Published online Jan 14, 2008. doi: 10.3748/wjg.14.224
Revised: October 26, 2007
Published online: January 14, 2008
AIM: To investigate the killing efficiency of a recombinant plasmid containing a thymidine kinase (TK) domain insert driven by the vascular endothelial growth factor receptor 2 (VEGFR2) promoter (KDR) on vascular endothelial cells.
METHODS: The KDR-TK fragment was extracted from pBluescript II KDR-TK plasmid by enzymatic digestion with XhoI and SalI. The enhanced green fluorescence protein (EGFP) carrier was extracted from pEGFP by the same procedure. The KDR-TK was inserted into the pEGFP carrier to construct pEGFP-KDR-TK. Using ultrasound irradiation and microbubble, pEGFP-KDR-TK was transferred into human umbilical vein endothelial cells (HUVECs). The transient infection rate was estimated by green fluorescent protein (GFP) expression. Transfected HUVECs, non-transfected HUVECs, and HepG2 cells were cultured in the presence of different concentrations of ganciclovir (GCV), and the killing efficacy of HSV-TK/GCV was analyzed by 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) assay.
RESULTS: The recombinant pEGFP-KDR-TK was successfully constructed by inserting the KDR-TK fragment into the pEGFP carrier. Transfected HUVECs showed cytoplasmic green fluorescence, and the transient transfection rate was about 20.3%. Pools of G418-resistant cells exhibited a higher sensitivity to the prodrug/GCV compared to non-transfected HUVECs or non-transfected HepG2 cells, respectively.
CONCLUSION: KDR promoter and the suicide gene/prodrug system mediated by diagnostic ultrasound combined with microbubble can significantly kill HUVECs. Such therapy may present a novel and attractive approach to target gene therapy on tumor vessels.
- Citation: Wang Y, Xu HX, Lu MD, Tang Q. Expression of thymidine kinase mediated by a novel non-viral delivery system under the control of vascular endothelial growth factor receptor 2 promoter selectively kills human umbilical vein endothelial cells. World J Gastroenterol 2008; 14(2): 224-230
- URL: https://www.wjgnet.com/1007-9327/full/v14/i2/224.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.224
Angiogenesis coincides with increased tumor cell entry into the blood circulation and thus facilitates metastasis. Previous reports revealed that targeting angiogenesis represents a new strategy for antitumor therapy[1].
Vascular endothelial growth factor-A (VEGF-A) is the major player in tumor angiogenesis, which activates two tyrosine kinase receptors VEGFR1 (Flt-1) and VEGFR2 (KDR in humans/Flk-1 in mice). KDR was found to be overexpressed on activated endothelial cells of newly formed vessels and strongly associated with invasion and metastasis in human malignant diseases[2].
Gene therapy has made significant progress in tumor therapy[3]. The effectiveness of suicide gene strategy directed against cancer cells has been shown for various tumor cell types[4]. Among several suicide gene prodrug combinations, the herpes simplex virus-thymidine kinase (HSV-TK) gene is a prototype. However, non-selective, ubiquitous expression of suicide genes may cause unexpected adverse effects such as bone marrow suppression[56]. Thus, prerequisite for achieving effective and safe gene therapy against cancer is the induction of a strong, tumor vascular-selective expression of suicide genes. The success of gene therapy largely depends on the development of a gene delivery vector that is safe, easy-performed, and efficient. Ultrasound exposure in combination with microbubble is a new non-viral delivery system that might improve transfection efficiency. Nie et al[7] have shown that a combination of microbubble contrast agents and ultrasound irradiation might be a beneficial and much easier way for non-viral gene therapy.
In the present study, we established a KDR promoter to drive TK gene expression in human umbilical vein endothelial cells (HUVECs). Transfection was achieved by diagnostic ultrasound combined with a microbubble contrast agent. Our purpose was to analyze the cytotoxic effect of TK/ganciclovir (GCV) under the control of the KDR promoter on HUVECs.
Primary HUVECs were isolated using the modified “irrigative digestion” technique as described by Jaffe et al[8]. Briefly, a transfusion silica gel tube was inserted into one end of new born aseptic umbilical cords after abdominal delivery from healthy pregnant women who had normal routine urinalysis and blood tests (women with any complications of pregnancy or viral infections were excluded) to flush the bloodstain remaining in the cavity repeatedly with PBS, and then 15-20 mL collagenase II (Sigma, St. Louis, MO, USA) was injected into the vein cavity and retained 10-12 min for digestion. The collection of umbilical vein was flushed with PBS and the endothelial cells were collected and centrifuged at 1000 rpm for 8 min. Cells were resuspended and seeded in human endothelial-serum free medium (SFM) (Gibco, California, USA) supplemented with 100 mL/L fetal bovine serum (FBS, Gibco), 2 ng/mL vascular endothelial cell growth factor (Sigma), 90 &mgr;g/mL heparin (Sigma), 100 U/mL penicillin, and 50 &mgr;g/mL streptomycin. Culture medium was changed after 24 h and then every three days (3 mL) till the cells became a confluent monolayer. The primary cells were allowed to grow for 3 passages (1:2 split). Viability was assessed by trypan blue dye exclusion staining (Sangong Biological Engineering technology, Shanghai, China). HUVECs were identified by staining with rabbit anti-human von Willebrand factor (Boshide Company, Wuhan, China), as well as by performing the acetylated low-density lipoprotein color reagent test (DiI-Ac-LDL) (Molecular Probes, Novato, Canada, Europe BV). Experiments were performed on cultures with a viability > 95%. The human hepatoma cell line HepG2 was obtained from Laboratory of Surgery of our institution and cultured in Dulbecco's modified Eagle medium (Sigma) supplemented with 10% FBS. The level of KDR expression in HUVEC and HepG2 cells was determined immunohistochemically.
