Published online Aug 7, 2008. doi: 10.3748/wjg.14.4677
Revised: June 27, 2008
Accepted: July 4, 2008
Published online: August 7, 2008
AIM: To investigate oxidative stress and lipid peroxidation in hepatic steatosis and the underlying implications in pathological mechanisms of non-alcoholic fatty liver disease (NAFLD).
METHODS: F2-isoprostanes (iPF2α-III) in blood and liver samples from steatotic (n = 9) and control (n = 7) rats were measured as in vivo marker of lipid peroxidation by a mass spectrometric approach. The lipid profile and endogenous antioxidant status (SOD and CAT) in the rats were also analyzed.
RESULTS: Significantly higher levels of iPF2α-III (mean 3.47 vs 2.40 pmol/mg tissue, P = 0.004) and lower activities of SOD (mean 1.26 U vs 1.40 U, P < 0.001) and CAT (mean 1026.36 U/mg vs 1149.68 U/mg protein, without significance) were observed in the livers of steatotic rats. Plasma total iPF2α-III was significantly correlated with the abnormalities of blood lipids as well as alanine aminotransferase (ALT) levels in the rats with simple steatosis, whereas no similar tendencies were observed in the control rats.
CONCLUSION: Enhancement of hepatic oxidative imbalance occurring at the steatotic stage of NAFLD suggests a possibility that manifestation of the local oxidative damage precedes that of systemic oxidative imbalance. Predominant metabolic features of the increased lipid peroxidation further suggest a close association of the oxidative imbalance and the dyslipidemia with functional deterioration of the steatotic liver. The findings need to be further evaluated, especially in human studies.
- Citation: Zhu MJ, Sun LJ, Liu YQ, Feng YL, Tong HT, Hu YH, Zhao Z. Blood F2-isoprostanes are significantly associated with abnormalities of lipid status in rats with steatosis. World J Gastroenterol 2008; 14(29): 4677-4683
- URL: https://www.wjgnet.com/1007-9327/full/v14/i29/4677.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4677
Non-alcoholic fatty liver disease (NAFLD), in the absence of alcohol abuse, represents a pathological spectrum of fatty liver disorders including simple steatosis and non-alcoholic steatohepatitis (NASH)[1]. The cause of the disease progression remains elusive. The “two hits” hypothesis addresses a requirement of one or more “second hits”, in addition to the excessive hepatic fat deposition (the “first hit”), for presumed transition of simple steatosis to NASH[12]. Despite the involvement of many other proinflammatory mediators, lipid peroxidation is one hypothesis as the “second hit” to explain the development of the disorder in humans[13–8].
Lipid peroxidation as an important mechanism of NAFLD has been a field of intense research but is not yet fully understood. Majority of previous researches were conducted mainly in NASH in both human subjects and animals. Only a few studies have focused on the effects of lipid peroxidation in simple steatosis, an earlier stage of NAFLD[910]. Given that ectopic lipids accumulation in the liver is primarily linked to hepatic mitochondrial dysfunction and insulin resistance[1], the steatosis stage of the disease might be of real importance in evaluating the cause-effect association of lipid peroxidation with the disease progress, thus providing clues for earlier pharmacological interventions against the development of NASH. Furthermore, malondialdehyde (MDA), a widely used index of lipid peroxidation, has been considered neither the sole end product of fatty peroxide formation and decomposition nor a substance generated exclusively through lipid peroxidation[11]. It remains uncertain if MDA in blood or tissues reflexes oxidative stress status associated with biological changes observed.
F2-isoprostanes, also referred to as iPF2α-III, are a group of prostaglandin-like isomers produced by free radical-catalyzed peroxidation of arachidonic acid[12]. Results from the Biomarkers of Oxidative Stress Study (BOSS) suggested that the most accurate marker to assess lipid peroxidation in vivo is the plasma or urinary iPF2α-III[13]. Elevated plasma or urinary levels of iPF2α-III have been reported in patients[1415] and in animals with NASH[16]. Information in the literatures with regard to iPF2α-III formation related to hepatic steatosis, however, is limited. Tong et al[17] found elevated iPF2α-III in plasma and liver of the rats with valproic acid-triggered liver injury.
The present study was conducted to investigate the oxidative imbalance by measuring local and circulating iPF2α-III and enzymatic antioxidant status in the rats with high-fat diet induced hepatic steatosis. The measures of the lipid peroxidation were then correlated with metabolic risk factors such as lipid profile, which are believed to be greatly involved in the pathogenesis of NAFLD among the steatotic rats.
