Published online Jun 7, 2008. doi: 10.3748/wjg.14.3403
Revised: February 25, 2008
Accepted: March 4, 2008
Published online: June 7, 2008
High soft tissue contrast resolution, acquisition of multi-planar images and the possibility to obtain functional information make magnetic resonance an interesting imaging technique to evaluate the small bowel disease. The absence of ionizing radiation is an important feature of magnetic resonance imaging (MRI) examinations because inflammatory diseases such as Crohn’s disease (CD) are studied most frequently, which are prevalent among children and young adults. MRI, using modern equipment and a rigorous technical approach, can offer detailed morphologic information and functional data on the small bowel. This article discusses the MRI protocols for small bowel and the MR imaging findings of small bowel diseases, such as CD and small bowel neoplasms.
- Citation: Zhu J, Xu JR, Gong HX, Zhou Y. Updating magnetic resonance imaging of small bowel: Imaging protocols and clinical indications. World J Gastroenterol 2008; 14(21): 3403-3409
- URL: https://www.wjgnet.com/1007-9327/full/v14/i21/3403.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3403
Magnetic resonance imaging (MRI) of the small bowel has been an unexplored field of application for years. Since 1998 the number of the publications has started to increase[1–6]. The reason was not lack of interest, but the technical inadequacy of the MR scanners to perform motion-free examinations. With the development of hardware (gradients, multi-channel coils) and software (fast and ultrafast sequences), which enabled breath-held studies, freezing voluntary (respiratory) and involuntary (peristaltic) motion artifacts, it opened the access to modern abdominal MRI.
High soft tissue contrast resolution, acquisition of multi-planar images and the possibility to obtain functional information make MR an interesting imaging technique to evaluate the small bowel disease. The absence of ionizing radiation is an important feature of MRI examinations because inflammatory diseases such as Crohn’s disease (CD) are studied most frequently, which are prevalent among children and young adults[7–9].
The major advantage of MRI, compared with conventional barium radiographic studies, is direct visualization of small bowel wall. This feature dramatically changes the image interpretation process. Radiologists must shift their attention from analysis of mucosal profile and lumen caliber to direct evaluation of bowel wall thickness and parietal inflammatory changes.
Bowel distension is a most important requisite for any method of the small bowel. A collapsed bowel loop can hide lesions or simulate pathologic wall thickening. The presence of the lesion that generates small bowel obstruction creates a natural distention of lumen and the possibility of examining the patient without any preparation[61011]. In contrast, the relative collapse of bowel loops under standard conditions has led researchers to study a variety of methods of luminal distension.
There are two main approaches for MRI of the small bowel: (1) study following oral administration of contrast material; and (2) study with distension of lumen obtained with contrast material that is introduced through a naso-jejunal tube (MR enteroclysis).
Oral contrast agents can be classified into positive, negative and biphasic categories according to their action on the signal intensity of bowel lumen.
A positive agent is a paramagnetic substance that produces a high signal intensity on T1-weighted sequences. It reduces T1 relaxation time without, or only minimally, influencing T2 relaxation time. Because of the water content of the contrast solution, it also results in high signal intensity on T2-weighted images. Positive contrast agents include paramagnetic substances, such as gadolinium chelates, ferrous and manganic ions and manganese ions[12–16]. The use of positive oral contrast agents has been abandoned almost completely because a hyperintense lumen does not enable a clear differentiation with inflammatory parietal enhancement.
A negative agent is a substance that produces a low signal intensity on T1- and T2-weighted sequences. These substances induce local inhomogeneity in the magnetic field that affects T1 and T2 relaxation time. T2 effects predominate and are caused by spin dephasing with a consequent loss of signal intensity. Negative contrast agents include perfluorooctyl bromide[17], iron oxides[1518], and oral magnetic particles[1415]. Barium sulfate, if used at high concentrations, can be considered a negative contrast agent[19]. Negative contrast agents are more favorable if hyperintense signal of the bowel wall and the surrounding fat tissue signs of acute inflammation have to be detected on T2-weighted sequences[15]. However, magnetic susceptibility on gradient echo sequences may alter image quality on breath-held T1-weighted images.
The term “biphasic” recently was introduced to define those substances that show different signal intensities depending on different sequences[20]. The first group (hyperintense signal on T1-weighted images and hypointense signal on T2-weighted images) included manganese and substances that contain manganese, and gadolinium chelates, which can act as biphasic contrast agents if administered at high concentrations[15]. The second group (hypointense signal on T1-weighted images and hyperintense signal on T2-weighted images) included water, hyperosmolar and isosmolar watery solutions, and barium sulfate[15]. Although water is the safest and cheapest agent, it has the limitation of intestinal absorption, which compromises an adequate distension of distal ileum in many patients[21]. To obviate this problem, hyperosmolar solutions, such as mannitol-based solutions, have been used. Mannitol reduces water absorption and distends the distal ileal loops well. Major drawbacks are undesirable side effects, such as diarrhea, meteorism and abdominal cramps[21–25].
