Published online Sep 14, 2008. doi: 10.3748/wjg.14.5290
Revised: June 20, 2008
Accepted: June 27, 2008
Published online: September 14, 2008
AIM: To assess whether in ulcerative colitis (UC) patients with ileo-rectal anastomosis (IRA), ileal lesions may develop in the neo-terminal-ileum and their possible relation with phenotypic changes towards colonic epithelium.
METHODS: A total of 19 patients with IRA under regular follow up were enrolled, including 11 UC and 8 controls (6 Crohn’s disease, CD; 1 familial adenomatous polyposis, FAP; 1 colon cancer, colon K). Ileal lesions were identified by ileoscopy with biopsies taken from the ileum (involved and uninvolved) and from the rectal stump. Staining included HE and immunohistochemistry using monoclonal antibodies against colonic epithelial protein CEP (Das-1) and human tropomyosin isoform 5, hTM5 (CG3). Possible relation between development of colonic metaplasia and ileal lesions was investigated.
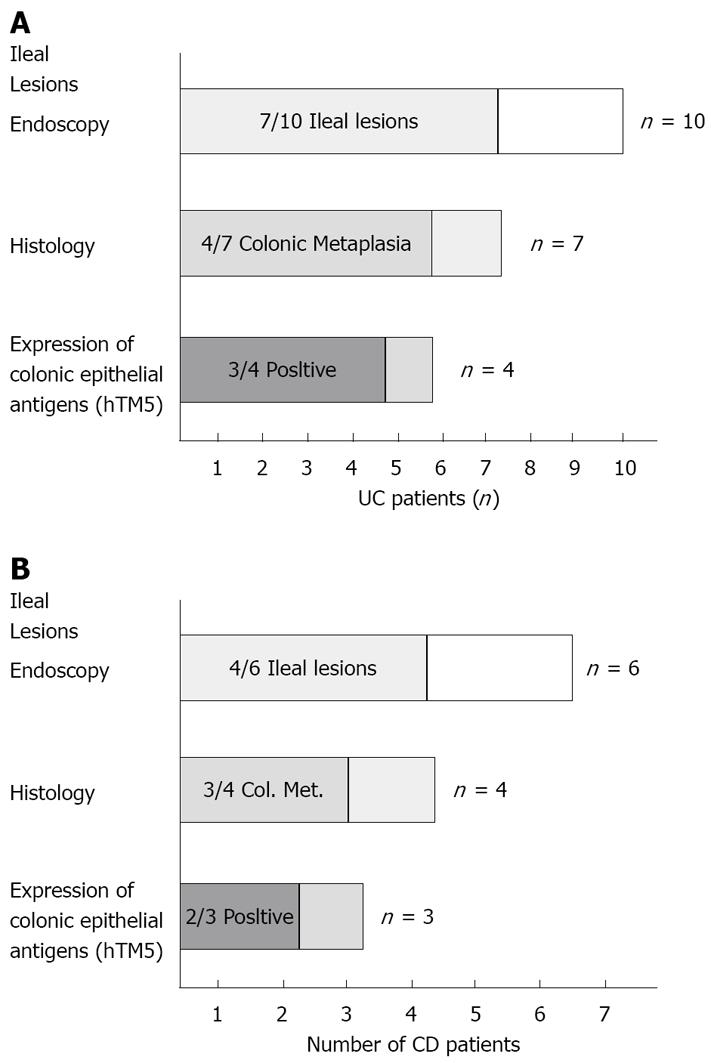
RESULTS: Stenosing adenocarcinoma of the rectal stump was detected in 1 UC patient. The neo-terminal ileum was therefore investigated in 10/11 UC patients. Ileal ulcers were detected in 7/10 UC, associated with colonic metaplasia in 4/7 (57.1%) and Das-1 and CG3 reactivity in 3/4 UC. In controls, recurrence occurred in 4/6 CD, associated with colonic metaplasia in 3/4 and reactivity with Das-1 and CG3 in 2/3.
CONCLUSION: Present findings suggest that in UC, ileal lesions associated with changes towards colonic epithelium may develop also after IRA. Changes of the ileal content after colectomy may contribute to the development of colonic metaplasia, leading to ileal lesions both in the pouch and in the neo-terminal ileum after IRA.
- Citation: Biancone L, Calabrese E, Palmieri G, Petruzziello C, Onali S, Sica GS, Cossignani M, Condino G, Das KM, Pallone F. Ileal lesions in patients with ulcerative colitis after ileo-rectal anastomosis: Relationship with colonic metaplasia. World J Gastroenterol 2008; 14(34): 5290-5300
- URL: https://www.wjgnet.com/1007-9327/full/v14/i34/5290.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5290
| Patient | Sex | Age | Disease duration before surgery (yr) | Indication for surgery |
| UC | ||||
| DLE | M | 50 | 7 | Refractory UC |
| LRA | F | 25 | 7 | CS-Refractory |
| DFML | F | 49 | 2 | CS-Refractory |
| ML | M | 37 | 1 | Refractory UC |
| BM | F | 46 | 1 | Toxic megacolon |
| TE | M | 47 | 8 | Refractory UC |
| SC | F | 39 | 1 | Refractory UC |
| CF | M | 36 | 8 | Toxic megacolon |
| LP | F | 50 | 20 | Refractory UC |
| NF | M | 63 | 8 | Refractory UC |
| TR | F | 47 | 11 | Toxic megacolon |
| CD | ||||
| DAD | F | 37 | 5 | Colonic stenosis |
| MA | M | 83 | 0 | Colonic stenosis |
| CP | F | 51 | 24 | Pelvic abscess |
| DCE | M | 30 | 5 | Obstruction |
| DLLV | M | 22 | 1 | Refractory CD |
| CRL | F | 59 | 1 | Recto-vaginal fistula |
| FAP | ||||
| ZG | F | 57 | 16 | FAP |
| Colon K | ||||
| ML | F | 68 | 1 | Colon cancer |
| Patient | Endo (n) | Ileal lesions at endoscopy | Histology | |||||||||
| Uninvolved ileum | Involved ileum | |||||||||||
| Conventional histology[23] | Immunohistochemistry | Conventional histology[23] | Immunohistochemistry | |||||||||
| A | B | Colonic metaplasia | CG3 MoAb | Das-1 MoAb | A | B | Colonic metaplasia | CG3 MoAb | Das-1 MoAb | |||
| LRA | 1 | Y | 1 | 0 | N | - | - | ND | ND | ND | ND | ND |
| 2 | Y | 0 | 0 | N | + | + | 12 | 12 | Y | + | + | |
| 3 | Y | 2 | 0 | N | + | + | 6 | 1 | N | + | + | |
| 4 | Y | 0 | 0 | N | + | + | 6 | 0 | N | + | + | |
| 5 | Y | 0 | 0 | N | + | + | 8 | 0 | N | + | + | |
| DFML | 1 | Y | ND | ND | ND | ND | ND | 0 | 0 | N | + | - |
| 2 | N | 0 | 0 | N | + | + | ND | ND | ND | ND | ND | |
| SC | 1 | N | 2 | 0 | N | + | + | ND | ND | ND | ND | ND |
| ML | 1 | Y | 0 | 0 | N | + | + | 10 | 4 | Y | + | + |
| 2 | N | 0 | 0 | N | ND | ND | ND | ND | ND | ND | ND | |
| TE | 1 | Y | ND | ND | ND | ND | ND | 0 | 0 | Y | + | - |
| LP | 1 | N | 0 | 0 | N | ND | ND | ND | ND | ND | ND | ND |
| BM | 1 | N | 0 | 0 | N | ND | ND | ND | ND | ND | ND | ND |
| 2 | N | 1 | 0 | N | + | - | ND | ND | ND | ND | ND | |
| 3 | N | 0 | 0 | N | ND | ND | ND | ND | ND | ND | ND | |
| DLE | 1 | Y | 0 | 0 | N | + | - | 6 | 2 | Y | + | - |
| 2 | Y | 0 | 2 | Y | + | + | 0 | 2 | Y | + | + | |
| 3 | N | 0 | 0 | N | ND | ND | ND | ND | ND | ND | ND | |
| CF | 1 | Y | ND | ND | ND | ND | ND | 2 | 0 | N | - | - |
| 2 | Y | 0 | 0 | N | ND | ND | ND | ND | ND | ND | ND | |
| NF | 1 | Y | ND | ND | ND | ND | ND | 6 | 3 | Y | ND | ND |
| Patient | Endo (n) | Ileal lesions at endoscopy | Histology | |||||||||
| Uninvolved ileum | Involved ileum | |||||||||||
| Conventional histology[23] | Immunohistochemistry | Conventional histology[23] | Immunohistochemistry | |||||||||
| Score | Score | Colonic | CG3 | Das-1 | Score | Score | Colonic | CG3 | Das-1 | |||
| A | B | metaplasia | MoAb | MoAb | A | B | metaplasia | MoAb | MoAb | |||
| DAD | 1 | Y | ND | ND | ND | ND | ND | 4 | 0 | N | + | - |
| (CD-1) | 2 | Y | ND | ND | ND | ND | ND | 0 | 0 | N | + | - |
| 3 | Y | 0 | 0 | N | + | - | 0 | 0 | N | + | - | |
| 4 | Y | 0 | 0 | N | + | - | 8 | 4 | Y | + | - | |
| MA | 1 | N | 4 | 4 | Y | + | + | 0 | 3 | Y | + | + |
| (CD-2) | ||||||||||||