Adult male Sprague-Dawley rats were obtained from the Slac Animal Center (Shanghai, China), and maintained with a 12 h light/dark cycle under constant temperature and humidity. The rats were chosen according to their lipid levels and then randomly assigned into a defined lard-based high-fat (HF) diet (n = 9) and standard laboratory chow (n = 7) groups (45% vs 10% kcal as fat, respectively). Both diets were made based on the ingredients of products D12451 and D12450B (Research Diets, New Brunswick, NJ, USA), respectively, and supplied by Double Lion Ltd. (Suzhou, China). Animals were fed ad libitum water and their corresponding diet for up to 16 wk. At the end of the study, two rats from each group were chosen randomly for the identification of hepatic steatosis. All rats were anesthetized and killed. Their fasting plasma samples were kept at -70°C, and the liver samples were thoroughly rinsed with ice-cold saline and frozen in liquid nitrogen and then stored at -70°C. The study was approved by the Animal Ethics Committee and all procedures complied with international standards of humane care in animal experimentation.
Fasting plasma glucose (GLU), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using a 7020 biochemical analyzer (Hitachi, Japan). The hepatic TC and TG were also determined in the same manner. The biochemical data for the control and HF rats are listed in Table 1.
| Parameter | HF (n = 9) | Control (n = 7) | P |
| Body weight (g) | 560.76 ± 5.59 | 482.37 ± 10.19 | < 0.001 |
| Liver | |||
| TC (mg/dL ) | 0.42 ± 0.04 | 0.46 ± 0.02 | |
| TG (mg/dL) | 0.89 ± 0.07 | 0.64 ± 0.07 | 0.023 |
| Plasma | |||
| GLU (mg/dL) | 9.18 ± 0.32 | 7.84 ± 0.15 | 0.004 |
| TG (mg/dL) | 0.98 ± 0.12 | 0.36 ± 0.16 | < 0.001 |
| TC (mg/dL) | 1.72 ± 0.11 | 1.39 ± 0.11 | |
| LDL-C (mg/dL) | 0.35 ± 0.03 | 0.18 ± 0.03 | 0.001 |
| HDL-C (mg/dL) | 0.96 ± 0.06 | 0.90 ± 0.05 | |
| ALT (U/L) | 58.11 ± 4.09 | 43.71 ± 3.17 | 0.019 |
| AST (U/L) | 70.22 ± 2.31 | 70.86 ± 4.76 |
Fragments of liver tissues were fixed in 10% formalin, embedded in paraffin, and sectioned (5 mm in thickness). The sections were mounted on the charged microscopic slides (Okando, USA). Hematoxylin-eosin (HE) staining was performed on all slides. The slides were then examined and pictured using a DM4000B flurescent microscopy (Leica, Switzerland).
The purification of iPF2α-III from the liver and plasma were performed as described[1819] with modifications. Briefly, the tissue sample (50-100 mg) was homogenized in 4 ml ice-cold chloroform:methanol (2:1, v/v). For plasma, the aliquots (500 &mgr;L) were transferred to Eppendorf tubes. The internal standard iPF2α-III-d4 (50 ng for liver and 5 ng for plasma) was added. For the liver sample, the homogenate was mixed at 4°C for 1 h, 1.6 mL of 0.9% NaCl was added to the sample followed by centrifuging at 2000 ×g for 10 min. The residual was dried under nitrogen. One mol/L KOH (1 mL for the liver residual and 500 &mgr;L for the plasma) was added to the tubes containing the samples. Both liver and plasma samples were hydrolyzed at 40°C for 45 min. One mol/L HCl (1 mL for the liver sample and 500 &mgr;L for the plasma sample) and 1 mL of 10 mmol/L formate buffer (pH 3.0) were then added. The sample was centrifuged at 12 000 ×g for 20 min; the supernatant was removed and applied to an Oasis HLB extraction cartridge[19]. The extraction steps were programmed into an ASPEC XL SPE System (Gilson S.A.S., France) and run automatically.
