage of CD25+CD62L- cells in RLNs was significantly higher than that in PLNs, but there was no difference in CD25+CD62L+ between RLNs and PLNs (Figure 9D and E), indicating that more Tregs in the RLNs are at the activation stage.
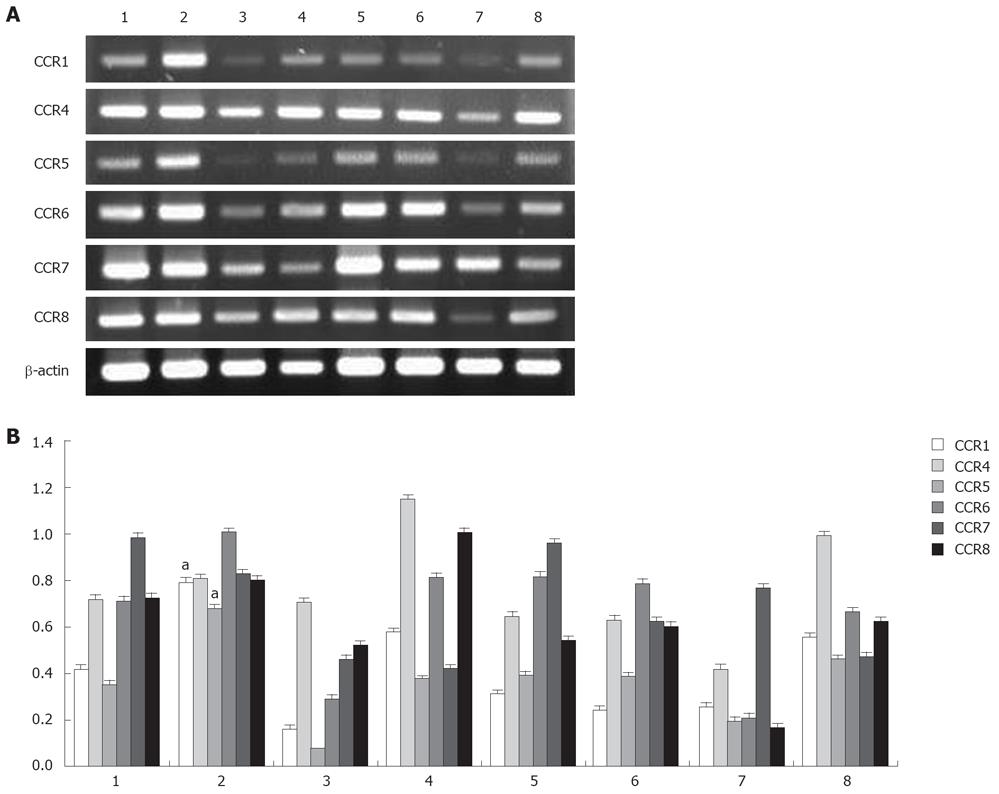
Because chemokine receptors are important for T cell migration to lymph nodes, the expression of these receptors might distinguish the characteristics of CD4+ cell subsets of RLNs and PLNs. To distinguish which factor attracts Tregs migration to RLNs, the expression of CCR1, CCR4, CCR5, CCR6, CCR7, and CCR8 transcripts in purified CD4+CD25+ and CD4+CD25- T cells of RLNs and PLNs was analyzed. According to the results of RT-PCR, CD4+CD25+ T cells of RLNs specifically expressed a higher level of CCR1 and CCR5 mRNA in mice of the BaP-only group at week 32 (Figure 10). Both CD4+CD25+ and CD4+CD25- T cells of RLNs and PLNs in mice of the BaP-only group exhibited a high expression of CCR4, CCR6, CCR7, and CCR8 mRNA. These results suggest that Tregs of RLNs in the forestomach tumors might possess a unique chemotactic profile for migration of Tregs, which is responsible for local immune suppression during tumor progression.