The pBluescript II KDR-TK plasmid, which contains Herpes simplex virus thymidine kinase (HSV-TK) and the restriction sites for XhoI and SalI and which is driven by the KDR promoter, was kindly provided by Dr Li Baojin. The 2.2-kb KDR-TK fragment was extracted from pBluescript II KDR-TK by a double digestion with XhoI and SalI (Takara Biotechnology Co.,Ltd, Dalian, China) and identified by electrophoresis and DNA sequencing. The pEGFP 4.7 kb carrier was released from EGFP plasmid DNA (donated by Doctor Zhang Ge, Laboratory of Molecular Medicine, Sun Yat-Sen University, China) containing the restriction sites for XhoI and SalI using the same technique. The KDR-TK was inserted into the same clone location of the pEGFP vector with T4 ligase (Takara) to construct a plasmid termed pEGFP-KDR-TK. Restriction enzyme digestion and DNA sequencing (Bioasia Biotech Ltd, Shanghai, China) were used to confirm correct construction. The plasmid EGFP-KDR-TK was propagated in E. coli strain DH5α and purified using a large-scale plasmid DNA purification kit (Plasmid Maxi Kit; Qiagen, Hilden, Germany). The purity of EGFP-KDR-TK plasmid DNA was assessed by ultraviolet spectrophotometry.
The ultrasound contrast agent SonoVueTM (Bracco SpA, Milan, Italy) that was used in this study consists of 59 mg of sulfur hexafluoride gas (SF6) and 25 mg of a freeze-dried white powder. Reconstitution of the material with 5 mL saline, yielded phospholipidstabilized microbubbles filled with sulfur hexafluoride with a diameter of less than 8 &mgr;m (mean, 2.5 &mgr;m)[9].
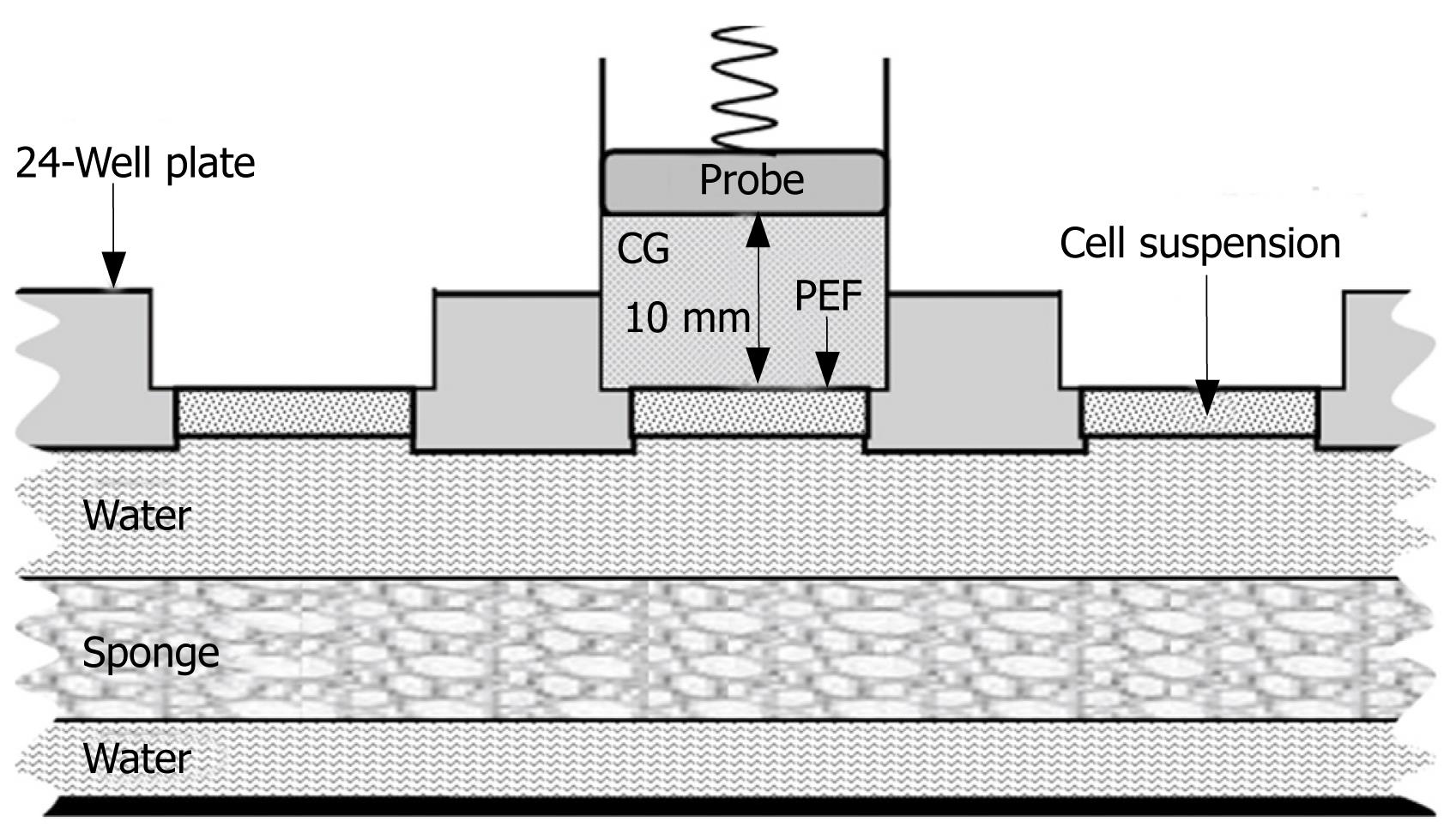
HUVECs were seeded in 24-well plates at a density of 1.5 × 105 cells/well together with 15.0 &mgr;g of pEGFP-KDR-TK (50 &mgr;g/mL) in a total volume of approximately 300 &mgr;L and incubated for 20 min at 37°C. The 24-well plates were randomly divided into three groups with 8 samples in each group: group A, sham-exposure to ultrasound without addition of SonoVue; group B, exposure to ultrasound without addition of SonoVue, and group C, exposure to ultrasound with the addition of 2% SonoVue (600 &mgr;g/well). The ultrasound machine used was HP-Viewpoint 2500 scanner (Phillips Medical System, Bothell, MA, USA). An E94K213 transcranial vector transducer probe (12 mm in diameter) was placed on a polyester film (thickness, 6 &mgr;m) that covered the 24-well plate with 10 mm thickened contact gel between the probe and the polyester film, and the cells were exposed to 1.9 MHz continuous ultrasound for 5 min at an 80.0 mW/cm2 output intensity (Figure 1). Five minutes after the first exposure, another 3 min exposure was performed under the same conditions. Cell viability was tested immediately after exposure by trypan blue dye exclusion staining. Cells were then incubated in SFM, 10% FBS, and 2 ng/mL vascular endothelial cell growth factor in 5% CO2 at 37°C for 48 h. Transfection efficiency was determined by fluorescence microscopy (Leica, Danaher Corporation, Wetzlar, Germany).
