Published online Aug 14, 2008. doi: 10.3748/wjg.14.4834
Revised: June 7, 2008
Accepted: June 14, 2008
Published online: August 14, 2008
A cerebral lipiodol embolism is an extremely rare complication of transcatheter arterial chemoembolization for hepatocellular carcinoma. We present a case of cerebral lipiodol embolism that occurred after the third arterial chemoembolization, report the clinical and radiological findings, and review the medical literature.
- Citation: Choi CS, Kim KH, Seo GS, Cho EY, Oh HJ, Choi SC, Kim TH, Kim HC, Roh BS. Cerebral and pulmonary embolisms after transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2008; 14(30): 4834-4837
- URL: https://www.wjgnet.com/1007-9327/full/v14/i30/4834.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4834
| Age | Sex | Symptoms | ABGA (PaO2 mmHg) | Shunt of evidence | Course of TACE (was occurred CLE) | Reference |
| 52 | Male | Headache | ||||
| Mental change | 74.7 | (-) | 2 | 8 | ||
| Motor weakness | ||||||
| 58 | Male | Visual loss | ||||
| Headache | ||||||
| Chest pain | 70.2 | (-) | 1 | 8 | ||
| Shortness of breath | ||||||
| 56 | Male | Dyspnea | 66 | ND | 3 | 8 |
| 81 | Female | Asleep, Tachypnea | ND | ND | 1.2 | 9 |
| Motor weakness | ||||||
| 76 | Male | Mental change | Hypoxia | ND | 16 | 7 |
| Hypoxia | ||||||
| 70 | Female | Mental change | ND | (-) | 1 | 10 |
| Disorientation | ||||||
| 62 | Female | Asleep | 56.6 | (-) | 3 | Present case |
| Doses of lipiodol (mL) | Tumor size | Pulmonary embolism | Injection artery of lipiodol | Recovery time (wk) | Reference |
| 35 | ND | (+) | ND | 3 | 8 |
| 8 | ND | (+) | ND | 3 | 8 |
| ND | ND | (+) | ND | 2 | 8 |
| 15 | 10 cm × 14 cm | (+) | RIPA, RMA | 2 | 9 |
| ND | Large | (+) | RIPA | 6 | 7 |
| Hepatic proper artery | |||||
| Epicholedocal artery | |||||
| 12 | Large | (-) | RCA, RHA, MHA | Death | 10 |
| 30 | 10 cm × 15 cm | (+) | RIPA, RHA | 6 | Present case |
Hepatocellular carcinoma (HCC) is a common malignancy in Asia[1,2]. Many patients with HCC present with advanced stage disease when first diagnosed. There are a variety of different therapeutic modalities used to treat advanced HCC. Transcatheter arterial chemoembolization (TACE) is used to confine tumors to the liver, in addition to shrinking their size, limiting the progression to vascular invasion, and decreasing the risk that viable tumor will embolize systemically, during hepatic manipulation at transplant hepatectomy[3-5]. Although TACE is an invasive procedure associated with several potential complications, it is a procedure that has been adopted worldwide and is the mainstay of treatment of patients with advanced HCC[6].
To date, the literature on embolic injury to the brain, associated with iodized oil after TACE, includes only four reports[7-10]. Here we report our experience with a patient who had HCC and a cerebral lipiodol embolism (CLE) after TACE and review the previous literature.
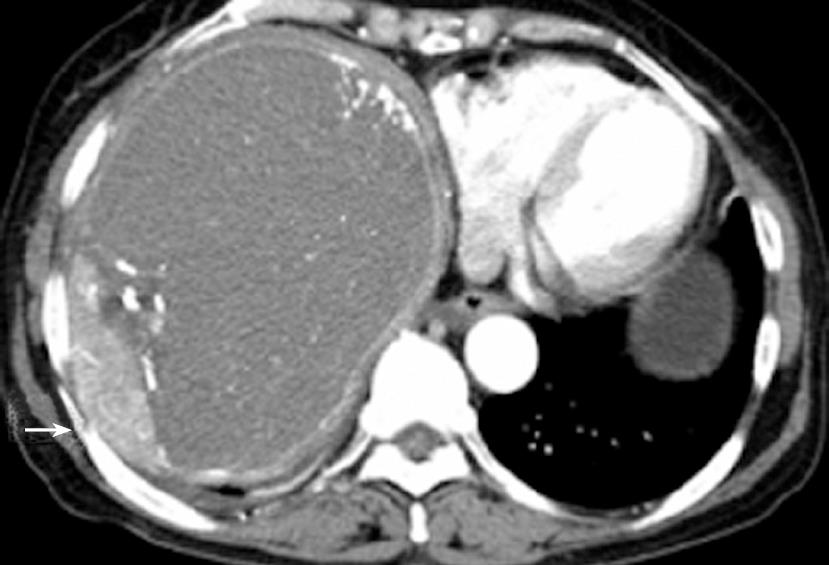
A 62-year-old woman with advanced HCC was admitted to the hospital for her third course of TACE. Six months previously, she was diagnosed with HCC. A 15 cm × 12 cm, well demarcated and exophytic growing tumor, at the right hepatic lobe without portal invasion, was initially identified. The exophytic HCC growth was contiguous to the diaphragm (Figure 1). Although serum alpha-fetoprotein (4514 ng/mL) was very elevated, metastatic evidence to the regional lymph nodes and bone marrow was not detected. The patient was initially treated with TACE and had a second course six months later.