In an attempt to reduce undesirable side effects and to obtain better distension of the distal ileum, some new oral mixtures, such as Polyethylene glycol, a water solution combined with low concentration sorbitol and locus bean gum (LBG), were used as the oral contrast agents[192627]. They are all hyperosmolar. Some of them can reduce the side effect and ensure optimal intestinal distension with appropriate concentration and reasonable transit time.
MR enteroclysis (MRE) is an emerging technique for the evaluation of small intestinal diseases. Administration of an iso-osmotic water solution through a nasojejunal catheter can guarantee adequate luminal distention, and in combination with ultrafast sequences, such as single shot TSE, true FISP, HASTE and 3D FLASH, resulting in excellent anatomic demonstration of the small intestine. MR fluoroscopy can be performed during MRE examination to monitor the filling process and might be useful in studying low-grade stenosis or motility related disorders. MRE is a very promising technique for the detection and characterization of involved small bowel segments in patients with Crohn’s disease while its diagnostic performance in disclosing lumen narrowing and extramural manifestations and complications of the disease is outstanding. Initial experience shows that MRE is very efficient in the diagnosis of small bowel tumors and can be used in the evaluation of small bowel obstruction[202829].
Fast sequences that are able to acquire T1- and T2-weighted images within a single breath-hold are essential requisites for MRI evaluation of small bowel. In T2-weighted images, several studies[1562829] support the validity of single-shot sequences, including half-Fourier single-shot turbo spin-echo (HASTE) and single-shot fast spin-echo. Because these sequences, based on the half-Fourier reconstruction technique, have extremely fast acquisition time (approximately 1 second per image), they are able to freeze motion artifacts. Single-shot sequences differ from each other depending on echo time (TE). Using long TE (e.g. about 600 m) can obtain selective images of fluids with cancellation of surrounding organs (similar to magnetic resonance cholangiopancreatography). Using shorter TE (60-90 m) can obtain simultaneous evaluation of fluids, bowel wall and surrounding structures. The use of fat saturation pulses is a useful complement to the acquisition of T2-weighted sequences[2131429–31]. Fat saturation causes an increase in contrast between bowel wall and the surrounding fat tissue. This can help assess the bowel wall inflammation and identify the inflammatory changes in peritoneal fat tissue.
The “balanced” or “hybrid” gradient-echo sequence has been introduced in clinical practice. This sequence, known as true fast imaging with steady-state precession (true-FISP), presents with an intermediate contrast between T1- and T2-weighted images[2931–33]. Shorter repetition times (TR) are used (< 3 m) and the acquisition time is short. The true FISP sequence provides motion-free, high-resolution images similar to T2-weighted images of the intestine, mesentery and vasculature in 1.5 s. However, this sequence is prone to susceptibility artifacts from intraluminal air and from “black boundary” artifact due to the chemical shift phenomenon, which may obscure the subtle bowel wall thickening. The black boundary artifact can be eliminated with use of fat suppression.
T1-weighted images are obtained with fast spoiled-gradient-echo sequences, using 2-D of 3-D acquisition. The acquisition time ranges from 15 to 20 seconds. T1-weighted sequences generally are used following intravenous injection of contrast material to evaluate enhancement, a useful parameter to assess disease activity, especially the inflammatory activity. T1-weighted images also benefit from the use of fat saturation. The gadolinium-enhanced fat-suppressed spoiled gradient-echo sequence provides T1-weighted images with excellent visualization of the enhanced bowel wall, which contrasts well with the low-signal-intensity mesenteric fat and negative intraluminal contrast material[3435].
The latest technical development to speed up the acquisition process is parallel imaging, based on simultaneous acquisition of spatial harmonics, or sensitivity encoding techniques. Marked improvement in spatial resolution can be achieved in shorter acquisition times[36]. Parallel imaging can reduce the number of phase encodings to be acquired per TR. Consequently, the spatial resolution can be increased while maintaining an acquisition time that is compatible with a single breath-hold, or the number of scans to be acquired, which allows a larger volume coverage. Alternatively, the acquisition time can be reduced drastically. The drawback in the use of parallel imaging is the reduction of signal-to-noise ratio (SNR) and the need to perform a calibration of equipment immediately before image acquisition[37].
Chronic inflammatory disease, and in particular, CD, represents the most common application of MRI of small bowel[1–5891528–3538].