Subsequently, the cells were incubated for another 4 h before they were subjected to selection with 600 &mgr;g/mL G418 (Gibco). At that time point when most of the cells in group A had died, G418 concentration was decreased to 200 &mgr;g/mL to maintain filtrate efficacy. After around three weeks of selection, a stable G418-resistant cell pool has formed as visualized by fluorescence microscopy. Cell colonies named KDR-TK/VEC were transferred with filter paper dipped trypsin to anther 24-well plate and cultured in an humidified atmosphere with 5% CO2 at 37°C for 3 d for subsequent experiments.
KDR-TK /VEC, HUVEC, and HepG2 cells in logarithm growth term were detached by amylopsin (Sigma), the number of viable cells was determined by trypan blue dye exclusion, and viable cells were seeded in 96-well plates at a density of 2 × 103 cells/well. One day later, the cells were treated with 500 &mgr;L of fresh medium in the absence or presence of varying concentrations of GCV (0, 1, 10, 50, or 100 &mgr;g/mL). After incubation for 3 d, the sensitivity of cells to GCV was assessed using conventional 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT) assay[10]. The percentage of suppressive cells was calculated with the following formula:
Relative suppressive rate = (1 - average OD570 of experiment/OD570 of control) × 100%.
Data were expressed as mean ± SD. The statistical significance of the data was compared by using two paired Student t test. ANOVA was used to analyze the differences among the different groups. Results were considered significant at P < 0.05.
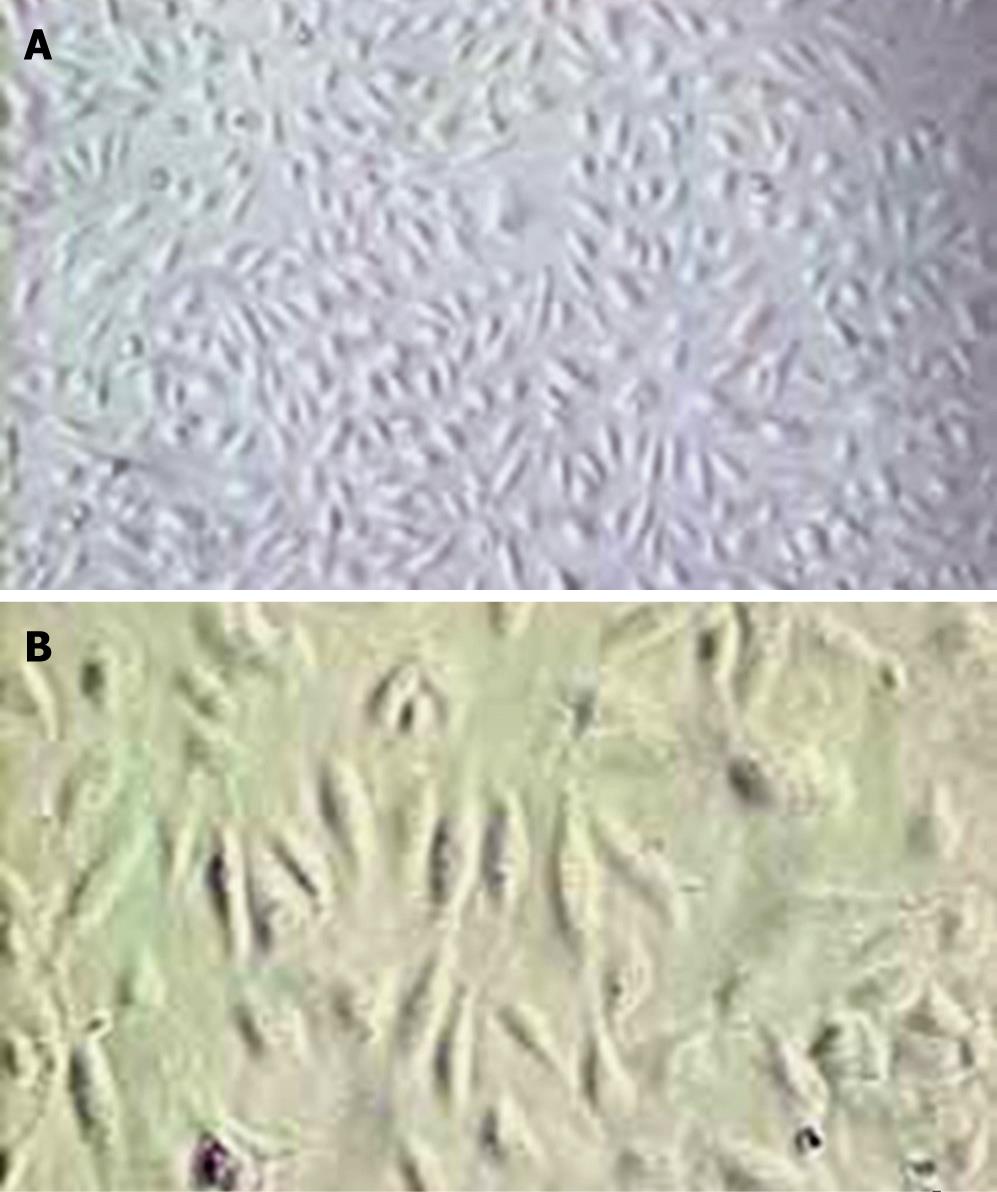
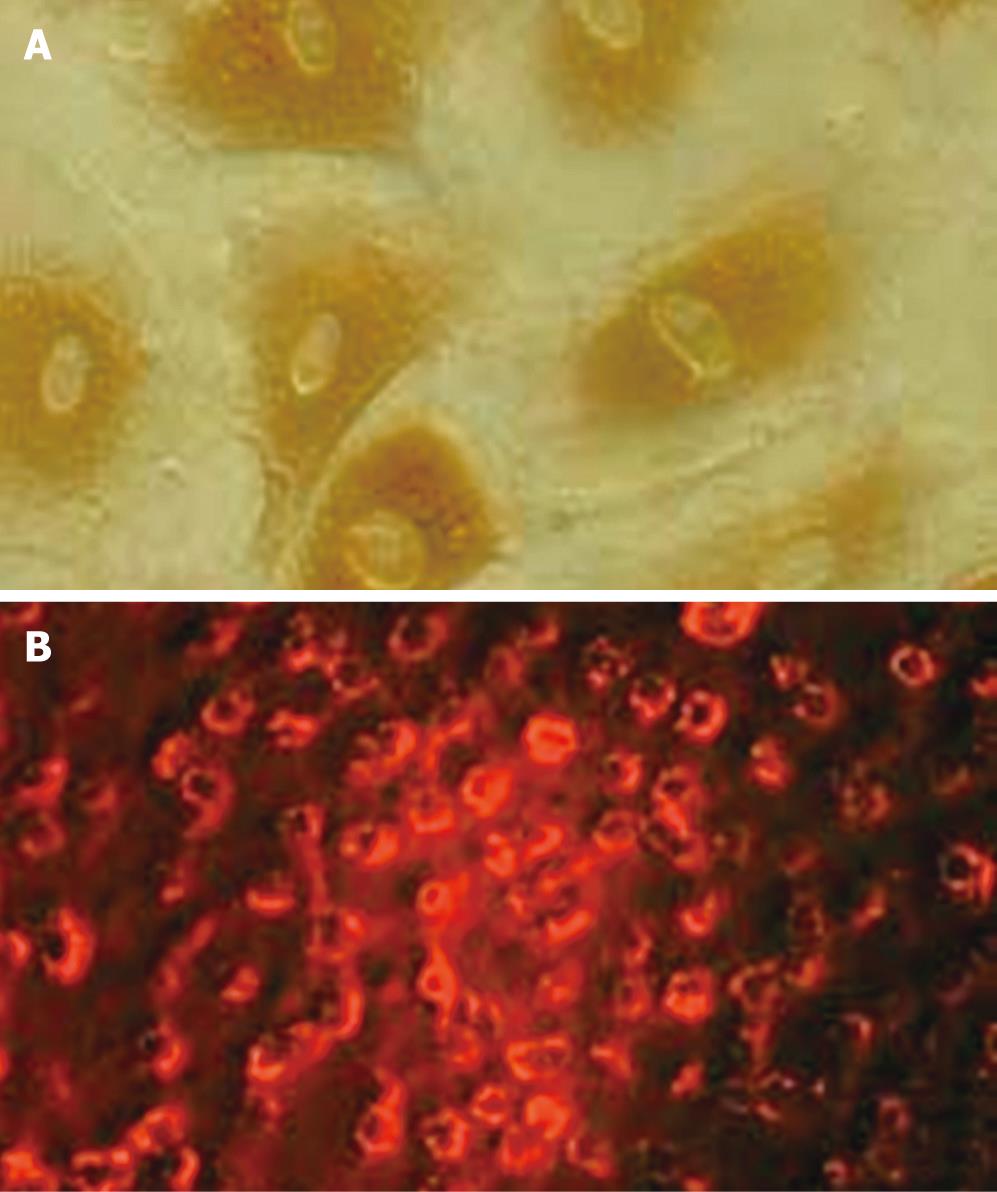
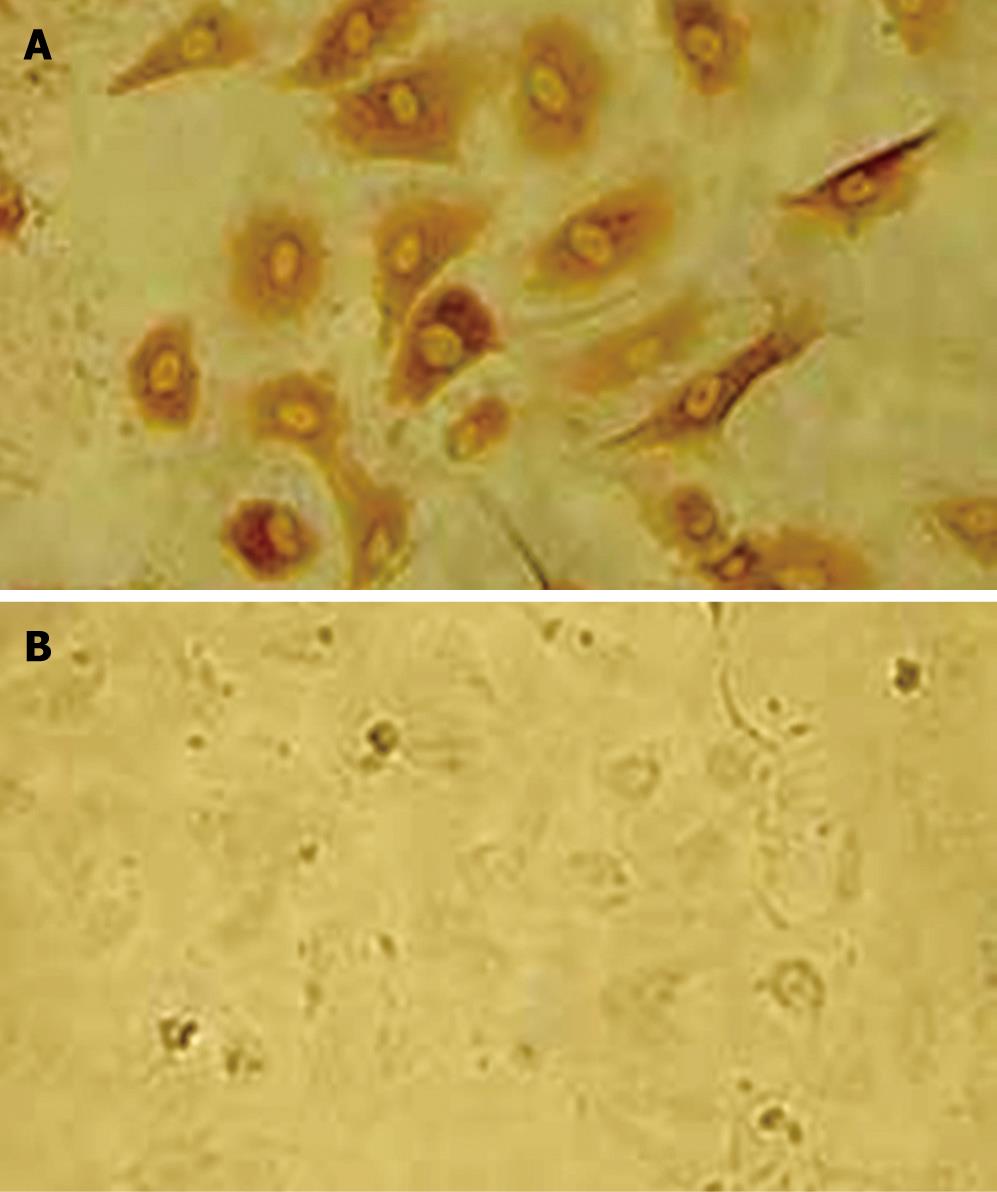
The average amounts of the endothelial cells isolated from umbilical cords using modified “irrigative digestion” technique were 1 × 106 cells. Light microscopic studies found that after 5 d in culture, HUVECs grew in confluent monolayer without a definable whirling pattern. The cells were homogenous, closely opposed, large, and polygonal with an oval centrally coated nucleus and indistinct cell border (Figure 2). Factor VIII is an important plasma marker of endothelium damage/dysfunction, which can also be detected in death or dysfunctional endothelial cells. The positive dye was brown in cytoplasm of HUVEC stained with the factor VIII related antigen (Figure 3A). Compared with the factor VIII, DiI-Ac-LDL can be taken up only by the living endothelial cells. The living HUVECs incubated with DiI-Ac-LDL are found to be red fluorescence under the fluorescent photomicroscope (Figure 3B), while the dead cells are uncolored. In the present study, the DiI-Ac-LDL stain revealed that more than 