Gastric cancer is one of the most common cancers in the world, and lymph node metastasis is the strongest determinant of patient survival. There are many submucosa-associated lymphoid tissues responsible for immunosurveillance in the gastrointestinal tract. An increase in Tregs has been correlated with immunosuppression and tumor progression in patients with gastrointestinal cancers[4]. So far, there is no evidence that addresses changes in Tregs of lymph nodes at the initial stage of gastric cancer development in human beings or animals. In this study, we clearly demonstrated that the proportion of Tregs increased in the RLNs during tumor progression in BaP-induced mouse forestomach carcinoma. These Tregs significantly displayed enhanced expression levels of Foxp3 mRNA and protein and down-regulation of CD62L, and exhibited a unique chemotactic receptor profile, including high CCR1 and CCR5 mRNA levels, for migration to inflammation sites. These inducible Tregs also expressed high levels of IL-10 for inhibition of host immunity. After depletion of Tregs, the tumor volume decreased, and more infiltrating and apoptotic cells in the tumors were found. Our results indicate that Tregs in RLNs may suppress local host immune response during tumor progression.
Tregs are known to be immunoregulatory, and play an important role in immunological tolerance to self-antigens and in inhibition of T-cell proliferation[2,29-32]. These observations demonstrate that Tregs are involved in immune dysfunction of cancer patients. Sutmuller et al[20] demonstrated that Treg depletion enhances T-cell reactivity to a known tumor-associated antigen. However, depletion of Tregs alone is not sufficient to treat any established cancer. In most of the relevant studies, anti-CD25 mAb was administered to deplete Tregs several days prior to tumor implantation or only 1 day afterwards[19,20,23,33-36]. In this study, although the CD25+ cells in lymph nodes were not completely depleted, forestomach tumor growth was still remarkably reduced, suggesting that increased Tregs in tumor-draining lymph nodes inhibit host local immune response during tumorigenesis. A consecutive course of depleted-antibody injections, for mice without histologically proven autoimmune disease, is sufficient to cause regression of the tumors. It was reported that depletion of CD25+ cells in tumor models could reduce tumor growth as I in our study[37].
Previous studies have shown that Tregs could suppress T cell priming in lymph nodes[30,38,39]. Depletion of Tregs could enhance T cell sensitization in tumor-draining lymph nodes and increase generation of specific immune T cells, and depletion of CD25+ T cells could facilitate infiltration of CD8+ T cells at tumor sites[19,23]. The Tregs are probably the main subset of CD4+ T cells responsible for the observed enhancement of tumor growth[40]. These studies suggest that removal of Tregs can generate tumor-specific CD8 T cells and tumor-nonspecific CD4-CD8- effector cells in vivo. In this study, Tregs were more abundant in RLNs around the forestomach carcinoma. After depletion, the number of Tregs in the RLNs decreased significantly, more granulocytes, CD4+, and CD8+ cells infiltrated into the tumors, and massive apoptotic cells were found in the tumor mass, proving that Tregs in the RLNs are strongly associated with tumor growth, and that depletion of Tregs can promote effective local tumor-specific immune responses.
Adhesion molecules (such as CD62L) and chemokine receptors are involved in lymphocyte trafficking. Tregs can be subdivided into different functional subsets based on the expression of CD62L. Loss of CD62L expression in Tregs could result from selective migration to forestomach cancer and accumulation at the tumor nodules or in draining lymph nodes after stimulation and activation. Iellem et al[40] reported that Tregs specifically express the chemokine receptors CCR4 and CCR8. Bystry et al[41] reported that Tregs express CCR5 and respond to the CCL4 ligand, and CCL4 is the most potent chemokine for Tregs. Chemokine receptor CCR1 and its two ligands, CCL5 and CCL7, or CCR5 and its ligand CCL4 may be involved in regulating the trafficking of Tregs. In this study, the expression profile of adhesion molecule and chemokine receptors differed from that of the RLN and PLN Tregs. There were more activated (CD62L negative) Tregs in RLNs, and these Tregs highly expressed the chemokine receptors CCR1 and CCR5, suggesting that a higher expression of CCR1 and CCR5 in activated Tregs may allow their migration toward RLNs or the tumor area to suppress local host immunity.