With MRI, both inflammatory changes of the bowel wall and extramural complications of Crohn’s disease can be assessed. The non-invasiveness of this technique, as well as its lack of ionizing radiation, has prompted many radiologists to perform systematic studies of MRI for evaluation of Crohn’s disease.
In patients having proved suspected CD, cross-sectional images, including CT and MRI, should be analyzed specifically for the presence and character of a pathologically altered bowel segment (wall thickness, pattern of attenuation, degree of enhancement, length of involvement), stenosis and prestenotic dilatation, skip lesions, fistulas, abscess, fibrofatty proliferation, increased vascularity of the vasa recta (comb sign), mesenteric adenopathy, and other extraintestinal disease involvement.
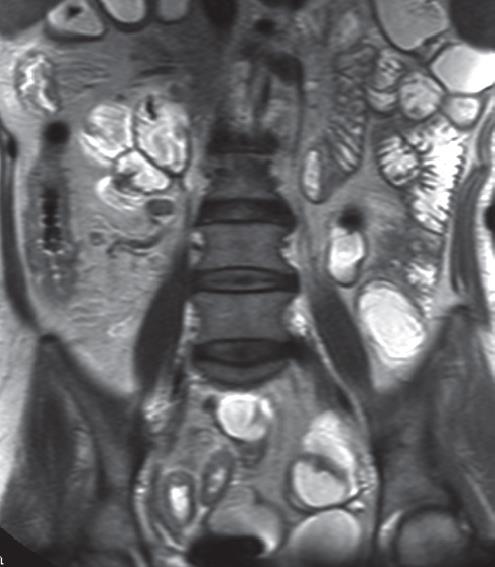
The normal small bowel wall thickness is between 1 mm and 3 mm when the lumen is well distended. Any portion of the bowel wall that exceeds 4-5 mm is considered abnormal[912171926293940]. An adequate intestinal distension is mandatory because collapsed loops or spastic intestinal segments may mimic wall thickening. Most optimal distension is obtained with MR enteroclysis with instillation of contrast medium after nasojejunal intubation under fluoroscopic guidance. Although many authors reporting on MR enteroclysis administer antiperistaltic drugs to reduce motion artifacts, reflex atony is induced by high flow rates, theoretically allowing images (almost) free of motion artifacts[4142]. Drawbacks are that this technique is uncomfortable for patients and exposes them to a considerable dose of ionizing radiation of up to 8 mSv during intubation[43]. To avoid such disadvantages, MRI has been performed by many researchers using oral contrast media. Many contrast media have been proposed, but no oral contrast medium has yet been accepted universally as optimal for use[23714–1922–26].
Small bowel wall thickening is a sensitive, but not pathognomonic, sign of CD. It is observed in several other intestinal diseases, such as ischemic disorders and infections.
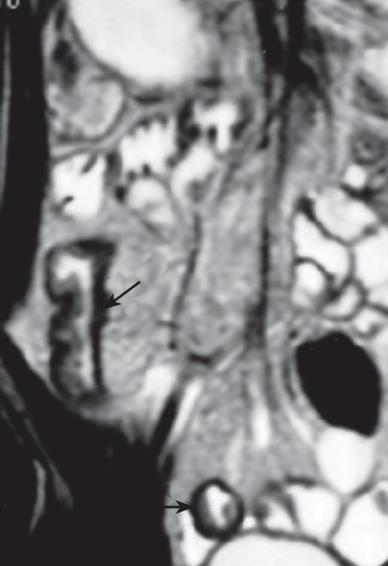
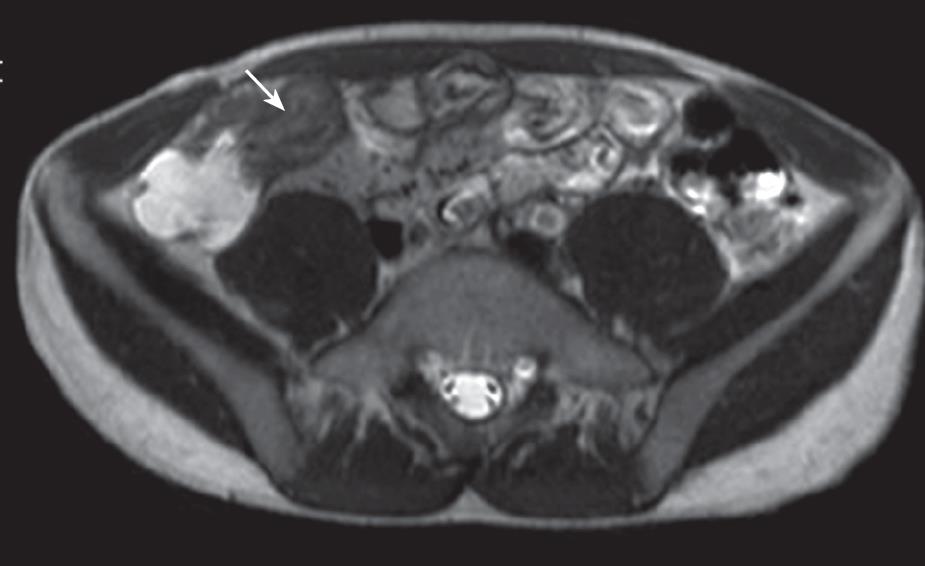
Although superficial mucosal lesions are missed easily as a result of inadequate spatial resolution, MR imaging can detect early inflammatory changes of the bowel wall, based on enhancement after intravenous injection of contrast medium. The bowel wall of the involved segment may have a homogeneous or stratified appearance at MR imaging after enhancement. The homogeneous enhancement is diffuse and transmural with no recognition of different bowel layers. The stratified appearance (so-called “target” or “double halo” appearance) is related to alternating layers of higher or lower attenuation or signal intensity. The stratified appearance also can be seen on T2-weighted imaging. “Target” or “double halo” appearance is often seen in active lesions, particularly after the intravenous administration of contrast medium, and related to submucosal edema. The intensity of enhancement correlates with the degree of inflammatory lesion activity. Inactive disease is characterized by no abnormalities or bowel wall thickening with relative low signal intensity representing fibrosis with limited, homogeneous contrast enhancement. Absence of stratification on T2-weighted images with stratified enhancement on T1-weighted images is often due to fibrosis, which is a typical long-standing CD[29333544]. This sign (stratified enhancement) also can be seen on MSCT (multi-slice CT) images[4546].