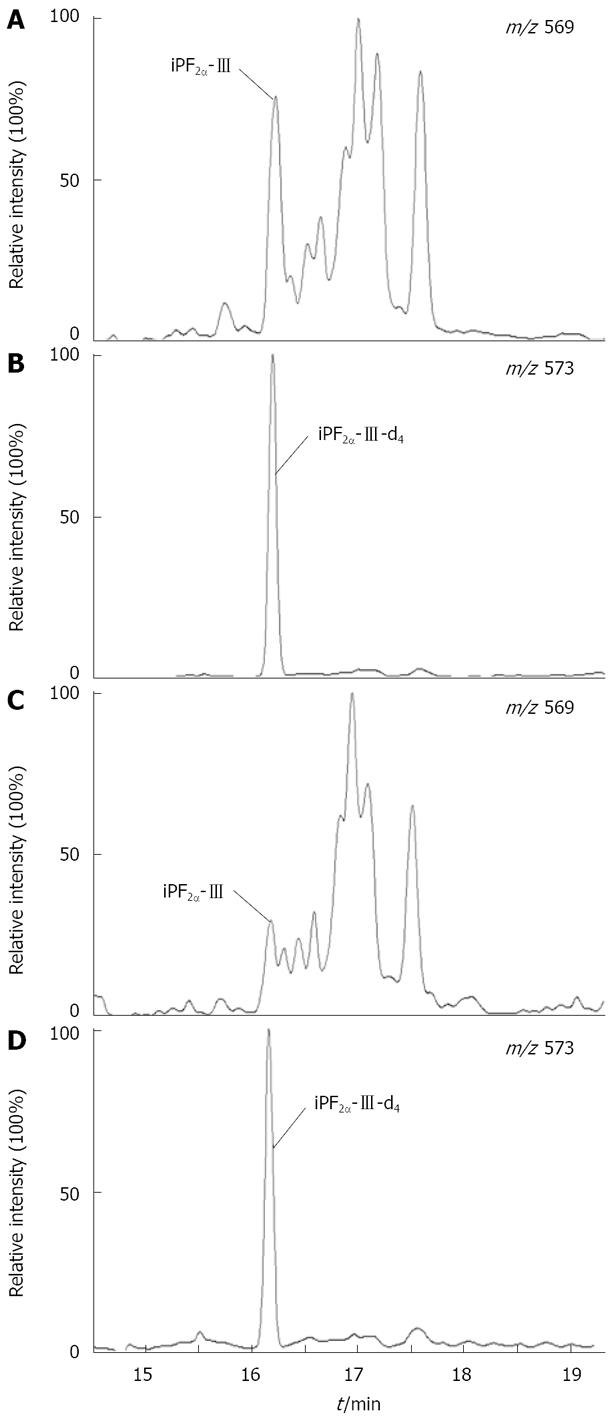
The iPF2α-III extracts were analyzed using gas chromatography-negative ion chemical ionization-mass spectrometry (GC-NICI-MS) as described[19]. After derivatizations with pentafluorobenzyl bromide (PFB) and N,O-bis-(trimethylsilyl)trifluoroacetamide (BSTFA), the PFB-TMS derivatives of iPF2α-III were analyzed on a TRACE DSQ gas chromatograph-mass spectrometer (ThermoFisher Scientific, USA). Selected ion monitoring (SIM) was performed to monitor the carboxylate anion (M-181) at m/z 569 for the iPF2α-III and m/z 573 for iPF2α-III-d4. The representative GC-NICI-MS chromatograms of hepatic and plasma F2-isoprostanes are shown in Figure 1.
Liver and erythrocyte SOD activities were measured with a SOD Assay Kit-WST (Dojindo Laboratories, Japan), and liver and blood CAT activities and the protein levels of the samples were determined using the Catalase Assay Kit and the BCA Protein Assay Kit (Beyotime Institute of Biotechnology, Jiangsu, China), using the protocols provided by the manufacturers, respectively.
Data were expressed as the mean ± SE. The differences between the mean values of two groups were determined by paired t test. Associations between the different variables were examined by Pearson correlation analysis. Statistical significance was set at P < 0.05.