95% HUVEC were alive. To estimate the selectivity of the strategy using the KDR promoter, it is necessary to identify KDR-positive and KDR-negative cell lines. As shown in Figure 4, immunohistochemical analysis revealed KDR expression in HUVECs but not in HepG2.
The positive clone of recombinant pEGFP-KDR-TK, which contains the neomycin resistance gene and the independent KDR promoters controlling the expression of TK, was identified by restriction endonuclease cleavage and sequencing. DNA sequencing and electrophoresis showed correct KDR-TK insertion into the pEGFP carrier (Figure 5).
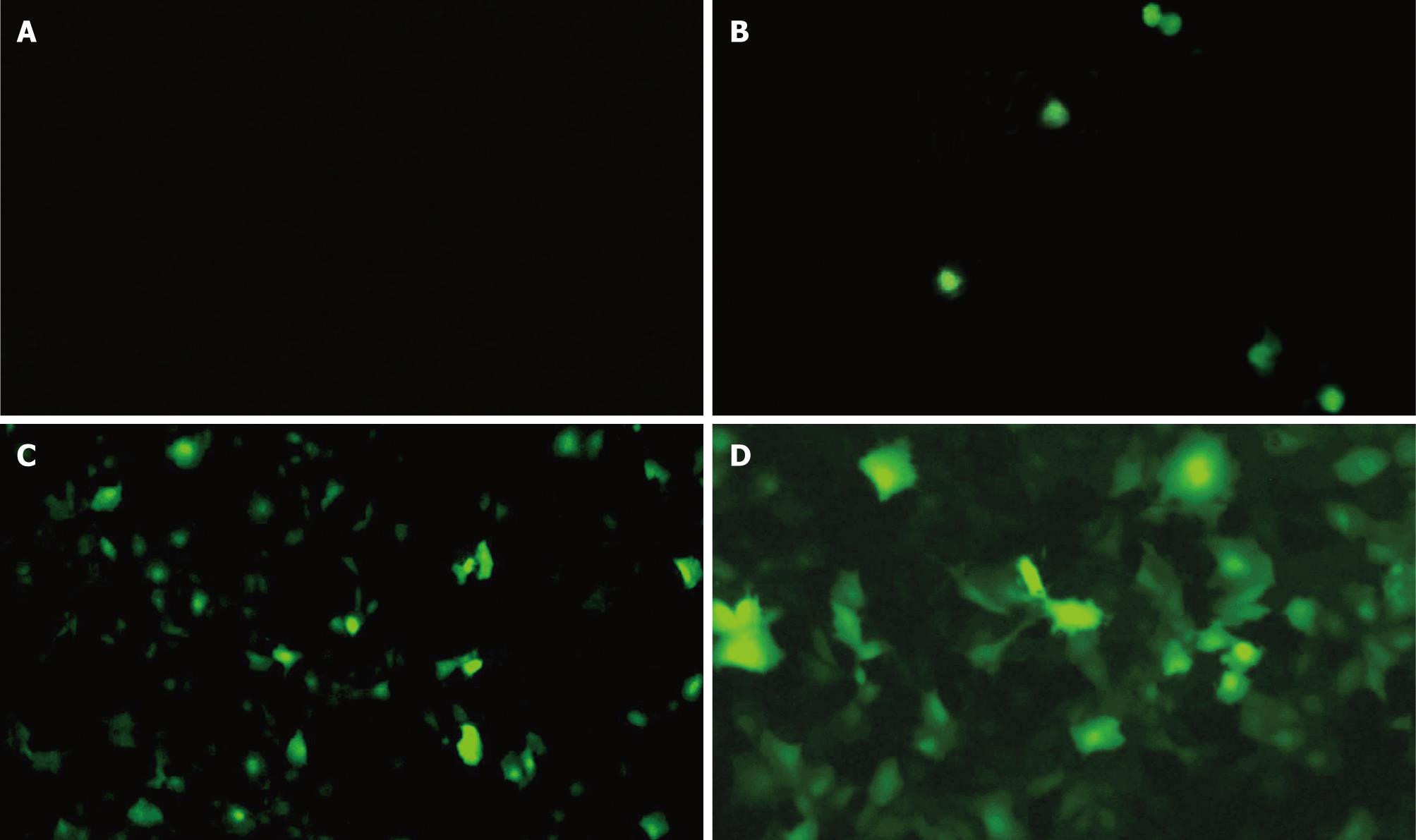
The transient transfection rate was assessed by fluorescence microscopy 48 h after transfection. Fluorescent microscopy revealed that the cells in group A had no obvious coloration (Figure 6A). Fluorescent staining was observed in few cells (1.7%) in group B (stimulated by ultrasound only, Figure 6B), whereas the HUVECs transfected by the pEGFP-KDR-TK in group C (stimulated by both SonoVue and ultrasound exposure) showed clear green fluorescence within 20.3% HUVECs indicating EGFP expression (Figure 6C and D). EGFP and TK were co-expressed in HUVECs by microbubble-enhanced ultrasound irradiation, followed by selection of permanent transfectants in the presence of G418. The G418-resistant cells were named KDR-TK/VEC.