Increased vascularity of the vasa recta (comb sign) is a sign of active inflammation. It arises from the combination of vascular engorgement of vasa recta and fibro-fatty proliferation and is demonstrated as multiple tubular, tortuous opacities on mesenteric side of ileum, aligned as the teeth of a comb[29]. “Comb sign” is frequently seen on enhanced MSCT images[4647]. Abscess and phlegmon can occur in the small bowel mesentery, abdominal wall, or psoas muscle or around the anus. Abscesses and phlegmon are well demonstrated at fat-saturated T2-weighted MR imaging and can be distinguished reliably, which aids in management planning. Fistulas and sinus tracts are also depicted, however, the reported sensitivity of MR imaging for depicting sinus tracts is 50%-75% when a conventional enteroclysis study is used as a reference[323945]. Mesenteric lymphadenopathy ranging from 3 to 8 mm in size is depicted at MR imaging with a true fast imaging with steady state precession (FISP) or T2-weighted turbo spin-echo sequence[3241]. If these sequences are not available, axial T1- or T2-weighted spin-echo imaging should be added. When lymph nodes are larger than 10 mm, lymphoma and carcinoma must be excluded.
Imaging techniques form a very important part of the evaluation of CD. However, several clinical scoring systems have been developed as well to assess disease activity and response to therapy, especially in trials. The Crohn’s disease activity index (CDAI) is currently the gold standard for clinical evaluation of disease activity[4748]. This index is relatively subjective since an important part of the total score is derived from items that reflect the patient’s perception of disease (general well being and "intensity of abdominal pain"). However, in many studies, this index has been used as gold standard for disease activity since it is a validated and extensively used clinical index. Scores ranging from 0 to approximately 600 with values below 150 are considered as remission and values over 150 as active disease. MR imaging was used to evaluate disease activity[930–353841444849]. Based on different experiences, contrast-enhanced(CE) fat-suppressed T1-weighted images offer the best correlation between MR findings and CDAI, although a correlation that used fat-suppressed T2-weighted images is also demonstrated. MRI can clearly distinct pathologic from normal bowel wall in CD, as it detects significant variations in bowel wall thickness with clinical improvement and is able to reflect pathologic inflammatory changes at the bowel wall based on variations in the CE. In most patients with active disease, abnormal bowel identified on MR imaging was isointense or slightly hypointense to the psoas muscles on T1-weighted imaging. On T2-weighted imaging, the abnormal bowel segments were usually isointense or slightly hyperintense compared with the psoas muscle. MR imaging can correctly identify active disease, the enhancement pattern of abnormal bowel is diffuse and layered. The layered pattern is seen only in patients with active disease. Consequently, this technique is reliably applicable to the follow-up of patients with CD. MRI is able to detect significant variations in bowel wall thickness and contrast enhancement (CE), reflecting favorable clinical response to medical treatment of CD’s relapse[303449].