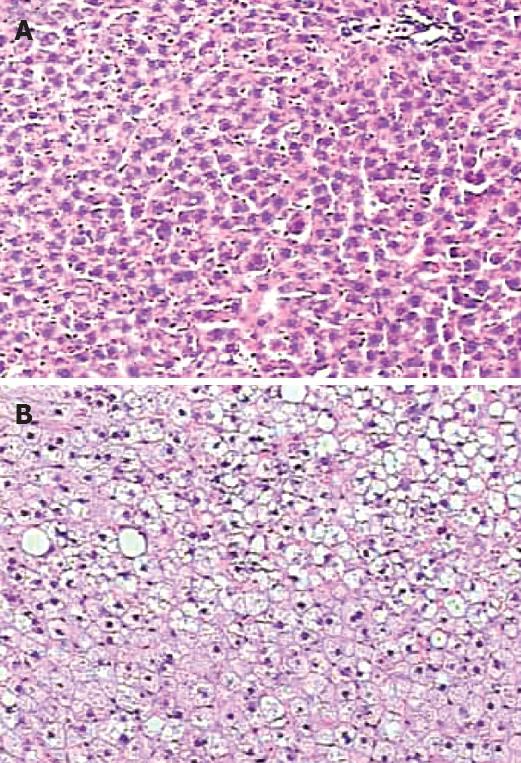
Prolonged feeding with the high-fat diet led to significant increases in body weight (P < 0.001) and fasting plasma glucose levels (P < 0.005) in HF rats compared to the control rats. Plasma LDL-C (P = 0.001) and TG (P < 0.001) were significantly higher in HF rats than those in the controls, whereas no difference in TC and HDL-C levels was found between the groups. Notably, feeding with high-fat diet increased the hepatic TG levels (P = 0.023), and typically resulted in steatosis in approximately 40% of hepatic lobules in the liver section examined (Figure 2). The hepatic steatosis was further supported by a significant increase in plasma ALT in HF rats (P = 0.019) (Table 1). No development of NASH was found in the rats.
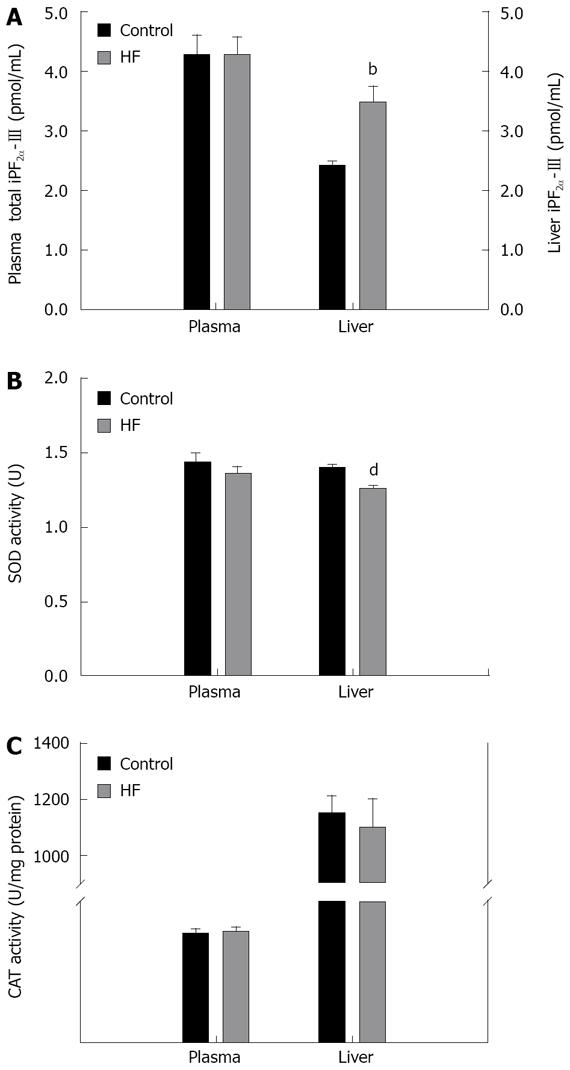
Hepatic and plasma total iPF2α-III levels and the activities of antioxidant enzymes were examined (Figure 3). While no significant difference appeared in circulating total iPF2α-III, an elevated hepatic iPF2α-III was observed in HF rats as compared to the control rats (mean 3.47 pmol/mg vs 2.40 pmol/mg tissue, P = 0.004) (Figure 3). Analysis of the endogenous antioxidant capacity revealed that the SOD activities in the liver, but not in circulation, were significantly reduced in the rats with steatosis as compared with that in the control (mean 1.26 U vs 1.40 U, P < 0.001) (Figure 3). Similar changes were also found for the liver CAT activities, however, the difference did not reach statistical significance.