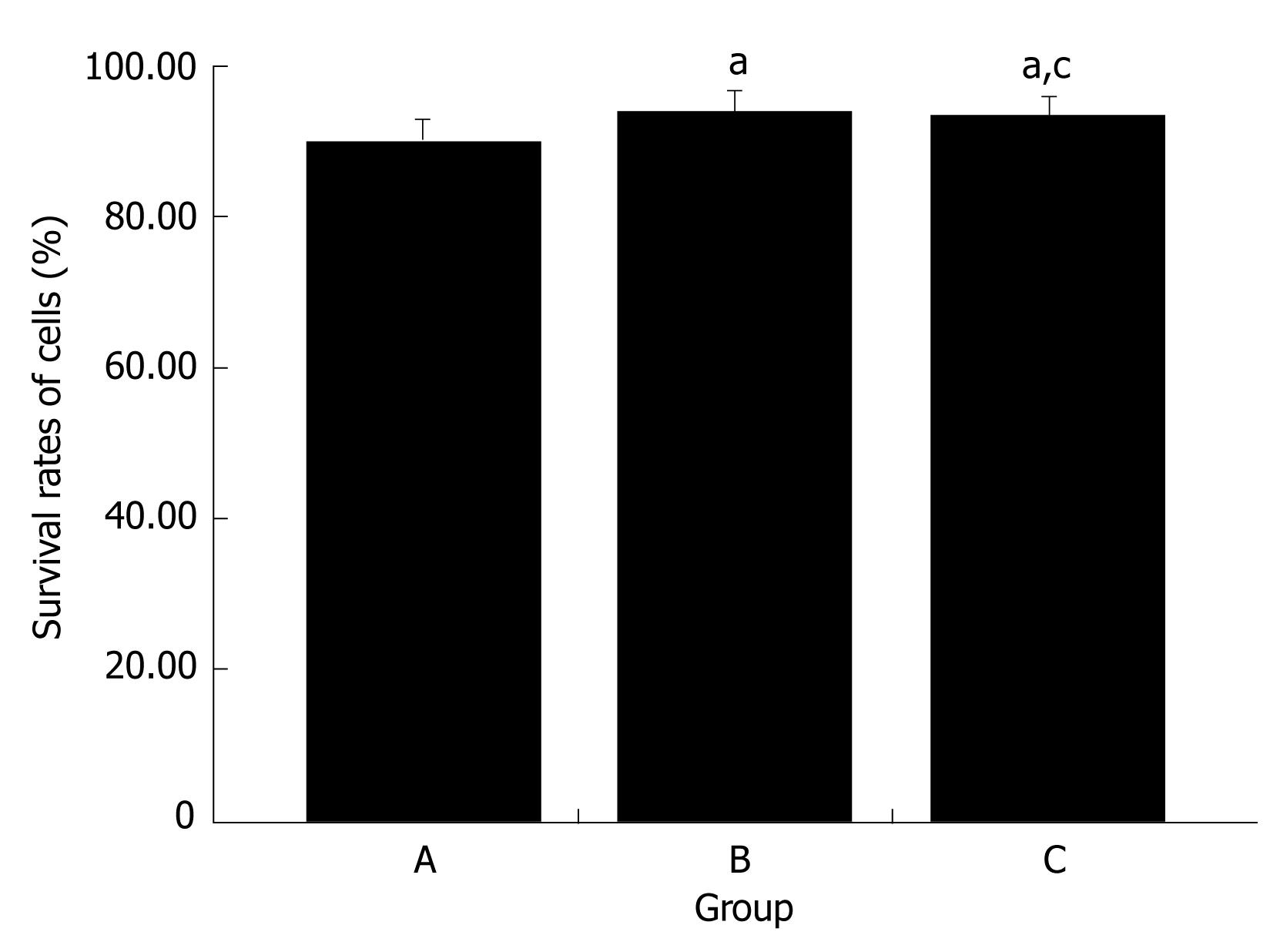
The percentage of surviving cells was examined by trypan blue dye exclusion after ultrasound exposure. The cell survival rates were 94.0% ± 1.1% in the group A, 93.5% ± 0.9% in the group A and 90.2% ± 0.9% in group B (Figure 7).