The above results highly suggest that, during tumor progression, tumor cells may recruit Tregs via a specific subset of chemokines and their receptors to control local host anti-tumor immunity. It is conceivable that it may be advantageous to use antibodies or fusion proteins to reduce the action of Tregs at the local sites in combination with chemoprevention drugs to gain the maximal benefit during treatment of gastric cancer, even though we did not perform functional inhibition studies of Treg cells on host cytototic cells. Chen et al[42] have induced apoptosis of Tregs in a lymphoma animal model using intratumorally injecting FasL protein to deplete Tregs in the tumor and tumor-draining lymph nodes. Viehl et al[43] have also depleted Tregs by intraperitoneal injection with PC61 three times a week, in combination with tumor vaccine. After depletion, the tumor-specific immune response was significantly increased in tumor-draining lymph nodes. Their results are consistent with our studies.
In conclusion, increased Tregs in draining regional lymph nodes suppress host local immunity during tumor growth and depletion of Tregs can promote host local immunity to prevent tumor progression, implying the significance of lymph node dissection in surgery for gastric cancer.
CD4+CD25+ regulatory T cells (Tregs) are correlated with the prognosis of malignant tumors and can compromise host immunity surveillance to cancer. Accumulation of Tregs in the drained lymph nodes may reduce host local immunity against gastric cancer. The role of Tregs in the drained lymph nodes was elucidated during cancer development was elucidated in this study.
Lymph node metastasis is the key factor for prognosis of gastric cancer patients. Lymph node dissection is the radical gastrectomy for gastric cancer, but it is a highly technique-demanding surgery. Intraperitoneal treatment with monoclonal antibody to deplete Tregs in the drained lymph nodes might assist in increasing the curative effect on gastric cancer.
The high incidence of lymph node metastasis is still the milestone of the prognosis of gastric cancer. Decreased host local immunity surveillance might be the determinative for lymph node metastasis. The study established mouse for stomach carcinoma by garage with benzo[a]pyrene (BaP). Then, the mice received anti-CD25 treatment. The results clearly demonstrate that depletion of Tregs with anti-CD25 antibody could decrease Tregs distributed in drained lymph nodes, increase local immune cell infiltration in tumor and reduce tumor growth. This is the first successful treatment of solid tumor with monoclonal antibody to deplete Tregs in mice, thus laying an experimental foundation of development of drugs for treatment of cancer.
Intraperitoneal injection of anti-CD25 monoclonal antibody is a promising therapeutic approach to forestomach carcinoma. The results implicate that anti-CD25 therapy could be used as a chemoprevention modality for malignan tumors and a local immunity therapy for gastric cancer.
The authors of this paper showed that depletion of Tregs can decrease growth of tumor in an animal model of forestomach carcinoma. The study was well designed and its findings are interesting and informative.
Peer reviewer: Ana J Coito, Associate Professor, Department of Surgery, The Dumont UCLA Transplant Center, 77-120 CHS BOX 90095-7054, Los Angeles 90095, United States
S- Editor Zhong XY L- Editor Wang XL E- Editor Lin YP
| 1. | Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606-612. |
| 2. | Piccirillo CA, Thornton AM. Cornerstone of peripheral tolerance: naturally occurring CD4+CD25+ regulatory T cells. Trends Immunol. 2004;25:374-380. |
| 3. | Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res. 2003;9:4404-4408. |
| 4. | Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer. 2003;98:1089-1099. |
| 5. | Kawaida H, Kono K, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Ooi A, Fujii H. Distribution of CD4+CD25high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res. 2005;124:151-157. |
| 6. | Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766-4772. |
| 7. | Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756-2761. |
| 8. | Woo EY, Yeh H, Chu CS, Schlienger K, Carroll RG, Riley JL, Kaiser LR, June CH. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272-4276. |
| 9. | Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942-949. |
| 10. | Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457-2464. |
| 11. | Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444-1453. |