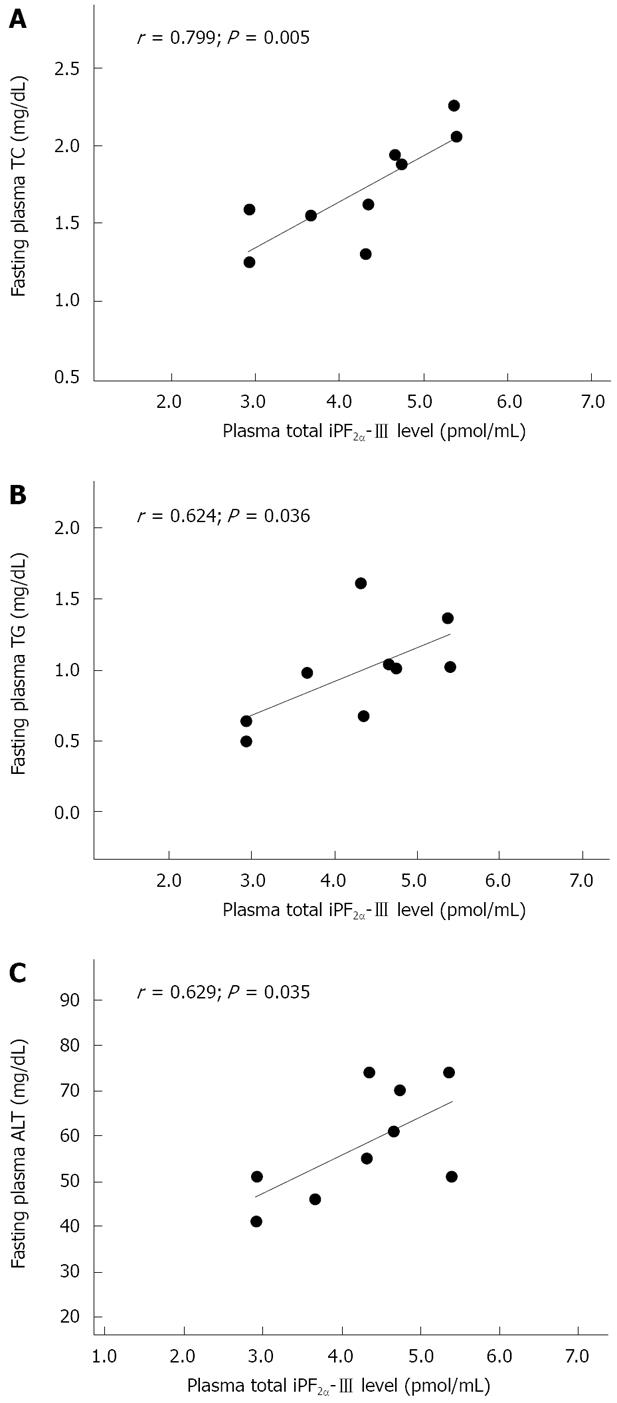
Pearson correlation analysis was performed between iPF2α-III and the metabolic risk factors, e.g. the abnormal lipid status, in HF rats. There were no significant associations of plasma total iPF2α-III with either erythrocyte SOD and blood CAT activities or fasting plasma glucose among HF rats. The significant associations were found between the plasma iPF2α-III concentrations and the plasma TC (r = 0.799, P = 0.005) as well as TG (r = 0.624, P = 0.036) in the HF rats (Figure 4A and B). A similar correlation but without significance was also found in the plasma LDL-C levels (r = 0.578, P = 0.052). Furthermore, there was a strong relationship between the iPF2α-III levels and the plasma ALT concentrations (r = 0.629, P = 0.035) (Figure 4C). In the liver, however, there were no relationships of iPF2α-III with either TC and TG or SOD and CAT in HF rats.
In the present study, a Sprague-Dawley rat model with simple steatosis was established by feeding the rats for up to 16 wk with a high-fat diet. The purpose of using such dietary regimen in the normal rats was, by resembling a common fat-rich diet in humans (60% kcal of fat) without toxin ingestion and alimentary deficiency, to naturally reproduce typical features of metabolic abnormalities at the simple steatotic stage of NAFLD seen in humans[2021]. As shown in Table 1, hepatic TG in the HF rats were elevated significantly, indicating an occurrence of lipotoxicity due to the imbalance between TG synthesis and degradation in the liver. The enhanced plasma ALT activity also suggested the lipotoxicity-induced impairment in the liver function. A recent cross-sectional and observational study in 257 Italians also showed that elevation of TG and ALT levels is the most predictive condition for hepatic steatosis[22]. In the present study, the presence of hepatic steatosis in HF rats was further confirmed histologically, showing mild to moderate macrovesicular fat accumulation in the liver samples observed (Figure 2). Despite of being multifactorial in nature in the onset of steatosis[23], these data clearly demonstrate that prolonged treatment with the high-fat diet can successfully cause liver steatosis in the experimental rats. Our results are supportive to the previous publications[2024]. Given that NAFLD is an important hepatic manifestation of metabolic syndrome[25], as expected, the steatotic rats in the present study were also featured with a spectrum of metabolic risk factors (Table 1), suggesting a close association of hepatic steatosis with obesity and insulin resistance, as commonly seen in humans[26].