On physical examination, a non-tender smooth surfaced mass was palpated at the right upper abdomen. Laboratory data (normal values in parentheses) on admission revealed a white blood cell count of 5.95× 109/L (normal, 4 × 109-10 × 109), a platelet count of 292 × 109/L (normal, 150 × 109-450 × 109), and hypoalbuminemia, 3.6 g/dL (normal, 3.8-5.1). The hemoglobin was low, 10.0 g/dL (normal, 12.0-18.0). The coagulation time, liver function studies and renal function were within normal range. Serological studies for hepatitis B were positive for HBsAg and HBeAb. The serum DNA level of hepatitis B virus was 64 400 copies/mL.
There was no evidence of cirrhosis. The distal substraction angiography (DSA) revealed the HCC was supplied from the right inferior phrenic artery and right hepatic artery. A third course of TACE was performed via two arteries using a mixture of 50 mg adriamycin and 30 mL lipiodol as well as gelatin sponge particles (Gelform; Upjohn, MI, USA).
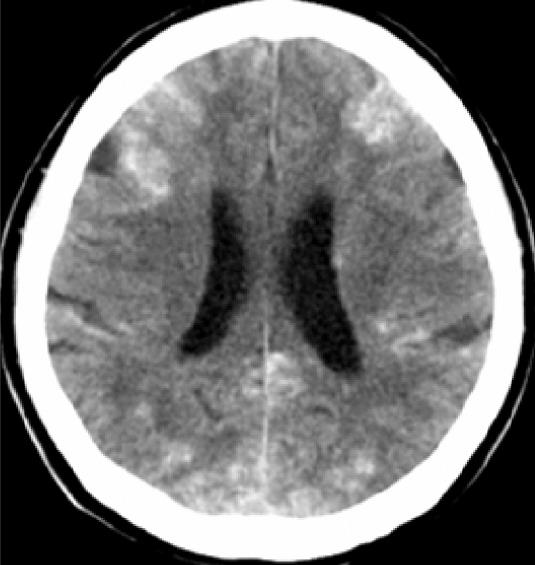
During the procedure, patient had no complaints, and the vital signs remained stable. Immediately after the procedure, the patients’ level of consciousness deteriorated; in addition, she had breathing difficulty. The arterial blood gas analysis showed a PaO2 of 55.7 mmHg, consistent with hypoxemia. A computed tomography (CT) scan of the brain, without contrast, 7 h after the TACE procedure revealed multiple lesions of increased attenuation in the cerebral cortex, basal ganglia, thalami, and cerebellum (Figure 2).