| DCE | 1 | N | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| (CD-3) | ||||||||||||
| CP | 1 | N | 0 | 0 | N | ND | ND | ND | ND | ND | ND | ND |
| (CD-4) | 2 | N | 2 | 0 | N | ND | ND | ND | ND | ND | ND | ND |
| 3 | Y | 1 | 1 | N | ND | ND | 2 | 1 | N | ND | ND | |
| DLV | 1 | Y | ND | ND | ND | ND | ND | 4 | 1 | Y | + | + |
| (CD-5) | 2 | N | 2 | 2 | Y | ND | ND | |||||
| 3 | Y | 4 | 2 | Y | ND | ND | 12 | 4 | Y | ND | ND | |
| CRL | 1 | Y | ND | ND | ND | ND | ND | 10 | 3 | Y | ND | ND |
| (CD-6) | ||||||||||||
| ZG | 1 | N | 0 | 0 | N | ND | ND | 8 | 2 | N | ND | ND |
| (FAP) | ||||||||||||
| ML | 1 | N | 0 | 0 | N | ND | ND | ND | ND | ND | ND | ND |
| (K) | ||||||||||||
Inflammatory changes of the distal ileum in ulcerative colitis (UC) may be observed in backwash ileitis[1-3] and after total proctocolectomy with ileal pouch (“pouchitis”)[4-8]. Chronic inflammation of the ileal pouch has been described not only in patients with UC[9-13], but also after total proctocolectomy for other conditions, including Familial Adenomatous Polyposis (FAP). The frequency of pouchitis is more frequent in UC (60%-70% at 1 years)[14-16] than in FAP (< 50% at 1 years), thus suggesting that the underlying UC may be involved in the pathogenesis of pouchitis[8,14,17]. Common bacterial flora also appears to play a major role in pouchitis, as suggested by both experimental and clinical evidences, supporting the efficacy of probiotics in subsiding symptoms of pouchitis in UC[18-22]. Although the etiology of pouchitis is unknown, the development of changes of the ileal mucosa lining the pouch, including flattening, reduced number and/or complete villar atrophy has been involved in UC[4,23,24]. These changes of the ileum, becoming similar to the colonic epithelium (“colonic metaplasia”) have been reported more frequently associated with pouchitis. Changes of the resident bacterial flora after proctocolectomy have therefore been involved in the development of both colonic metaplasia and pouchitis.
Proctocolectomy represented the procedure of choice in severe UC over the past 30 years[25], and permanent ileostomy[26,27] is still a valid option in an elderly patient in whom either an ileal pouch or an ileo-rectal anastomosis (IRA) is contraindicated. Nowadays, colectomy with IRA[28-30] must be compared with the more recent sphincter saving procedure, namely restorative proctocolectomy. Ravitch and Sabiston[31] are rightly given the credit for the development of total colectomy and ileo-anal pouch, representing the preferred surgical option in referral IBD surgical unit[32].
IRA for UC determines the persistence of the diseased rectal stump requiring local treatment and cancer surveillance, thus being rarely performed in UC, due to technical feasibility or clinical requirements. It is conceivable that not only after ileal pouch, but also after IRA, the neo-terminal ileum may develop inflammatory changes.
In order to investigate this issue, we aimed to assess whether inflammatory changes of the ileum in UC may also be observed after IRA. We also explored whether these changes of the neo-terminal ileum are associated with the development of colonic metaplasia of the ileum itself. For this purpose, colonic metaplasia of the ileum was investigated in biopsy samples taken from the neo-terminal ileum of patients with IRA for UC, by both conventional histological assessment and immunohistochemistry, using monoclonal antibodies (MoAb) against colonic epithelial antigens (CEP and hTM5)[23,33-36]. In one UC patient, ileal lesions were also assessed by wireless capsule endoscopy (WCE). As controls, a small group of patients with IRA for Crohn’s disease (CD), FAP or colon cancer (colon K) were also investigated as patients with IRA for UC.
A total of 19 patients with IRA in regular follow up at the University “Tor Vergata” of Rome, Italy, were studied. Clinical characteristics of each patient are reported in Table 1. The study population included patients with IRA for UC (n = 11), and, as control group, 6 patients with IRA for CD, 1 for FAP and 1 for adenocarcinoma of the sigmoid colon (colon K). In all UC patients and controls the diagnosis was confirmed by macroscopic and histological analysis of the surgical specimen. Clinical history, endoscopic, surgical and histological findings were reviewed for the possibility that endoscopic findings after surgery would suggest possible changes of previous diagnosis (i.e. ileal lesions in UC). The study group included 11 UC patients (5 M; median age 46; range 25-63), with a median UC duration of 7 years (range 1-20), a median time interval from surgery of 4 years (range 1-12). Indication for surgery was adenocarcinoma in the rectal stump (n = 1), medical intractability (n = 8), toxic megacolon (n = 2). Among the 8 controls, there were 6 patients with CD (3 M, median age 51, range 22-83), with a median CD duration of 3 years (range 0-24), a median time interval from surgery of 4 years (range 1-20). Indication was stenosis of the sigmoid colon (n = 2), recto-vaginal fistula (n = 1), obstruction (n = 1), pelvic abscess (n = 1), refractory CD (n = 1). Control group also included 2 patients with IRA for FAP (F, age 57) (n = 1) and adenocarcinoma of the sigmoid colon (F, age 68) (n = 1).