With the rat model established, we investigated the oxidative imbalance status specifically at the earlier steatotic stage of NAFLD. The hepatic and blood iPF2α-III and enzymatic antioxidants (SOD and CAT) were measured to characterize the effects of oxidative imbalance on the underlying pathogenesis of NAFLD. The main finding is that hepatic iPF2α-III concentrations were significantly higher in HF rats than that in the control rats (P = 0.004). Associated with this marked elevation in iPF2α-III, hepatic activities of SOD (P < 0.001) and CAT (P = 0.068) of the HF rats were declined as compared to the control, though the decrease of CAT activity did not show statistical significance. In contrast, circulating levels of iPF2α-III as well as the activities of SOD and CAT were not different between two groups of rats (Figure 3). The results demonstrate that there was a local but possibly not systemic enhancement of oxidative imbalance in the rats with steatosis. It has been postulated that abnormal hepatic accumulation of TG has implication as the “first hit” in the formation of steatosis[2]. This accumulation is believed to be mediated, not exclusively, by alteration in intracellular fatty acid trafficking and the decrease in mitochondrial fatty acid β-oxidation[27]. All of these mediators can trigger oxidative imbalance by excessive production of free oxygen radicals capable of inducing lipid peroxidation of hepatocyte membranes. In this regard, while being consistent with those postulations, increased iPF2α-III in the steatotic livers observed in the present study is also compatible with the F2-isoprostanes results[17] and the MDA results[9] derived from the animals with simple steatosis. Taking together with these observations, our data also demonstrate enhanced oxidative imbalance mechanisms especially at the steatotic stage of NAFLD. The discrepancy in circulating status of oxidative stress exists between our study and others in steatotic animal models[17]. One explanation for the disagreement could be the way to induce steatosis used in the present study is different from that of Tong et al[17], possibly resulting in two distinct pathways towards different biochemical and physiological manifestations of systemic oxidative stress. In addition, in the study by Tong et al, the local as well as systemic antioxidant status was not characterized as to whether the valproic acid treatment would also parallelly lead to significant damages in antioxidative capacities of the rats with liver injury is unclear. Given that in the present study the circulating SOD and CAT activities were not changed among the experimental rats, it should make sense to postulate that the absence of increase in plasma iPF2α-III in HF rats may simply reflect an apparent systemic balance between oxidative stress and antioxidant defense existing temporally in simple steatosis before NASH develops. Indeed, as in the NASH mice fed with a choline-deficient diet, Yoshida et al[16] reported a more prominent elevation of iPF2α-III in the liver than that in plasma. Machado et al[28] also observed that there was no significant difference in plasma concentrations of 8-OHdG, a DNA oxidation marker, and 4-HNE, a toxic lipid peroxidation product, between patients with NASH and the control. These findings from both animals and human studies suggest a possibility that manifestation of oxidative imbalance in the local might precede that in circulation in the steatotic stage of NAFLD. This possibility certainly needs to be validated further.
In agreement with the present observation, there have also been increasingly concerns as to whether circulating oxidative stress status can accurately be a reflection of hepatic oxidative damage in NAFLD[29]. Despite of the absence of increased circulating iPF2α-III in HF rats, the present study found that plasma iPF2α-III concentrations were strongly correlated not only with plasma TC and TG (and LDL, but without significance) but also with plasma ALT in rats with steatosis (Figure 4), whereas such a correlation was not found in the control rats. Thus, the abnormal lipid profile could be, at least in part, important contributors to oxidation imbalance related to the high-fat induced steatosis, whereas systemic relationship of lipid peroxidation with lipid status could be an important reflection of the functional decline of steatotic liver. While it might be expected that enhanced lipid peroxidation in NAFLD would be dependent, at least partially, on some lipidamic measures, there was little published support for such a correlation to date. Konishi et al[30] found no significant associations of plasma iPF2α-III with TC and TG in patients with NAFLD. In addition, Madan et al[31] failed to find relationship between oxidant stress and ALT levels in either NAFLD patients or diseased controls. Nevertheless, the present data suggest that oxidative imbalance and lipid peroxidation play a significant role in pathogenesis of NAFLD, especially at the steatotic stage. The results may indicate a potential value of measuring circulating total iPF2α-III to assess the extent of hepatic lipotoxicity during in vivo oxidative imbalance and its relevance to the progression of simple steatosis to NASH. This might further provide the rationale for interventions to slow the steatosis in human studies.