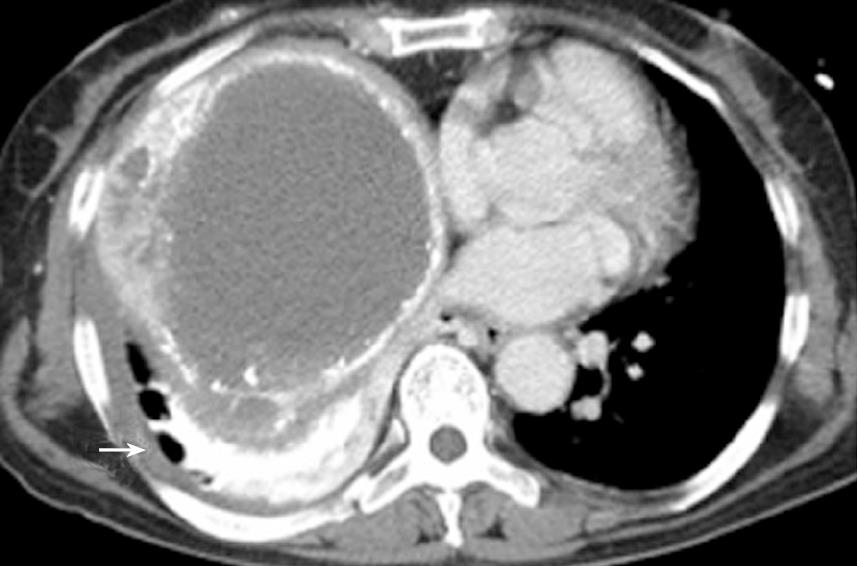
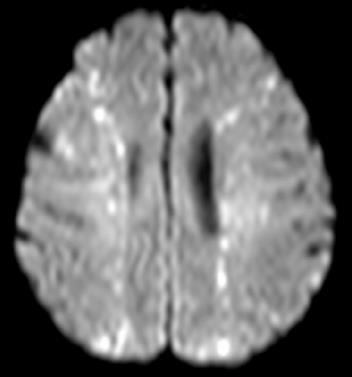
At the same time, a CT of the chest revealed hyper-attenuated images at the right lung base (Figure 3). Forty-eight hours after the procedure, magnetic resonance imaging (MRI) of the brain was performed. Diffusion-weighted (DW) images demonstrated disseminated hyper-attenuated and hyper-intense punctate-patchy lesions in the cerebrum and cerebellum (Figure 4). The laboratory data was not remarkable except for a leukocytosis. The chest X-ray showed a right pleural effusion and diffuse parenchymal infiltration one day post TACE. Echocardiography revealed no atrial septal defect or other intracardiac shunt. Three weeks later, a follow up brain CT scan revealed complete resolution of the lesions. The patients’ level of consciousness gradually had improved. The neurological symptoms recovered completely by discharge 6 wk later.
TACE is an invasive procedure associated with several potential complications, including the postembolization syndrome, septicemia, hepatic insufficiency, liver abscess formation, intrahepatic biloma, embolization of extrahepatic organs, cholecystitis, tumor rupture, multiple intrahepatic aneurysms, gastrointestinal mucosal lesions, variceal bleeding, and iatrogenic dissection or perforation of the vessel[11]. However, a CLE, following TACE in patients with HCC, is an extremely rare complication[7-10].
The symptoms associated with CLE are variable and include visual loss, headache, motor dysfunction, and mental status changes; the severity of such symptoms varies with the site of lipiodol deposition. Occasionally, patients present with dispend and hypoxia when the lungs are involved in lipiodol embolization (Table 1).
It is known that CLE is associated with a right-to-left shunt, and infusion of a large dose of lipiodol[12,13]. Right-to-left shunt is often undetectable on routine examination, including chest or abdominal CT, DSA, and routine echocardiography. Evidence of a shunt has not been reported in previous studies (Table 2). It is not necessary to routinely examine for detection of shunt prior to the TACE. But, if a condition is present that increases the risk of a shunt[14], further evaluation to detect the shunt is indicated.
In most prior cases of CLE including present case, the HCC was a large tumor[7,9,10]. Determination of the optimal dose of lipiodol is of critical importance. The lipiodol dose is determine by a variety of factors including the blood supply of the tumor, the tumor size, the patients’ condition, procedure tolerance, catheter position, and liver function reserve[15,16]. Generally, it is known that lipiodol dose should not exceed 15 mL to 20 mL in order to prevent the risk of an extrahepatic embolism[13]. However, there were prior reported cases of CLE that had appropriate doses of lipiodol[8-10]. Moreover, there was a study that recommend the use of a high dose iodized oil (more than 20 mL) TACE for patients with large HCC[17]. Therefore, all procedures must be individualized.
Another risk factor of a CLE is the communication between inferior phrenic artery (IPA) and the pulmonary artery; this might occur with adhesive pleura or tumor invasion[10,11]. In the previous reports, including present case, the vessels used for the lipiodol infusion described for four of seven cases. Among the four cases, there were three where lipiodol was infused into the right IPA[7,9]. In addition, in one of the four cases, where the IPA was not used, the lipiodol was infused into the right renal capsular artery (RCA), which might have been anastomosed to the IPA. Evidence of pulmonary embolism was noted in six of seven cases.