Endoscopy: Endoscopy was performed according to clinical criteria, including possible treatment changes, cancer surveillance, or recurrence assessment (in CD). According to these criteria, 9 IBD patients underwent repeated endoscopic assessments of the rectum and ileum, in a median follow up of 4 years (range 1-20). The numbers of endoscopic assessment were: 21 in the 11 UC, 12 in the 6 CD, and 1 in the only FAP and colon cancer patients. Persistence or healing of the ileal lesions was searched in patients undergoing repeated endoscopies. All endoscopies were performed by the same gastroenterologist (LB), with pictures taken from the rectum, anastomosis and neo-terminal ileum. In UC, the endoscopic degree of inflammation was graded according to the Mayo score (0-4)[37]. The presence and degree of ileal lesions visualized by the endoscope was searched and described as: erosions, aphthoid or deep ulcers, strictures and/or stenosis. The extent of the lesions (cm), the number of ulcerations (few or diffuse: < 5 or ≥ 5) and macroscopic aspect of the ileum between ulcers were also reported. In CD, endoscopic recurrence was graded according to Rutgeerts et al (from 0 to 4)[38].
Histology: In all patients biopsies (n = 2) were taken from the neo-terminal ileum (uninvolved and involved area), anastomosis and rectal stump. In all patients, at least 10 cm of the neo-terminal ileum were visualized. When considering all biopsy samples taken during repeated endoscopies, 90 biopsy samples (180 biopsies) were taken from the ileum, anastomotic area and rectum. In UC, biopsies were taken from the neo-terminal ileum (macroscopically uninvolved n = 17, involved n = 12), anastomosis (n = 3) and rectum (n = 18). In controls, biopsies were taken: in CD from the ileum (uninvolved n = 8; involved n = 8), anastomosis (n = 6) and rectal stump (n = 11), in FAP (n = 1) and colon cancer patients (n = 1) from the uninvolved n = 2 or involved n = 1 ileum, anastomosis (n = 2) and rectum (n = 2). Biopsy samples were kept in 10% formalin. Paraffin blocks were used for routine histology by HE staining and for immunohistochemistry by immunoperoxidase staining. Histological assessment was made by one single histopathologist (GP), in order to: (1) Confirm the diagnosis of UC or CD; (2) Assess the presence and degree of inflammation; (3) Detect dysplasia/neoplasia in the rectal stump; (4) Detect changes of the epithelium lining the ileum towards colonic epithelium. Inflammatory changes were assessed according to conventional criteria[4,23,39]. Histologic elements of inflammatory and colonic metaplasia were assessed according to Fruin et al[24], including: (1) An inflammatory score considering histological characteristics of the villi epithelia, crypt epithelia, stroma and ulceration (A score: range 0-28); (2) Colonic metaplasia score considering characteristics of villous atrophy and crypt hyperplasia (B score: range 0-6).