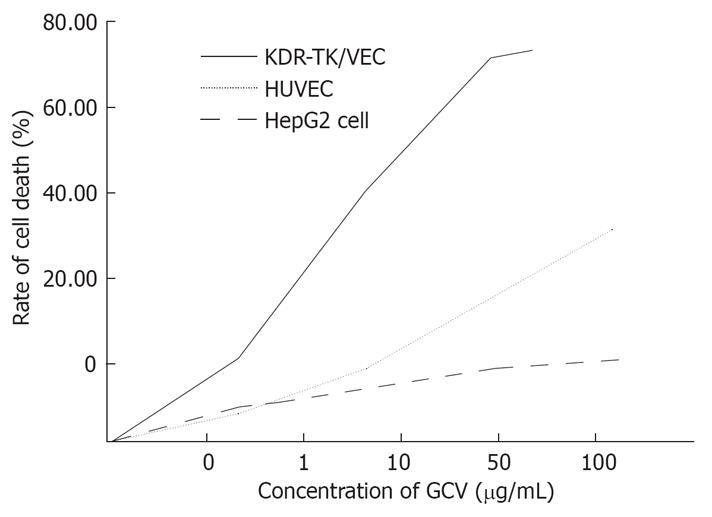
We selected GCV as a prodrug and examined the sensitivity of KDR-TK/VEC, HUVEC, and HepG2 to GCV. As shown in Figure 8, HepG2 cells were found to be resistant to GCV, and the value of IC 50, defined as the dose required for 50% cytotoxicity, was > 100 &mgr;g/mL (the highest concentration tested). HUVECs exhibited a higher sensitivity to GCV in comparison to HepG2, when the concentration tested was above 50 &mgr;g/mL, the IC 50 value for HUVECs was still more than 100 &mgr;g/mL. Conversely, KDR-TK/VEC cells were susceptible to GCV in a dose-dependent manner and exhibited approximately 75% suppression at a GCV concentration of 100 &mgr;g/mL, with the IC 50 value being approximately 10 &mgr;g/mL. KDR-TK/VEC cells also showed significantly higher sensitivity to GCV compared to non-transfected HUVECs, resulting in 2 to 5-fold higher suppression to GCV.
Acquired drug resistance is a major problem in cancer therapy. Many deaths from cancer may follow the development of resistance to chemotherapy. The emergence of resistance depends in part on the genetic instability, heterogeneity, and high mutational rate of tumor cells[1]. In contrast, endothelial cells have relative genomic stability. Therefore, antiangiogenic therapy targeting tumoral endothelial cells may avoid the problem of drug resistance. In addition, the growth and persistence of solid tumors and their metastases are angiogenesis dependent[11], thus angiogenesis provides a target for therapeutic approaches to solid tumor, and the inhibition of angiogenesis is very promising.
Most angiogenic inhibitors are biologically active peptides. It is difficult to express proteins persistently and stably in vivo, and the complexity and time requirement for protein purification make their clinical application infeasible[12]. Gene therapy is an attractive means of delivering these antiangiogenic agents to tumor site, and targeting the tumor vessels could avoid potential side effects. Although several previous studies have shown the effectiveness of TK targeting in endothelium as antiangiogenic strategy, few of them have reported with suicide gene therapy by targeting TK in the vasculature and not in the human tumor cells[1314]. Ozalki et al have shown that vWF2 promoter combined with HSV-TK suicide gene can exert an endothelial-preferential killing effect on the transduced HUVECs in vitro[15]. Von Willebrand factor (vWF) is a blood glycoprotein which is excreted by endothelium and megakarcytes, and the elevation of vWF is not only found in cancer, but also in other cardiovascular diseases[16]. Consequently, tumor vessels are not accessible targets for antiangiogenic gene therapy, when the vWF2 promoter and HSV-TK/prodrug are administrated systemically. Compared with vWF, KDR has a major role in tumor angiogenesis, and in adulthood, KDR appears mostly restricted to vascular endothelial/lymph endothelial cells[2]. Because of the overexpression of KDR in tumors vasculature, KDR-promoter did not yield high TK protein expression in normal vasculature, whereas in endothelial cells within tumors. Dancer and colleagues indicated that expression of TK driven by an endothelial-specific promoter can cause significant decrease in microvessel density and inhibit tumor growth[17].
The suicide gene used in this study has been widely used in gene therapy of animal tumors, and it encodes an enzyme that can effectively phosphorylate the antiviral nucleoside analogue ganciclovir (GCV) to its monophosphase form, which is further phosphorylated by cellular kinases to DNA polymerase inhibitors[18]. Apoptosis has been demonstrated as the major mechanism of cell death caused by the HSV-TK/GCV system in different cells[1920]. The present study demonstrated that KDR promoter and the suicide gene/prodrug system showed a targeted killing effect on transfected HUVECs. The sensitivity of KDR-TK-transfected HUVECs to GCV was found to be about 2 to 5-fold higher when compared to non-transfected HUVEC and HepG2 cells.
Viral delivery systems have been developed as highly efficient carriers for gene delivery to a variety of tissues. However, viral gene carriers have some major disadvantages such as possible immune response, severe inflammation reactions, the risk for insertional mutagenesis, and interference with expression of cellular genes[2122]. Recently, ultrasound together with microbubble has been recognized as a useful means for controlled delivery of drugs. Although the mechanism of enhanced transfection efficiency by ultrasound is not yet fully understood, cavitation is an important event that increase capillary permeability and produce transient nanopores in cell membranes. SonoVue as well as other microbubble can be rapidly destroyed by the sonication, and the threshold of cavitation decreases after addition of microbubbles. In this study, we demonstrate that widely used diagnostic ultrasound and microbubble-based ultrasound contrast agent can also be applied for the delivery of DNA. The expression of EGFP in HUVECs was just 1.7% in the group only treated with ultrasound, whereas the expression rate significantly increased to 20.3% (P < 0.001) when treated with ultrasound and SonoVue. In contrast, therapeutic or high-intensity focused ultrasound has been used for most of the described ultrasound-facilitated gene delivery and gene transfection experiments[23–25]. An advantage of using diagnostic ultrasound instead of therapeutic ultrasound allows visualization of the site of interest and microbubble possible by using conventional ultrasound imaging systems. In this study, more than 90% of the cells treated with ultrasound and SonoVue were viable. Though the longer exposure time used in our experiment, the cell viability in this study is also higher than that using therapeutic ultrasound[26]. Although the transient transfection rate of KDR-TK in this study is relatively low compared with other studies using viral vectors, we believe this disadvantage can be offset by lower acoustic frequency, higher ultrasound output, or longer exposure time.