| 13. | Halliwell B, Gutteridge JM. Free radicals, ageing and disease. Free radicals in biology and medicine, 2nd edition. New York: Oxford University, Claredon Press 1989; 446-493. |
| 14. | Deshpande SS, Ingle AD, Maru GB. Inhibitory effects of curcumin-free aqueous turmeric extract on benzo[a]pyrene-induced forestomach papillomas in mice. Cancer Lett. 1997;118:79-85. |
| 15. | Agha AM, El-Fattah AA, Al-Zuhair HH, Al-Rikabi AC. Chemopreventive effect of Ginkgo biloba extract against benzo(a)pyrene-induced forestomach carcinogenesis in mice: amelioration of doxorubicin cardiotoxicity. J Exp Clin Cancer Res. 2001;20:39-50. |
| 16. | Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA. 2002;99:7610-7615. |
| 17. | Goswami UC, Sharma N. Efficiency of a few retinoids and carotenoids in vivo in controlling benzo[a]pyrene-induced forestomach tumour in female Swiss mice. Br J Nutr. 2005;94:540-543. |
| 18. | Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128-3133. |
| 19. | Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211-5218. |
| 20. | Sutmuller RP, van Duivenvoorde LM, van Elsas A, Schumacher TN, Wildenberg ME, Allison JP, Toes RE, Offringa R, Melief CJ. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823-832. |
| 21. | Wattenberg LW, Lam LK, Fladmoe AV. Inhibition of chemical carcinogen-induced neoplasia by coumarins and alpha-angelicalactone. Cancer Res. 1979;39:1651-1654. |
| 22. | Tanaka H, Tanaka J, Kjaergaard J, Shu S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J Immunother. 2002;25:207-217. |
| 23. | Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693-699. |
| 24. | Chen YL, Wang JY, Chen SH, Yang BC. Granulocytes mediates the Fas-L-associated apoptosis during lung metastasis of melanoma that determines the metastatic behaviour. Br J Cancer. 2002;87:359-365. |
| 25. | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057-1061. |
| 26. | Gough M, Crittenden M, Thanarajasingam U, Sanchez-Perez L, Thompson J, Jevremovic D, Vile R. Gene therapy to manipulate effector T cell trafficking to tumors for immunotherapy. J Immunol. 2005;174:5766-5773. |
| 27. | Matsuura M, Okazaki K, Nishio A, Nakase H, Tamaki H, Uchida K, Nishi T, Asada M, Kawasaki K, Fukui T. Therapeutic effects of rectal administration of basic fibroblast growth factor on experimental murine colitis. Gastroenterology. 2005;128:975-986. |
| 28. | Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816-822. |
| 29. | Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunol Rev. 2001;182:18-32. |
| 30. | Gavin M, Rudensky A. Control of immune homeostasis by naturally arising regulatory CD4+ T cells. Curr Opin Immunol. 2003;15:690-696. |
| 31. | Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81-88. |
| 32. | Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. |
| 33. | Tawara I, Take Y, Uenaka A, Noguchi Y, Nakayama E. Sequential involvement of two distinct CD4+ regulatory T cells during the course of transplantable tumor growth and protection from 3-methylcholanthrene-induced tumorigenesis by CD25-depletion. Jpn J Cancer Res. 2002;93:911-916. |
| 34. | Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diaz de Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931-5939. |
| 35. | Li J, Hu P, Khawli LA, Epstein AL. Complete regression of experimental solid tumors by combination LEC/chTNT-3 immunotherapy and CD25(+) T-cell depletion. Cancer Res. 2003;63:8384-8392. |
| 36. | Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389-400. |
| 37. | Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295-307. |
| 38. | von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol. 2003;3:223-232. |
| 39. | Lin CC, Chou CW, Shiau AL, Tu CF, Ko TM, Chen YL, Yang BC, Tao MH, Lai MD. Therapeutic HER2/Neu DNA vaccine inhibits mouse tumor naturally overexpressing endogenous neu. Mol Ther. 2004;10:290-301. |
| 40. | Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D'Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847-853. |
| 41. | Bystry RS, Aluvihare V, Welch KA, Kallikourdis M, Betz AG. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126-1132. |
| 42. | Chen A, Liu S, Park D, Kang Y, Zheng G. Depleting intratumoral CD4+CD25+ regulatory T cells via FasL protein transfer enhances the therapeutic efficacy of adoptive T cell transfer. Cancer Res. 2007;67:1291-1298. |
| 43. | Viehl CT, Moore TT, Liyanage UK, Frey DM, Ehlers JP, Eberlein TJ, Goedegebuure PS, Linehan DC. Depletion of CD4+CD25+ regulatory T cells promotes a tumor-specific immune response in pancreas cancer-bearing mice. Ann Surg Oncol. 2006;13:1252-1258. |