Immunohistochemistry: In order to detect possible ileal changes towards colonic epithelium, the expression of colonic antigens was assessed by immunoperoxidase using 2 MoAbs against human tropomyosin isoform 5 (hTM5) (CG3) and against the > 200 kDa colonic epithelial antigen (Das-1)[33-36]. Sections were stained as reported previously[33-36].
Clinical assessment: Clinical assessment was made according to the Mayo score in UC[37] and to the CD Activity Index (CDAI) in CD[40].
WCE: In one compliant UC patient, the presence and extent of the lesions in the neo-terminal ileum was also investigated by WCE. WCE examination was performed using the Given M2A capsule (Given Imaging, Yoqneam, Israel[41,42]) as described[43], by using bowel preparation with PEG (2 L). Exclusion criteria included: low compliance, diverticula, blind loop, pace-maker, neurological disorders, intestinal strictures. WCE images were assessed by one gastroenterologist. The detection of the following lesions was reported: erosions, ulcers, strictures or stenosis. Any other lesion was also reported.
The study was approved by the Local Ethic committee.
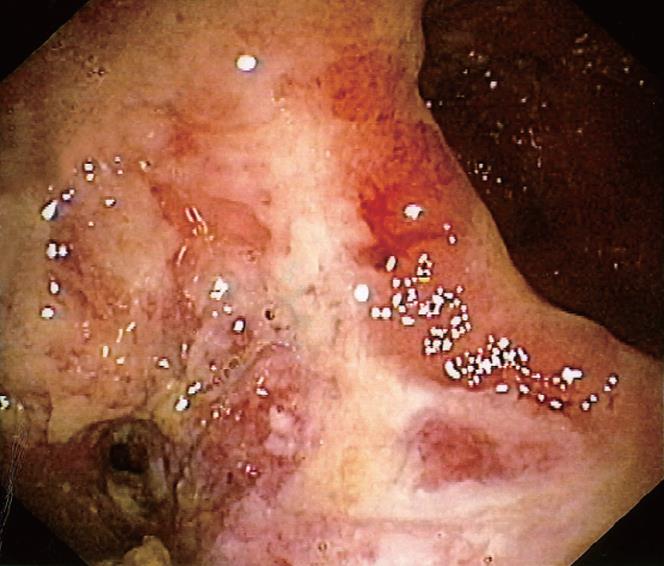
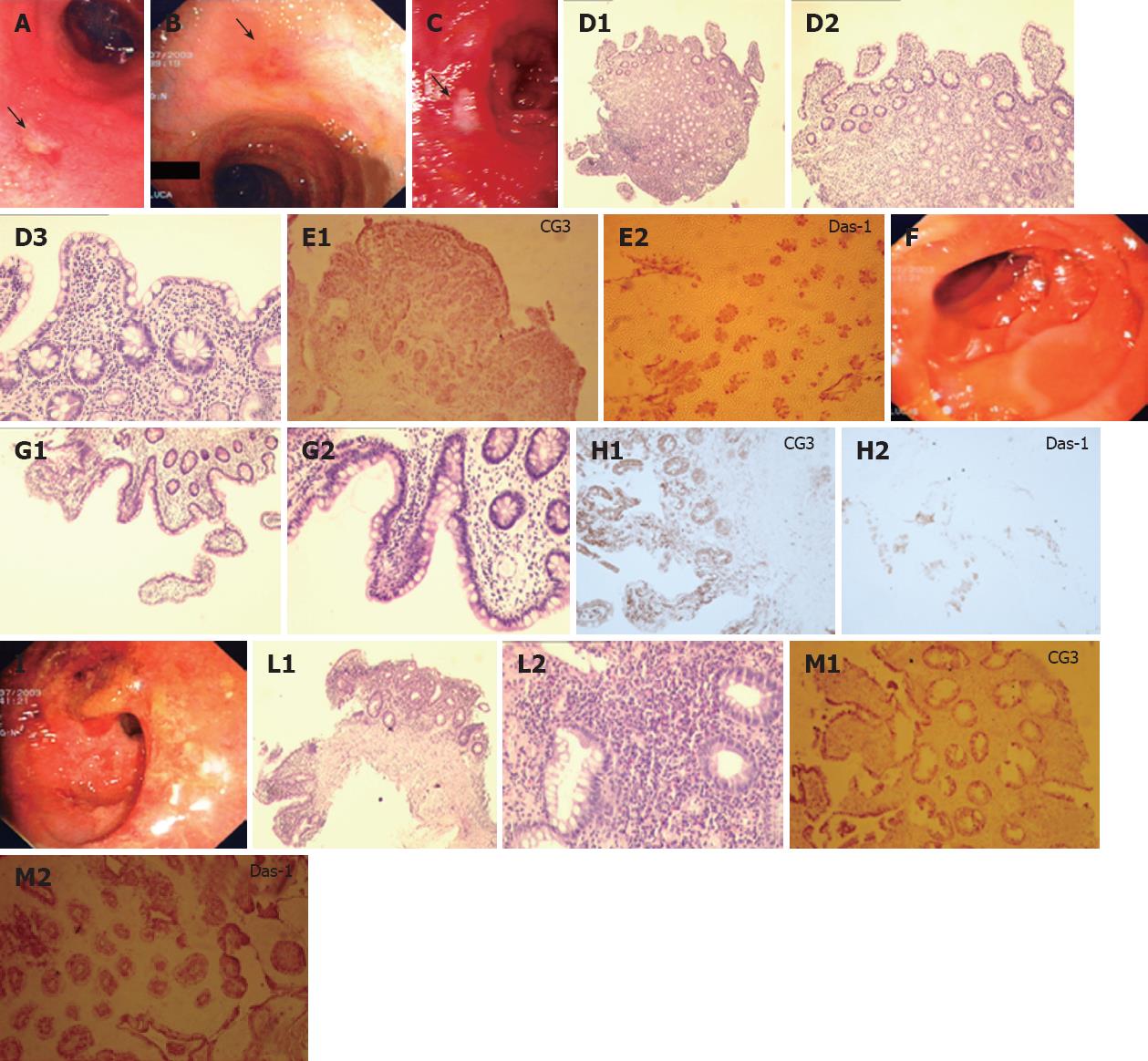
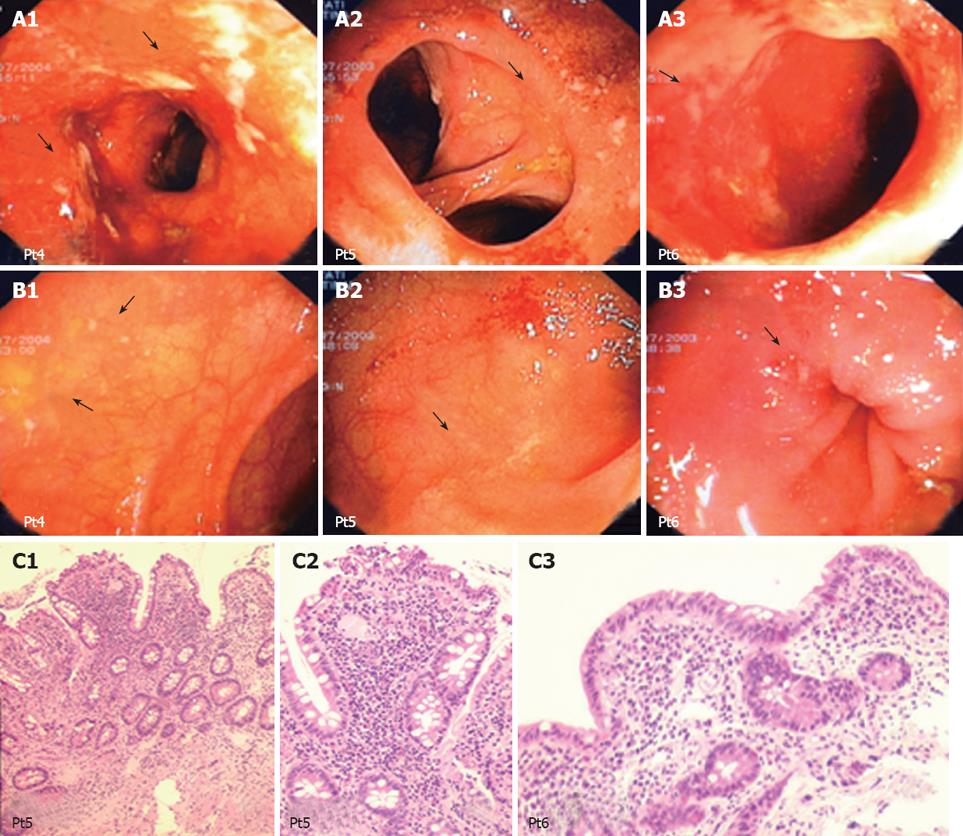
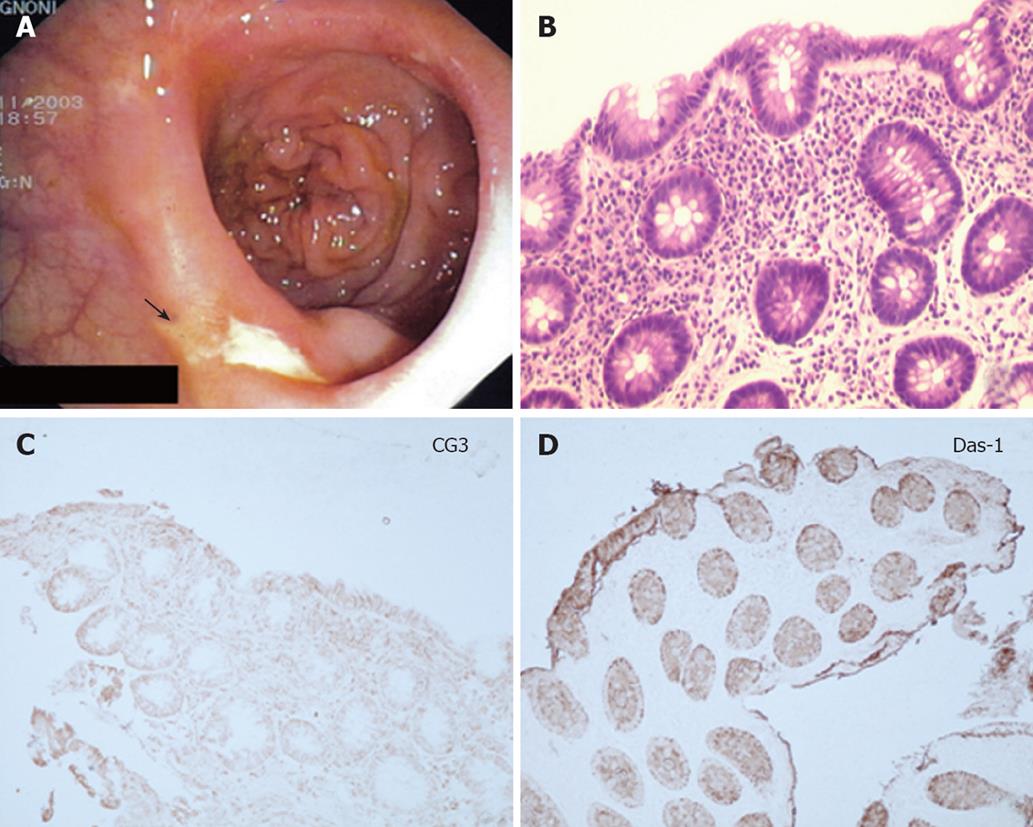
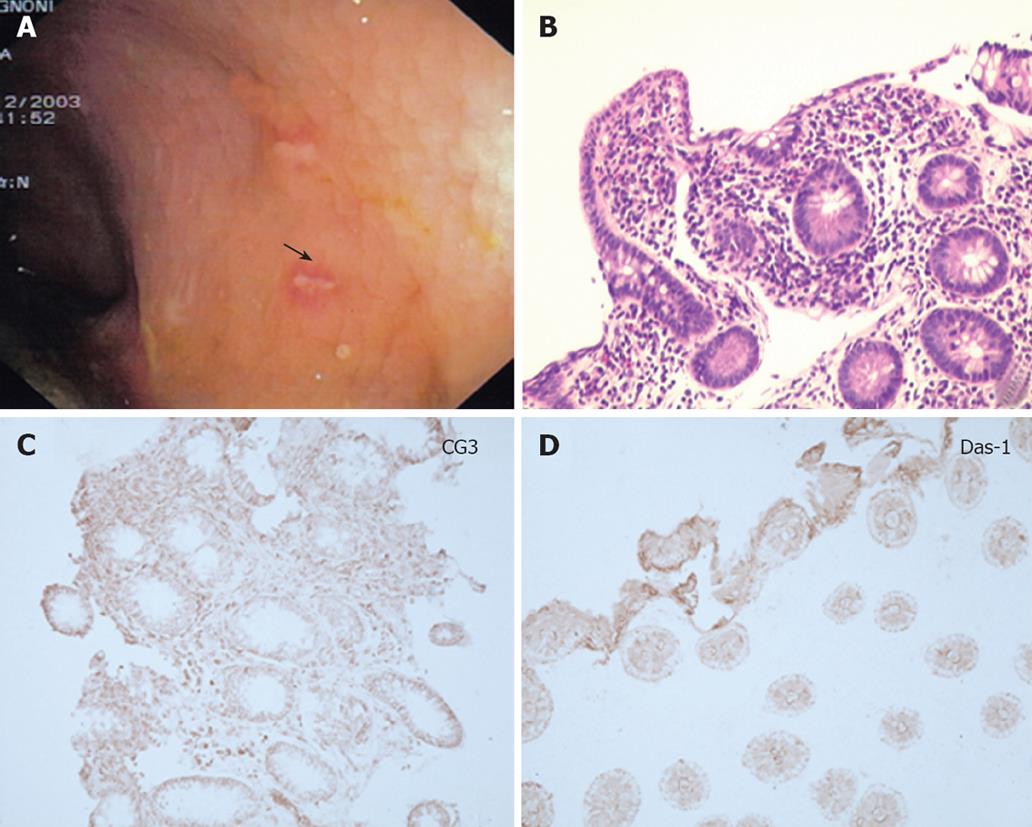
In one UC patient, an anastomotic stricture that could not be passed by the endoscope was detected. A hard and ulcerated area was present in the rectal stump, compatible with adenocarcinoma, confirmed by histology (Figure 1). The ileum was therefore visualized in 10/11 patients. Figure 2A shows the percentage of UC patients with endoscopic lesions in the neo-terminal ileum, together with changes towards colonic epithelium, as detected by both conventional histology and staining using CG3 and Das-1 MoAbs. As indicated, ileal lesions were detected at first endoscopy in 7/10 patients. Histological analysis of the macroscopically involved ileum detected changes towards colonic epithelium in 4/7 patients. CG3 and Das-1 MoAbs staining were observed in ileal samples from 3 out of these 4 patients. The endoscopic and histological views of the neo-terminal ileum and rectal stump from 2 UC patients with lesions associated or not with changes towards colonic epithelium are shown in Figures 3 and 4, respectively.
Clinical assessment: At first endoscopy, UC was clinically active (Mayo score) in 5 and inactive in 6 patients.