Therefore, we think that right IPA to pulmonary artery shunt is also a potential route for right-to-left shunt.
In the present case, because we performed echocardiography and DSA, we confirmed the absence of intracardiac and intratumoral shunts. The communication between IPA and pulmonary vessels occurred via adherent pleura and tumor recurrence. Moreover, a large dose of lipiodol was used and, therefore, the patient had a greater risk for a CLE.
As previous reports recommended[10], the total dose of lipiodol should not exceed 20 mL and injection of lipiodol via the IPA should be used with caution during TACE procedure.
To prevent a CLE, an individualized plan of therapy including the lipiodol dose, evaluation for a shunt and choice of the vessels used for the lipiodol infusion prior to TACE are important considerations.
Peer reviewer: Osman C Ozdogan, Associate Professor, Department of Gastroenterology, Liver Unit, Marmara University School of Medicine, Istanbul 34662, Turkey
S- Editor Zhong XY L- Editor Rippe RA E- Editor Zhang WB
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. |
| 2. | El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745-750. |
| 3. | Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, Miller CM, Schwartz ME. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533-539. |
| 4. | Harnois DM, Steers J, Andrews JC, Rubin JC, Pitot HC, Burgart L, Wiesner RH, Gores GJ. Preoperative hepatic artery chemoembolization followed by orthotopic liver transplantation for hepatocellular carcinoma. Liver Transpl Surg. 1999;5:192-199. |
| 5. | Graziadei IW, Sandmueller H, Waldenberger P, Koenigsrainer A, Nachbaur K, Jaschke W, Margreiter R, Vogel W. Chemoembolization followed by liver transplantation for hepatocellular carcinoma impedes tumor progression while on the waiting list and leads to excellent outcome. Liver Transpl. 2003;9:557-563. |
| 6. | Ramsey DE, Geschwind JF. Chemoembolization of hepatocellular carcinoma--what to tell the skeptics: review and meta-analysis. Tech Vasc Interv Radiol. 2002;5:122-126. |
| 7. | Takao H, Makita K, Doi I, Watanabe T. Cerebral lipiodol embolism after transcatheter arterial chemoembolization of hepatocellular carcinoma. J Comput Assist Tomogr. 2005;29:680-682. |
| 8. | Yoo KM, Yoo BG, Kim KS, Lee SU, Han BH. Cerebral lipiodol embolism during transcatheter arterial chemoembolization. Neurology. 2004;63:181-183. |
| 9. | Wu RH, Tzeng WS, Chang CM. Iodized oil embolization to brain following transcatheter arterial embolization of liver. J Gastroenterol Hepatol. 2005;20:1465-1467. |
| 10. | Matsumoto K, Nojiri J, Takase Y, Egashira Y, Azama S, Kato A, Kitahara K, Miyazaki K, Kudo S. Cerebral lipiodol embolism: a complication of transcatheter arterial chemoembolization for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2007;30:512-514. |
| 11. | Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, Iwanaga S, Mori M, Morikawa M, Fukuda T. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18:605-619. |
| 12. | Sato M, Kishi K, Shioyama Y, Tsuda M, Terada M, Tanaka H, Nakatani K, Sonomura T, Maeda M, Daimon M. [Effects of experimental hepatic artery embolization with lipiodol and gelatin sponge on liver tissue]. Nippon Igaku Hoshasen Gakkai Zasshi. 1990;50:107-113. |
| 13. | Chung JW, Park JH, Im JG, Han JK, Han MC. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993;187:689-693. |
| 14. | Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994;105:1528-1537. |
| 15. | Cheng HY, Shou Y, Wang X, Xu AM, Chen D, Jia YC. Adjustment of lipiodol dose according to tumor blood supply during transcatheter arterial chemoembolization for large hepatocellular carcinoma by multidetector helical CT. World J Gastroenterol. 2004;10:2753-2755. |
| 16. | Nakao N, Uchida H, Kamino K, Nishimura Y, Ohishi H, Takayasu Y, Miura K. Determination of the optimum dose level of lipiodol in transcatheter arterial embolization of primary hepatocellular carcinoma based on retrospective multivariate analysis. Cardiovasc Intervent Radiol. 1994;17:76-80. |
| 17. | Chen MS, Li JQ, Zhang YQ, Lu LX, Zhang WZ, Yuan YF, Guo YP, Lin XJ, Li GH. High-dose iodized oil transcatheter arterial chemoembolization for patients with large hepatocellular carcinoma. World J Gastroenterol. 2002;8:74-78. |