In summary, the present study demonstrates an enhanced oxidative imbalance at the steatotic stage of NAFLD. Such oxidative imbalance was not observed in the circulation, suggesting a possibility that manifestation of the local oxidative damage precedes that of systemic oxidative imbalance. The association analysis shows predominant metabolic features of the increased lipid peroxidation and also suggests a close association of the oxidative imbalance and the dyslipidemia with functional deterioration of the steatotic liver. Although there is no confirmatory conclusion in terms of the cause-effect relationship between oxidative stress and occurrence of steatosis from the present study, the results suggest that the steatotic stage of NAFLD might be a valuable model for evaluating the roles of oxidative imbalance in pathogenesis of NAFLD and for providing clues for earlier interventions against the development of NASH in human studies.
Oxidative imbalance as an important mechanism of non-alcoholic fatty liver disease (NAFLD) has been a field of intense research but is not yet fully understood. While majority of previous researches were conducted mainly in NASH, only a few studies have focused on the effects of lipid peroxidation in simple steatosis, an earlier stage of NAFLD. As ectopic lipids accumulation in the liver is primarily linked to hepatic mitochondrial dysfunction and insulin resistance, it is reasonable to postulate that the steatotic stage of NAFLD might be of real importance in evaluating the cause-effect association of lipid peroxidation with the disease progression, thus providing clues for earlier pharmacological interventions against the development of NASH.
This study utilized the quantitative assay of F2-isoprostanes (i.e. iPF2α-III), which has been considered to be the most accurate in vivo lipid peoxidation marker, along with endogenous enzymatic antioxidants SOD and CAT to characterize hepatic as well as peripheral oxidative imbalance and its metabolic relevance in a rat model with simple steatosis.
F2-isoprostanes, also referred to as iPF2α-III, are a group of prostaglandin F2-like isomers produced by free radical-catalyzed peroxidation of arachidonic acid. Increased plasma or urinary levels of iPF2α-III have been reported both in humans and animal models with NASH. Information in the literatures with regard to iPF2α-III production related to hepatic steatosis, however, is limited. The basic findings of the study are (1) significantly higher levels of iPF2α-III and lower activities of SOD and CAT were observed in the steatotic livers as compared with the control rats, while no such oxidative imbalance was found in the circulation; and (2) the formation of iPF2α-III in the circulation was significantly correlated with the abnormalities of blood lipids as well as ALT levels in the rats with simple steatosis, whereas no similar tendencies were evident in the control rats.
The findings of the present study suggest an enhanced oxidative imbalance at the steatotic stage of NAFLD prior to the appearance of NASH, and it may further suggest a possibility that manifestation of the local oxidative damage precedes that of systemic oxidative imbalance. Furthermore, the results reveal not only a predominant metabolic feature of the increased lipid peroxidation, but also a close association of the oxidative imbalance and the dyslipidemia with functional deterioration of the steatotic liver in the rats with simple steatosis. We believe that these findings do provide supports to the postulation mentioned above.
This study performed a fine measurement of iPF-III using GC-MS to show a strong evidence for oxidative imbalance in the steatotic liver. It demonstrated in this paper that F2-isoprostanes (iPF2-III) in the liver increased when steatosis was induced in rats by treatment of high fat diet. This result together with decreased in SOD and catalase levels led to the conclusion that oxidative imbalance proceeds in early stages of NAFLD.