Endoscopy: As the neo-terminal ileum was not visualized in 1 UC patient due to an adenocarcinoma of the rectal stump (Figure 1), the ileum was visualized in 10/11 patients. Among these 10 patients, macroscopic lesions were detected in the rectal stump in 8, in the anastomosis in 6, in the ileum in 7. Rectal lesions in the 8 patients included erosions (n = 2) and ulcers (n = 6). In 2/6 patients with anastomotic lesions, a stricture that could be passed by the endoscope was observed. Table 2 shows the endoscopic and histological findings in each of the 11 UC patients studied. Among the 10 patients, 7 showed macroscopic lesions in the ileum proximal to the IRA at first endoscopy. Lesions were localized within 15 cm above the anastomosis in all patients, including ulcers, sporadic (< 5) in 5 (apthoid, n = 3; deep, n = 2) and diffuse (> 5) in 2 UC. In all 7 patients, ileal lesions were surrounded by macroscopically uninvolved areas, while the rectal stump showed typical UC lesions. All 6 UC with anastomotic lesions also showed ileal lesions, while 1 patient with ileal lesions showed a normal anastomosis.
Histology: HE staining. Adenocarcinoma of the rectal stump was detected in 1 patient (Figure 1) and inflammatory changes in the remaining 10. In the macroscopically uninvolved ileum, biopsies were taken from 7/10 UC, showing inflammatory changes (n = 2), lamina propria oedema (n = 3) or no lesions (n = 2) (Table 2). In the macroscopically involved ileum, histology detected inflammation in all 7 UC (acute and chronic inflammation n = 3; villous atrophy n = 4). The anastomotic area showed inflammation in 3/6 UC.
Assessment of changes of the ileal epithelium toward colonic epithelium. Changes towards colonic epithelium are observed in the macroscopically uninvolved ileum from 7/10 UC, showing an inflammatory infiltrate in 3 (A1, n = 1; A2, n = 2), no inflammation in 4 and colonic metaplasia in 1 (B2) (Table 2). Changes toward colonic epithelium were found in the 7/10 UC with ileal lesions, showing inflammatory changes in 4 (A12, n = 1; A10, n = 1; A6, n = 2). Ileal changes towards colonic epithelium have been detected in the 4/7 UC with ileal lesions (A12, n = 1; A4, n = 1; A3, n = 1; A2, n = 1).
Figures 3A to C and 4A to C show the endoscopic, histologic and immunohistochemistry analysis of biopsy samples taken from 4 additional UC patients with IRA.
Immunohistochemistry: Expression of hTM5-related antigens was seen in the uninvolved ileum from 6/10 UC, showing CG3 staining in all 6 and Das-1 staining in 5/6 UC. The involved ileum showed CG3 staining in 5/6 UC and Das-1 staining in 3/6 UC.