Benign and malignant small intestinal tumors are uncommon. Adenomas, leiomyomas and lipomas constitute the three most common primary benign small intestinal tumors[50]. In general, benign tumors occur less commonly in the duodenum and increase in frequency in the ileum. The term “polyp” is a clinical term for any tumorous mass that projects above the surrounding normal mucosa. Hamartomatous, hyperplastic and inflammatory polyps are benign, non-neoplastic lesions and adenomatous polyps are true neoplastic tumors containing dysplastic epithelium and are precursors of carcinoma. Polyps are infrequently symptomatic and are usually incidental findings at autopsy. Current convention is that leiomyomas should be classified as gastrointestinal stromal tumors (GIST), and benignancy can never be determined with absolute certainty. Small bowel GIST accounts for 25% of these tumors. As in the stomach, these may be large and ulcerating.
Adenocarcinomas account for 50% of all small bowel malignancies, but only account for less than 1% of all gastrointestinal malignancies[51]. The most common site for small bowel adenocarcinoma is the duodenum. This tumor frequently occurs in close proximity to the ampulla and as a result may cause obstructive jaundice[52]. Adenocarcinoma and metastases can be seen rarely in the jejunum.
Most primary gastrointestinal non-Hodgkin lymphomas are of B-cell type, and appear to arise from B cells of mucosa-associated lymphoid tissue (MALT). In the small intestine, the terminal ileum is the most common site affected, which may reflect the relatively greater amount of lymphoid tissue present in this segment compared with the duodenum and jejunum.
Carcinoids are the most common primary neoplasm of the small bowel. They are well-differentiated neuroendocrine neoplasms that occur primarily in the distal ileum. Men and women are affected with equal frequency. Most patients present with tumor-related symptoms of bleeding and bowel obstruction or intussusception. Ileal carcinoids are regional mesenteric metastases and vascular sclerosis. The primary tumor may be quite small with the accompanying lymphadenopathy and desmoplastic reaction in the root of the mesentery presenting as the only visible manifestation of disease. Liver metastases are responsible for the “carcinoid syndrome”, which is characterized by vasomotor instability, intestinal hypomotility and bronchoconstriction[53].
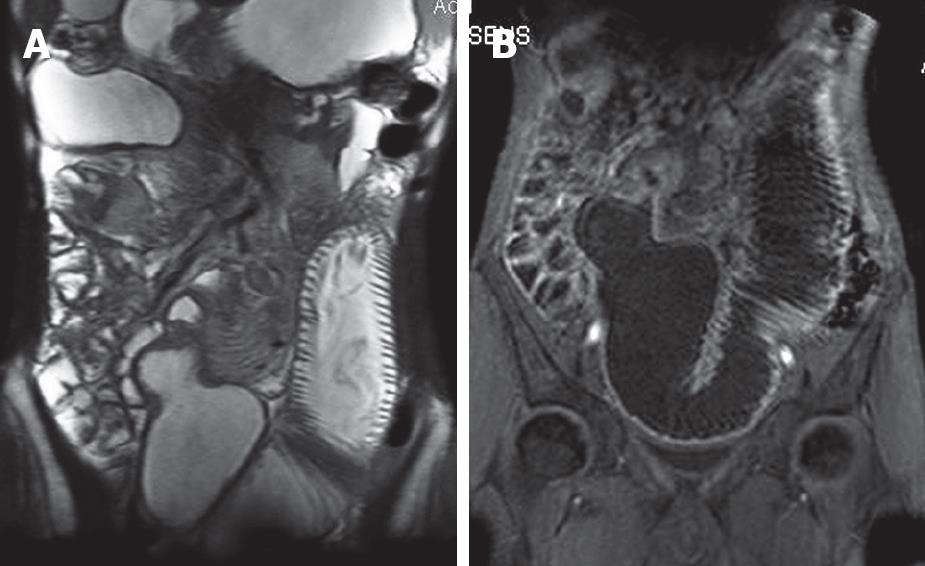
Tumors had similar signal intensity to normal small bowel on precontrast images. Tumors showed heterogeneous enhancement greater than adjacent bowel on gadolinium-enhanced images. Tumor local extent was best shown on precontrast-spoiled gradient-echo images and postgadolinium T1-weighted fat-suppressed images. Image quality was most consistent on breath-hold images. Precontrast breath-hold T1-weighted spoiled gradient-echo images and gadolinium-enhanced fat suppressed images demonstrate tumor extent most reliably. The accuracy of the technique in cases of non-occlusive tumors of the lumen is not known, given the lack of large case series[2854].
MR imaging, using modern equipment and a rigorous technical approach, can offer detailed morphologic information and functional data on the small bowel. The optimal study technique is debatable, although the oral administration of contrast material as a first-line approach is less expensive, faster, easier to perform and better tolerated by patients. MR enteroclysis might be reserved for selected cases as a second-line study.