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. |
| 2. | Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842-845. |
| 3. | Berson A, De Beco V, Letteron P, Robin MA, Moreau C, El Kahwaji J, Verthier N, Feldmann G, Fromenty B, Pessayre D. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology. 1998;114:764-774. |
| 5. | Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol. 2001;13:777-784. |
| 6. | McClain CJ, Mokshagundam SP, Barve SS, Song Z, Hill DB, Chen T, Deaciuc I. Mechanisms of non-alcoholic steatohepatitis. Alcohol. 2004;34:67-79. |
| 7. | Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987-1000. |
| 8. | Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197-208. |
| 9. | Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200-208. |
| 10. | Oliveira CP, da Costa Gayotto LC, Tatai C, Della Bina BI, Janiszewski M, Lima ES, Abdalla DS, Lopasso FP, Laurindo FR, Laudanna AA. Oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, in rats fed with a choline-deficient diet. J Cell Mol Med. 2002;6:399-406. |
| 11. | Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515-540. |
| 12. | Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ 2nd. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA. 1990;87:9383-9387. |
| 13. | Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221-226. |
| 14. | Haukeland JW, Damas JK, Konopski Z, Loberg EM, Haaland T, Goverud I, Torjesen PA, Birkeland K, Bjoro K, Aukrust P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44:1167-1174. |
| 15. | Kojima H, Sakurai S, Uemura M, Fukui H, Morimoto H, Tamagawa Y. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2007;31:S61-S66. |
| 16. | Yoshida Y, Itoh N, Hayakawa M, Habuchi Y, Inoue R, Chen ZH, Cao J, Cynshi O, Niki E. Lipid peroxidation in mice fed a choline-deficient diet as evaluated by total hydroxyoctadecadienoic acid. Nutrition. 2006;22:303-311. |
| 17. | Tong V, Chang TK, Chen J, Abbott FS. The effect of valproic acid on hepatic and plasma levels of 15-F2t-isoprostane in rats. Free Radic Biol Med. 2003;34:1435-1446. |
| 18. | Musiek ES, Cha JK, Yin H, Zackert WE, Terry ES, Porter NA, Montine TJ, Morrow JD. Quantification of F-ring isoprostane-like compounds (F4-neuroprostanes) derived from docosahexaenoic acid in vivo in humans by a stable isotope dilution mass spectrometric assay. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;799:95-102. |
| 19. | Zhao Z, Hjelm NM, Lam CW, Ho CS. One-step solid-phase extraction procedure for F(2)-isoprostanes. Clin Chem. 2001;47:1306-1308. |
| 20. | Gauthier MS, Couturier K, Latour JG, Lavoie JM. Concurrent exercise prevents high-fat-diet-induced macrovesicular hepatic steatosis. J Appl Physiol. 2003;94:2127-2134. |
| 21. | Zou Y, Li J, Lu C, Wang J, Ge J, Huang Y, Zhang L, Wang Y. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sci. 2006;79:1100-1107. |
| 22. | Bellentani S, Saccoccio G, Masutti F, Croce LS, Brandi G, Sasso F, Cristanini G, Tiribelli C. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112-117. |
| 23. | Cave M, Deaciuc I, Mendez C, Song Z, Joshi-Barve S, Barve S, McClain C. Nonalcoholic fatty liver disease: predisposing factors and the role of nutrition. J Nutr Biochem. 2007;18:184-195. |
| 24. | Han LK, Takaku T, Li J, Kimura Y, Okuda H. Anti-obesity action of oolong tea. Int J Obes Relat Metab Disord. 1999;23:98-105. |
| 25. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. |
| 26. | Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89-104. |
| 27. | Reid AE. Nonalcoholic steatohepatitis. Gastroenterology. 2001;121:710-723. |
| 28. | Machado MV, Ravasco P, Jesus L, Marques-Vidal P, Oliveira CR, Proenca T, Baldeiras I, Camilo ME, Cortez-Pinto H. Blood oxidative stress markers in non-alcoholic steatohepatitis and how it correlates with diet. Scand J Gastroenterol. 2008;44:95-102. |
| 29. | Bonnefont-Rousselot D, Ratziu V, Giral P, Charlotte F, Beucler I, Poynard T. Blood oxidative stress markers are unreliable markers of hepatic steatosis. Aliment Pharmacol Ther. 2006;23:91-98. |
| 30. | Konishi M, Iwasa M, Araki J, Kobayashi Y, Katsuki A, Sumida Y, Nakagawa N, Kojima Y, Watanabe S, Adachi Y. Increased lipid peroxidation in patients with non-alcoholic fatty liver disease and chronic hepatitis C as measured by the plasma level of 8-isoprostane. J Gastroenterol Hepatol. 2006;21:1821-1825. |
| 31. | Madan K, Bhardwaj P, Thareja S, Gupta SD, Saraya A. Oxidant stress and antioxidant status among patients with nonalcoholic fatty liver disease (NAFLD). J Clin Gastroenterol. 2006;40:930-935. |