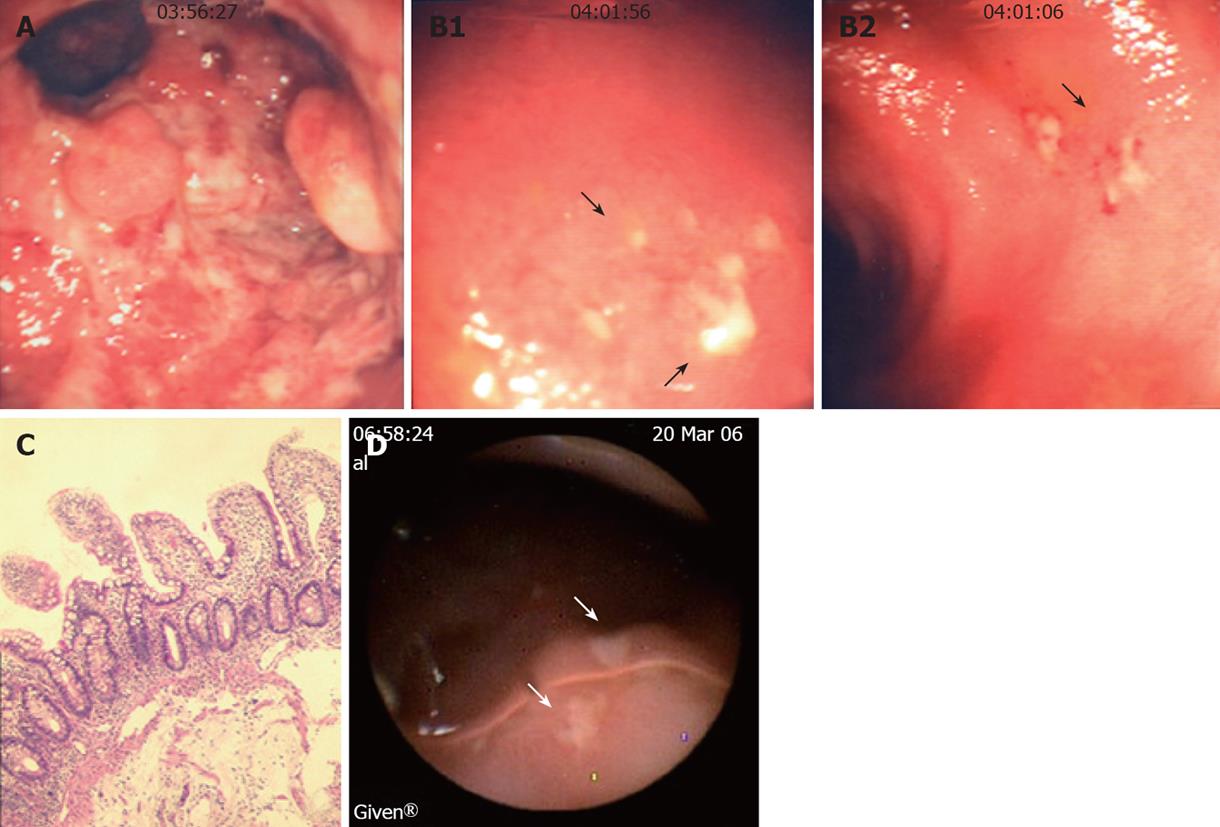
Longitudinal study: Endoscopy. Among the 7 patients with ileal lesions, 6 underwent repeated endoscopies (n = 5 in 1; n = 2 in 4; n = 3 in 1). Ileal lesions were detected at all endoscopies in 3/6 UC, at first but not at second endoscopy in 2, healing at third endoscopy in 1 UC. Figure 5 shows the endoscopic, histologic and immunohistochemical analysis of the rectum, involved and uninvolved ileum from one UC patient at 3 consecutive endoscopies.
Histology. Ileal lesions at endoscopy were confirmed by histology in all UC patients. One patient showed ileal changes toward colonic epithelium at all endoscopies (Figure 3).
Immunohistochemistry. In UC, biopsy samples were taken during repeated endoscopies from the uninvolved (n = 11) and involved ileum (n = 10). In the uninvolved ileum, 10/11 biopsies showed CG3 staining (strong in 4) and 8/11 Das-1 staining (glandular n = 5; epithelial cells n = 3). In the involved ileum 9/10 biopsies showed CG3 staining (glandular n = 2; epithelial cells n = 7, strong in 3) and 6/10 showed Das-1 staining (glandular n = 5; epithelial cells, n = 1).
WCE. In the only UC patient studied by WCE, this procedure confirmed the endoscopic findings, showing multiple erosions and ulcers covered by fibrin in the distal 10 cm of the neo-terminal ileum (Figure 3D). These lesions appeared focal, being surrounded by macroscopically uninvolved mucosa. No other lesions, active bleeding or strictures were detected by WCE in the entire small bowel.
CD patients: Figure 2B shows the percentage of CD patients with ileal recurrence detected by endoscopy and histology, together with changes towards colonic epithelium assessed by histology and immunohistochemistry. Recurrence was detected in 4 out of 6 patients: histology detected changes towards colonic epithelium in 3 out of these 4 patients, associated with the expression of colonic epithelial antigens in 2 out of 3 patients.
Clinical assessment. At first endoscopy, disease was active in 3 (CDAI > 150) and inactive in 3 patients.
Endoscopy. Lesions were detected in the rectum in 2/6 patients, in the anastomosis in 5/6 and in the ileum in 4/6 patients. Lesions included CD recurrence of grade 0 (n = 2), 2 (n = 2), 3 (n = 1) or 4 (n = 1) (Figures 6 and 7).
Histology. (1) HE staining: In rectal samples, inflammatory changes were detected in 3/6 CD. The macroscopically uninvolved ileum from 4/6 patients showed changes towards colonic metaplasia (n = 1), inflammation and villous atrophy (n = 2) or no lesions (n = 1) (Figures 6 and 7). Among the 4 patients with ileal recurrence, inflammation was detected in 3 (with villous atrophy in 2) and colonic metaplasia in 1. The anastomosis showed inflammation in 3 (glandular hyperplasia n = 1, villous atrophy n = 1, colonic metaplasia n = 1). (2) Assessment of changes of the ileal epithelium toward colonic epithelium: In 4/6 CD, biopsy samples were taken from the macroscopically uninvolved ileum, showing inflammation in 3/4 (A2, n = 1; A4, n = 2) and changes towards colonic metaplasia in 2/4 (B2, n = 1; B4, n = 1) (Table 3). Ileal lesions at endoscopy were confirmed by histology in all 4 CD (A2, n = 1; A8, n = 1; A10, n = 1; A12, n = 1) showing colonic metaplasia in 3/4 patients (B3, n = 1; B4, n = 2). (3) Immunohistochemistry: hTM5-related antigens expression in the uninvolved ileum was searched in 2 CD, showing staining against CG3 in both and against Das-1 in 1 patient. Biopsies from the involved ileum were taken from 3 CD, showing CG3 staining in all 3, and Das-1 staining in 2/3 patients.
Longitudinal study: Endoscopy: Repeated endoscopies (n = 4 in 1; n = 3 in 2) were performed in 3/6 patients, showing persistent lesions. Histology: In 5/6 patients, ileal inflammatory changes were detected at all endoscopies. Immunohistochemistry: The only patient with repeated immunohistochemical analysis showed persistent CG3 positivity and Das-1 negativity.
Endoscopy: One anastomotic ulcer was detected. Histology: Inflammation were detected only in the anastomosis (A = 0; B = 2).
Endoscopy: No lesions were detected. Histology: Mild inflammation was detected in the rectum only.