The major clinical indication is the evaluation of patients who have suspected or known CD. The absence of ionizing radiation, in view of the young age of most of the patients and the frequency of the examinations, is an important advantage over other techniques (radiography and CT enteroclysis).
| 1. | Ernst O, Asselah T, Cablan X, Sergent G. Breath-hold fast spin-echo MR imaging of Crohn's disease. AJR Am J Roentgenol. 1998;170:127-128. |
| 2. | Rieber A, Wruk D, Nussle K, Aschoff AJ, Reinshagen M, Adler G, Brambs HJ, Tomczak R. [MRI of the abdomen combined with enteroclysis in Crohn disease using oral and intravenous Gd-DTPA]. Radiologe. 1998;38:23-28. |
| 3. | Holzknecht N, Helmberger T, von Ritter C, Gauger J, Faber S, Reiser M. [MRI of the small intestine with rapid MRI sequences in Crohn disease after enteroclysis with oral iron particles]. Radiologe. 1998;38:29-36. |
| 4. | Ha HK, Lee EH, Lim CH, Shin YM, Jeong YK, Yoon KH, Lee MG, Min YI, Auh YH. Application of MRI for small intestinal diseases. J Magn Reson Imaging. 1998;8:375-383. |
| 5. | Lee JK, Marcos HB, Semelka RC. MR imaging of the small bowel using the HASTE sequence. AJR Am J Roentgenol. 1998;170:1457-1463. |
| 6. | Regan F, Beall DP, Bohlman ME, Khazan R, Sufi A, Schaefer DC. Fast MR imaging and the detection of small-bowel obstruction. AJR Am J Roentgenol. 1998;170:1465-1469. |
| 7. | Adamek HE, Breer H, Karschkes T, Albert J, Riemann JF. Magnetic resonance imaging in gastroenterology: time to say good-bye to all that endoscopy? Endoscopy. 2000;32:406-410. |
| 8. | Wong SH, Wong VW, Sung JJ. Virtual colonoscopy-induced perforation in a patient with Crohn’s disease. World J Gastroenterol. 2007;13:978-979. |
| 9. | Saibeni S, Rondonotti E, Iozzelli A, Spina L, Tontini GE, Cavallaro F, Ciscato C, de Franchis R, Sardanelli F, Vecchi M. Imaging of the small bowel in Crohn’s disease: a review of old and new techniques. World J Gastroenterol. 2007;13:3279-3287. |
| 10. | Chou CK, Liu GC, Chen LT, Jaw TS. The use of MRI in bowel obstruction. Abdom Imaging. 1993;18:131-135. |
| 11. | Beall DP, Fortman BJ, Lawler BC, Regan F. Imaging bowel obstruction: a comparison between fast magnetic resonance imaging and helical computed tomography. Clin Radiol. 2002;57:719-724. |
| 12. | Hirohashi S, Uchida H, Yoshikawa K, Fujita N, Ohtomo K, Yuasa Y, Kawamura Y, Matsui O. Large scale clinical evaluation of bowel contrast agent containing ferric ammonium citrate in MRI. Magn Reson Imaging. 1994;12:837-846. |
| 13. | Rieber A, Nussle K, Reinshagen M, Brambs HJ, Gabelmann A. MRI of the abdomen with positive oral contrast agents for the diagnosis of inflammatory small bowel disease. Abdom Imaging. 2002;27:394-399. |
| 14. | Vlahos L, Gouliamos A, Athanasopoulou A, Kotoulas G, Claus W, Hatziioannou A, Kalovidouris A, Papavasiliou C. A comparative study between Gd-DTPA and oral magnetic particles (OMP) as gastrointestinal (GI) contrast agents for MRI of the abdomen. Magn Reson Imaging. 1994;12:719-726. |
| 15. | Rieber A, Aschoff A, Nussle K, Wruk D, Tomczak R, Reinshagen M, Adler G, Brambs HJ. MRI in the diagnosis of small bowel disease: use of positive and negative oral contrast media in combination with enteroclysis. Eur Radiol. 2000;10:1377-1382. |
| 16. | Hiraishi K, Narabayashi I, Fujita O, Yamamoto K, Sagami A, Hisada Y, Saika Y, Adachi I, Hasegawa H. Blueberry juice: preliminary evaluation as an oral contrast agent in gastrointestinal MR imaging. Radiology. 1995;194:119-123. |
| 17. | Brown JJ, Duncan JR, Heiken JP, Balfe DM, Corr AP, Mirowitz SA, Eilenberg SS, Lee JK. Perfluoroctylbromide as a gastrointestinal contrast agent for MR imaging: use with and without glucagon. Radiology. 1991;181:455-460. |