In UC, inflammatory changes of the ileum in UC may be observed in pouchitis[4-8] and in backwash ileitis[1-3]. In pouchitis, ileal lesions have been related to the development of changes of the epithelium towards colonic epithelium (“colonic metaplasia”)[4,23,24]. Although the etiology of pouchitis remains unknown, the resident bacterial flora is involved in the pathogenesis of this condition[15,18,19,21,22]. In particular, the efficacy “in vivo” of probiotic preparations in UC patients with pouchitis suggests a pathogenetic role for changes of the resident bacterial flora after proctocolectomy[18,19,21]. As proctocolectomy is required for both ileal pouch and IRA, we aimed to assess whether UC patients may develop inflammatory changes of the ileum not only in the ileal pouch, but also above the IRA. We also explored whether these changes are associated with the development of colonic metaplasia of the epithelium lining the ileum. Ileal changes have been examined by histology and immunohistochemistry using MoAbs against the major cytoskeletal microfilament protein, tropomyosin isoform 5 (hTM5) in colon epithelium[33-36]. Evidence indicates that bacterial-host interactions may induce the expression of cryptic cytoskeletal proteins on human cells surface[23,44]. Cytoskeletal proteins include a family of intracytoplasmatic proteins (α-actinin, talin, ezrin, villin, F-actin, myosin II, calpactin, gelsolin, laminin, tropomyosin), modulating the structure, shape, and motility of several cell types, including human colonic epithelial cells[45]. The enteropathogenic Escherichia coli binds to enterocytes by injecting a translocated intimin receptor in the host cells membrane, linking to the intimin receptor of the bacterium itself. This binding is followed by a rearrangement of the cytoskeletal proteins within the cytoplasm of the enterocytes (α-actinin, talin, ezrin, villin, F-actin, myosin II, TMs), thus forming pedestals linking the bacterium itself to colonic epithelial cells[44,45]. These observations prompted us to also assess whether hTM5 may be expressed on the epithelial cells lining the neo-terminal ileum in UC patients with IRA, due to changes of the ileum towards colonic epithelium after colectomy and the possible relation between the development of colonic metaplasia and ileal lesions. Our findings from a limited number of patients with IBD suggest that ileal lesions may be observed after IRA, although more frequently in UC (7 out of 10 patients). The presence of scattered erosions and ulcers in the neo-terminal ileum above the anastomosis was confirmed by WCE images, acquired as described[41-43], from the only patient performing this procedure. Despite the focal inflammation of the ileum, histological analysis of the surgical colonic specimen confirmed the diagnosis of UC in all 7 patients. Ileal lesions were associated with epithelial changes towards colonic epithelium in 4 out of these 7 patients, associated with the expression of hTM5-related antigens in 3 out of these 4 patients. The same findings were not detected in any of the 7 UC patients with no ileal lesions. Surprisingly, colonic metaplasia and the expression of colonic epithelial antigens were also observed in some of the few tested patients with IRA for CD. Although “colonization” of the ileum after total colectomy for any indication has been described, its possible relation with the development of ileal lesions is unknown. The score from Fruin et al[24] was used for assessing colonic metaplasia, as for pouchitis. Major limits of our study include the low number of patients and the cross-sectional study plan, not allowing comparisons before versus after surgery. Although it is said that colectomy cures UC, this study underscores the fact that surgery is not the final answer due to the high incidence of pouchitis and other functional problems experience by these patients. Of particular concern is the asymptomatic neoplasia that can arise in the residual rectal stump required for these surgical procedures, which was seen in one of the study patients.
Although not conclusive, our findings suggest that lesions may be observed in the neo-terminal ileum of UC and CD patients following IRA. These changes are towards colonic epithelium phenotype and with the expression of colonic epithelial antigens in some patients. Longitudinal studies are ongoing for further characterization of the molecular mechanisms leading to ileal changes in UC. Present findings suggest that changes of the ileal content after colectomy may contribute to the development of colonic type of metaplasia, leading to ileal lesions both in the pouch and in the neo-terminal ileum after IRA.
Inflammatory changes of the distal ileum in ulcerative colitis (UC) may be observed in backwash ileitis and after total proctocolectomy with ileal pouch (“pouchitis”). Although total proctocolectomy with ileal pouch currently represents the most frequently performed surgical procedure in UC, colectomy with ileo-rectal anastomosis (IRA) is still in these patients. The persistence of the diseased rectal stump after IRA requires endoscopical surveillance. Ileal inflammation may be observed in UC patients with pouchitis, being related to changes of the ileal epithelium towards colonic epithelium (“colonic metaplasia”). Whether the ileum above IRA in patients with IRA for UC may develop inflammatory changes as observed in pouchitis is unknown and this observation may be useful for proper follow up of patients after surgery.
The etiology of pouchitis in patients with UC is unknown. However, the development of changes of the ileal mucosa lining the pouch, including flattening, reduced number and/or complete villar atrophy has been involved in the pathogenesis of pouchitis. These changes of the ileum, becoming similar to the colonic epithelium (“colonic metaplasia”) have been reported more frequently associated with pouchitis. Changes of the resident bacterial flora after proctocolectomy therefore have been involved in the development of both colonic metaplasia and pouchitis. It is conceivable that after total colectomy for UC and related changes of the common bacterial flora, ileal lesions may develop not only after ileal pouch, but also after IRA.
The present study showed that In UC, ileal lesions associated with changes towards colonic epithelium may develop after IRA. Changes of the ileal content after colectomy may contribute to the development of colonic metaplasia, leading to ileal lesions also after IRA.
Results from our study suggest that patients with IRA for UC need endoscopical assessment not only for cancer surveillance but also for assessing the possible development of ileal lesions above anastomosis. Present findings also add new insights regarding the natural history of UC after IRA.
Tropomyosin isoform 5 (hTM5) is a cytoskeletal microfilament protein present in the epithelium from human colon, but not from the ileum. Mucosal and circulating antibodies against hTM5 have been demonstrated in patients with UC. Evidences indicate that bacterial-host interactions may induce the expression of cryptic cytoskeletal proteins on human cells surface. Cytoskeletal proteins include a family of intracytoplasmatic proteins (α-actinin, talin, ezrin, villin, F-actin, myosin II, calpactin, gelsolin, laminin, tropomyosin), modulating the structure, shape, and motility of several cell types, including human colonic epithelial cells.
This is a clinical and immunohistochemical study supporting the need of continued endoscopical follow up of UC patients after IRA.
Peer reviewer: Jay Pravda, MD, Inflammatory Disease Research Center, Gainesville, Florida 32614-2181, United States
S- Editor Li DL L- Editor Li M E- Editor Zhang WB
| 1. | Abdelrazeq AS, Wilson TR, Leitch DL, Lund JN, Leveson SH. Ileitis in ulcerative colitis: is it a backwash? Dis Colon Rectum. 2005;48:2038-2046. |
| 2. | Gustavsson S, Weiland LH, Kelly KA. Relationship of backwash ileitis to ileal pouchitis after ileal pouch-anal anastomosis. Dis Colon Rectum. 1987;30:25-28. |
| 3. | Haskell H, Andrews CW Jr, Reddy SI, Dendrinos K, Farraye FA, Stucchi AF, Becker JM, Odze RD. Pathologic features and clinical significance of "backwash" ileitis in ulcerative colitis. Am J Surg Pathol. 2005;29:1472-1481. |
| 4. | Apel R, Cohen Z, Andrews CW Jr, McLeod R, Steinhart H, Odze RD. Prospective evaluation of early morphological changes in pelvic ileal pouches. Gastroenterology. 1994;107:435-443. |
| 5. | Arai K, Koganei K, Kimura H, Akatani M, Kitoh F, Sugita A, Fukushima T. Incidence and outcome of complications following restorative proctocolectomy. Am J Surg. 2005;190:39-42. |
| 6. | Brunel M, Penna C, Tiret E, Balladur P, Parc R. Restorative proctocolectomy for distal ulcerative colitis. Gut. 1999;45:542-545. |
| 7. | Chambers WM, McC Mortensen NJ. Should ileal pouch-anal anastomosis include mucosectomy? Colorectal Dis. 2007;9:384-392. |
| 8. | Madden MV, Farthing MJ, Nicholls RJ. Inflammation in ileal reservoirs: 'pouchitis'. Gut. 1990;31:247-249. |
| 9. | Meagher AP, Farouk R, Dozois RR, Kelly KA, Pemberton JH. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. Br J Surg. 1998;85:800-803. |
| 10. | Meuwissen SG, Hoitsma H, Boot H, Seldenrijk CA. Pouchitis (pouch ileitis). Neth J Med. 1989;35 Suppl 1:S54-S66. |
| 11. | Michelassi F, Lee J, Rubin M, Fichera A, Kasza K, Karrison T, Hurst RD. Long-term functional results after ileal pouch anal restorative proctocolectomy for ulcerative colitis: a prospective observational study. Ann Surg. 2003;238:433-441; discussion 442-445. |
| 13. | Salemans JM, Nagengast FM, Lubbers EJ, Kuijpers JH. Postoperative and long-term results of ileal pouch-anal anastomosis for ulcerative colitis and familial polyposis coli. Dig Dis Sci. 1992;37:1882-1889. |
| 14. | Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a Pouchitis Disease Activity Index. Mayo Clin Proc. 1994;69:409-415. |
| 15. | Shen B, Fazio VW, Remzi FH, Lashner BA. Clinical approach to diseases of ileal pouch-anal anastomosis. Am J Gastroenterol. 2005;100:2796-2807. |
| 16. | Zuccaro G Jr, Fazio VW, Church JM, Lavery IC, Ruderman WB, Farmer RG. Pouch ileitis. Dig Dis Sci. 1989;34:1505-1510. |
| 17. | Shen B, Fazio VW, Remzi FH, Delaney CP, Bennett AE, Achkar JP, Brzezinski A, Khandwala F, Liu W, Bambrick ML. Comprehensive evaluation of inflammatory and noninflammatory sequelae of ileal pouch-anal anastomoses. Am J Gastroenterol. 2005;100:93-101. |
| 18. | Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:305-309. |
| 19. | Gionchetti P, Rizzello F, Helwig U, Venturi A, Lammers KM, Brigidi P, Vitali B, Poggioli G, Miglioli M, Campieri M. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124:1202-1209. |
| 20. | Hurst RD, Molinari M, Chung TP, Rubin M, Michelassi F. Prospective study of the incidence, timing and treatment of pouchitis in 104 consecutive patients after restorative proctocolectomy. Arch Surg. 1996;131:497-500; discussion 501-502. |
| 21. | Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M, Kamm MA. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53:108-114. |
| 22. | Sandborn WJ, McLeod R, Jewell DP. Medical therapy for induction and maintenance of remission in pouchitis: a systematic review. Inflamm Bowel Dis. 1999;5:33-39. |
| 23. | Biancone L, Palmieri G, Lombardi A, Colantoni A, Tonelli F, Das KM, Pallone F. Tropomyosin expression in the ileal pouch: a relationship with the development of pouchitis in ulcerative colitis. Am J Gastroenterol. 2003;98:2719-2726. |
| 24. | Fruin AB, El-Zammer O, Stucchi AF, O'Brien M, Becker JM. Colonic metaplasia in the ileal pouch is associated with inflammation and is not the result of long-term adaptation. J Gastrointest Surg. 2003;7:246-253; discussion 253-254. |
| 25. | Kettlewell MGW. Proctocolectomy for ulcerative colitis. Inflammatory Bowel Diseases. Edinburgh: Churchill Livingstone 1991; 439-445. |
| 27. | Larson DW, Pemberton JH. Current concepts and controversies in surgery for IBD. Gastroenterology. 2004;126:1611-1619. |
| 28. | Bulow C, Vasen H, Jarvinen H, Bjork J, Bisgaard ML, Bulow S. Ileorectal anastomosis is appropriate for a subset of patients with familial adenomatous polyposis. Gastroenterology. 2000;119:1454-1460. |
| 29. | Lepisto A, Jarvinen HJ. Fate of the rectum after colectomy with ileorectal anastomosis in ulcerative colitis. Scand J Surg. 2005;94:40-42. |
| 30. | Watts JM, Hughes ES. Ulcerative colitis and Crohn's disease: results after colectomy and ileorectal anastomosis. Br J Surg. 1977;64:77-83. |
| 31. | Roggo A, Brunner U, Ottinger LW, Largiader F. The continuing challenge of aneurysms of the popliteal artery. Surg Gynecol Obstet. 1993;177:565-572. |
| 32. | Nicholls RJ, Holt SD, Lubowski DZ. Restorative proctocolectomy with ileal reservoir. Comparison of two-stage vs. three-stage procedures and analysis of factors that might affect outcome. Dis Colon Rectum. 1989;32:323-326. |
| 33. | Kesari KV, Yoshizaki N, Geng X, Lin JJ, Das KM. Externalization of tropomyosin isoform 5 in colon epithelial cells. Clin Exp Immunol. 1999;118:219-227. |
| 34. | Das KM, Dasgupta A, Mandal A, Geng X. Autoimmunity to cytoskeletal protein tropomyosin. A clue to the pathogenetic mechanism for ulcerative colitis. J Immunol. 1993;150:2487-2493. |
| 35. | Lin JJ, Hegmann TE, Lin JL. Differential localization of tropomyosin isoforms in cultured nonmuscle cells. J Cell Biol. 1988;107:563-572. |
| 36. | Takahashi F, Das KM. Isolation and characterization of a colonic autoantigen specifically recognized by colon tissue-bound immunoglobulin G from idiopathic ulcerative colitis. J Clin Invest. 1985;76:311-318. |
| 37. | Tremaine WJ, Sandborn WJ, Wolff BG, Carpenter HA, Zinsmeister AR, Metzger PP. Bismuth carbomer foam enemas for active chronic pouchitis: a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 1997;11:1041-1046. |
| 38. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn's disease. Gastroenterology. 1990;99:956-963. |
| 39. | Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Lofberg R, Modigliani R, Present DH, Rutgeerts P, Scholmerich J, Stange EF. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122:512-530. |
| 40. | Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439-444. |
| 41. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. |
| 43. | Biancone L, Calabrese E, Petruzziello C, Onali S, Caruso A, Palmieri G, Sica GS, Pallone F. Wireless capsule endoscopy and small intestine contrast ultrasonography in recurrence of Crohn's disease. Inflamm Bowel Dis. 2007;13:1256-1265. |
| 44. | Vallance BA, Finlay BB. Exploitation of host cells by enteropathogenic Escherichia coli. Proc Natl Acad Sci USA. 2000;97:8799-8806. |