| 18. | Johnson WK, Stoupis C, Torres GM, Rosenberg EB, Ros PR. Superparamagnetic iron oxide (SPIO) as an oral contrast agent in gastrointestinal (GI) magnetic resonance imaging (MRI): comparison with state-of-the-art computed tomography (CT). Magn Reson Imaging. 1996;14:43-49. |
| 19. | Ajaj W, Goyen M, Schneemann H, Kuehle C, Nuefer M, Ruehm SG, Goehde SC, Lauenstein TC. Oral contrast agents for small bowel distension in MRI: influence of the osmolarity for small bowel distention. Eur Radiol. 2005;15:1400-1406. |
| 20. | Maglinte DD, Siegelman ES, Kelvin FM. MR enteroclysis: the future of small-bowel imaging? Radiology. 2000;215:639-641. |
| 21. | Lomas DJ, Graves MJ. Small bowel MRI using water as a contrast medium. Br J Radiol. 1999;72:994-997. |
| 22. | Grubnic S, Padhani AR, Revell PB, Husband JE. Comparative efficacy of and sequence choice for two oral contrast agents used during MR imaging. AJR Am J Roentgenol. 1999;173:173-178. |
| 23. | Ajaj W, Goehde SC, Schneemann H, Ruehm SG, Debatin JF, Lauenstein TC. Dose optimization of mannitol solution for small bowel distension in MRI. J Magn Reson Imaging. 2004;20:648-653. |
| 24. | Kuehle CA, Ajaj W, Ladd SC, Massing S, Barkhausen J, Lauenstein TC. Hydro-MRI of the small bowel: effect of contrast volume, timing of contrast administration, and data acquisition on bowel distention. AJR Am J Roentgenol. 2006;187:W375-W385. |
| 25. | Lauenstein TC, Schneemann H, Vogt FM, Herborn CU, Ruhm SG, Debatin JF. Optimization of oral contrast agents for MR imaging of the small bowel. Radiology. 2003;228:279-283. |
| 26. | Ajaj W, Goehde SC, Schneemann H, Ruehm SG, Debatin JF, Lauenstein TC. Oral contrast agents for small bowel MRI: comparison of different additives to optimize bowel distension. Eur Radiol. 2004;14:458-464. |
| 27. | McKenna DA, Roche CJ, Murphy JM, McCarthy PA. Polyethylene glycol solution as an oral contrast agent for MRI of the small bowel in a patient population. Clin Radiol. 2006;61:966-970. |
| 28. | Umschaden HW, Szolar D, Gasser J, Umschaden M, Haselbach H. Small-bowel disease: comparison of MR enteroclysis images with conventional enteroclysis and surgical findings. Radiology. 2000;215:717-725. |
| 29. | Wiarda BM, Kuipers EJ, Heitbrink MA, van Oijen A, Stoker J. MR Enteroclysis of inflammatory small-bowel diseases. AJR Am J Roentgenol. 2006;187:522-531. |
| 30. | Low RN, Sebrechts CP, Politoske DA, Bennett MT, Flores S, Snyder RJ, Pressman JH. Crohn disease with endoscopic correlation: single-shot fast spin-echo and gadolinium-enhanced fat-suppressed spoiled gradient-echo MR imaging. Radiology. 2002;222:652-660. |
| 31. | Albert JG, Martiny F, Krummenerl A, Stock K, Lesske J, Gobel CM, Lotterer E, Nietsch HH, Behrmann C, Fleig WE. Diagnosis of small bowel Crohn's disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005;54:1721-1727. |
| 32. | Prassopoulos P, Papanikolaou N, Grammatikakis J, Rousomoustakaki M, Maris T, Gourtsoyiannis N. MR enteroclysis imaging of Crohn disease. Radiographics. 2001;21 Spec No:S161-S172. |
| 33. | Furukawa A, Saotome T, Yamasaki M, Maeda K, Nitta N, Takahashi M, Tsujikawa T, Fujiyama Y, Murata K, Sakamoto T. Cross-sectional imaging in Crohn disease. Radiographics. 2004;24:689-702. |
| 34. | Maccioni F, Bruni A, Viscido A, Colaiacomo MC, Cocco A, Montesani C, Caprilli R, Marini M. MR imaging in patients with Crohn disease: value of T2- versus T1-weighted gadolinium-enhanced MR sequences with use of an oral superparamagnetic contrast agent. Radiology. 2006;238:517-530. |
| 35. | Florie J, Wasser MN, Arts-Cieslik K, Akkerman EM, Siersema PD, Stoker J. Dynamic contrast-enhanced MRI of the bowel wall for assessment of disease activity in Crohn‘s disease. AJR Am J Roentgenol. 2006;186:1384-1392. |
| 36. | Zhang J, Israel GM, Hecht EM, Krinsky GA, Babb JS, Lee VS. Isotropic 3D T2-weighted MR cholangiopancreatography with parallel imaging: feasibility study. AJR Am J Roentgenol. 2006;187:1564-1570. |
| 37. | Lomas DJ. Techniques for magnetic resonance imaging of the bowel. Top Magn Reson Imaging. 2002;13:379-387. |
| 38. | Holzknecht N, Helmberger T, Herrmann K, Ochsenkuhn T, Goke B, Reiser M. [MRI in Crohn's disease after transduodenal contrast administration using negative oral MRI contrast media]. Radiologe. 2003;43:43-50. |
| 39. | Gourtsoyiannis N, Papanikolaou N, Grammatikakis J, Prassopoulos P. MR enteroclysis: technical considerations and clinical applications. Eur Radiol. 2002;12:2651-2658. |
| 40. | Laghi A, Carbone I, Catalano C, Iannaccone R, Paolantonio P, Baeli I, Trenna S, Passariello R. Polyethylene glycol solution as an oral contrast agent for MR imaging of the small bowel. AJR Am J Roentgenol. 2001;177:1333-1334. |
| 41. | Rieber A, Wruk D, Potthast S, Nussle K, Reinshagen M, Adler G, Brambs HJ. Diagnostic imaging in Crohn’s disease: comparison of magnetic resonance imaging and conventional imaging methods. Int J Colorectal Dis. 2000;15:176-181. |
| 42. | Schmidt S, Lepori D, Meuwly JY, Duvoisin B, Meuli R, Michetti P, Felley C, Schnyder P, van Melle G, Denys A. Prospective comparison of MR enteroclysis with multidetector spiral-CT enteroclysis: interobserver agreement and sensitivity by means of “sign-by-sign” correlation. Eur Radiol. 2003;13:1303-1311. |
| 43. | Thoeni RF, Gould RG. Enteroclysis and small bowel series: comparison of radiation dose and examination time. Radiology. 1991;178:659-662. |
| 44. | Frokjaer JB, Larsen E, Steffensen E, Nielsen AH, Drewes AM. Magnetic resonance imaging of the small bowel in Crohn’s disease. Scand J Gastroenterol. 2005;40:832-842. |
| 45. | Macari M, Megibow AJ, Balthazar EJ. A pattern approach to the abnormal small bowel: observations at MDCT and CT enterography. AJR Am J Roentgenol. 2007;188:1344-1355. |
| 46. | Colombel JF, Solem CA, Sandborn WJ, Booya F, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Bodily KD, Fletcher JG. Quantitative measurement and visual assessment of ileal Crohn’s disease activity by computed tomography enterography: correlation with endoscopic severity and C reactive protein. Gut. 2006;55:1561-1567. |
| 47. | Wiarda BM, Kuipers EJ, Houdijk LP, Tuynman HA. MR enteroclysis: imaging technique of choice in diagnosis of small bowel diseases. Dig Dis Sci. 2005;50:1036-1040. |
| 48. | Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Lofberg R, Modigliani R, Present DH, Rutgeerts P, Scholmerich J, Stange EF. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn’s disease. Gastroenterology. 2002;122:512-530. |
| 49. | Sempere GA, Martinez Sanjuan V, Medina Chulia E, Benages A, Tome Toyosato A, Canelles P, Bulto A, Quiles F, Puchades I, Cuquerella J. MRI evaluation of inflammatory activity in Crohn‘s disease. AJR Am J Roentgenol. 2005;184:1829-1835. |
| 50. | Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Listrom MB, Rilke FO. Gastrointestinal Pathology. Philadelphia: Lippincott 1999; 154. |
| 51. | Trenkner SW, Halvorsen RA Jr, Thompson WM. Neoplasms of the upper gastrointestinal tract. Radiol Clin North Am. 1994;32:15-24. |
| 52. | Teplick SK, Glick SN, Keller MS. The duodenum. Textbook of Diagnostic Imaging. Philadelphia: Saunders 1988; 808-846. |
| 53. | Rubesin SE, Gilchrist AM, Bronner M, Saul SH, Herlinger H, Grumbach K, Levine MS, Laufer I. Non-Hodgkin lymphoma of the small intestine. Radiographics. 1990;10:985-998. |
| 54. | Semelka RC, John G, Kelekis NL, Burdeny DA, Ascher SM. Small bowel neoplastic disease: demonstration by MRI. J Magn Reson Imaging. 1996;6:855-860. |