Our observation also suggested that non-transfected HUVECs have more sensitivity to GCV, compared with non-transfected HepG2 cells. The mechanism was not yet understood; we speculated that the lower sensitivity to GCV of non-transfected HepG2 cells might partly involve the deficiency of TK in tumor cells[27].
In summary, our study revealed that, on the basis of the KDR-mediated invasion, TK/GCV can accomplish a selective killing effect on HUVECs in vitro. KDR promoter and the suicide gene/prodrug system mediated by diagnostic ultrasound combined with microbubble may present a novel and attractive approach for gene therapy to target tumor vessels.
Tumor angiogenesis plays a critical role in the development and progression of the solid tumor. Targeting angiogenesis represents a new strategy for antitumor therapies. Ultrasound exposure in combination with microbubble is a new non-viral delivery system.
Gene therapy to target tumor vessels can cause a significant decrease in microvessel density and inhibit tumor growth. Just as therapeutic ultrasound, diagnostic ultrasound in conjunction with microbubbles can also be a useful gene delivery system.
Diagnostic ultrasound-facilitated gene delivery on targeting the vascular endothelial cells has not been used. This study established a specific promoter in order to target gene expression in human umbilical vein endothelial cells and demonstrated that diagnostic ultrasound combined with microbubble can be a useful gene delivery system.
This study showed KDR promoter and the suicide gene/prodrug system mediated by diagnostic ultrasound can significantly kill human umbilical vein endothelial cells. This may present a novel and attractive approach to target gene therapy on tumor vessel.
Microbubble: microscopically gas bubbles encapsulated by an elastic shell, can be used in diagnostic and therapeutic applications.
This study is well-designed and very interesting, and the manuscript is well-written.
| 1. | Boehm T, Folkman J, Browder T, O'Reilly MS. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature. 1997;390:404-407. |
| 2. | Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469-478. |
| 3. | Chen QR, Zhang L, Gasper W, Mixson AJ. Targeting tumor angiogenesis with gene therapy. Mol Genet Metab. 2001;74:120-127. |
| 4. | Ross G, Erickson R, Knorr D, Motulsky AG, Parkman R, Samulski J, Straus SE, Smith BR. Gene therapy in the United States: a five-year status report. Hum Gene Ther. 1996;7:1781-1790. |
| 5. | Cao G, Kuriyama S, Gao J, Kikukawa M, Cui L, Nakatani T, Zhang X, Tsujinoue H, Pan X, Fukui H. Effective and safe gene therapy for colorectal carcinoma using the cytosine deaminase gene directed by the carcinoembryonic antigen promoter. Gene Ther. 1999;6:83-90. |
| 6. | Cao G, Kuriyama S, Cui L, Nagao S, Pan X, Toyokawa Y, Zhang X, Nishiwaki I, Qi Z. Analysis of the human carcinoembryonic antigen promoter core region in colorectal carcinoma-selective cytosine deaminase gene therapy. Cancer Gene Ther. 1999;6:572-580. |
| 7. | Nie F, Xu HX, Tang Q, Lu MD. Microbubble-enhanced ultrasound exposure improves gene transfer in vascular endothelial cells. World J Gastroenterol. 2006;12:7508-7513. |
| 8. | Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745-2756. |
| 9. | Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, Zheng YL, Liang JY. Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med. 2006;25:23-33. |
| 10. | Kuriyama S, Mitoro A, Yamazaki M, Tsujinoue H, Nakatani T, Akahane T, Toyokawa Y, Kojima H, Okamoto S, Fukui H. Comparison of gene therapy with the herpes simplex virus thymidine kinase gene and the bacterial cytosine deaminase gene for the treatment of hepatocellular carcinoma. Scand J Gastroenterol. 1999;34:1033-1041. |
| 12. | Wu Y, Li ZY, Zhao X, Kan B, Wei YQ. Inhibition of ovarian tumor growth by gene therapy with recombinant soluble vascular endothelial growth factor receptor 2. Hum Gene Ther. 2006;17:941-948. |
| 13. | Ram Z, Walbridge S, Shawker T, Culver KW, Blaese RM, Oldfield EH. The effect of thymidine kinase transduction and ganciclovir therapy on tumor vasculature and growth of 9L gliomas in rats. J Neurosurg. 1994;81:256-260. |
| 14. | Pramudji C, Shimura S, Ebara S, Yang G, Wang J, Ren C, Yuan Y, Tahir SA, Timme TL, Thompson TC. In situ prostate cancer gene therapy using a novel adenoviral vector regulated by the caveolin-1 promoter. Clin Cancer Res. 2001;7:4272-4279. |
| 15. | Ozaki K, Yoshida T, Ide H, Saito I, Ikeda Y, Sugimura T, Terada M. Use of von Willebrand factor promoter to transduce suicidal gene to human endothelial cells, HUVEC. Hum Gene Ther. 1996;7:1483-1490. |
| 16. | Blann AD. Plasma von Willebrand factor, thrombosis, and the endothelium: the first 30 years. Thromb Haemost. 2006;95:49-55. |
| 17. | Dancer A, Julien S, Bouillot S, Pointu H, Vernet M, Huber P. Expression of thymidine kinase driven by an endothelial-specific promoter inhibits tumor growth of Lewis lung carcinoma cells in transgenic mice. Gene Ther. 2003;10:1170-1178. |
| 18. | Moolten FL, Wells JM. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990;82:297-300. |
| 19. | Krohne TU, Shankara S, Geissler M, Roberts BL, Wands JR, Blum HE, Mohr L. Mechanisms of cell death induced by suicide genes encoding purine nucleoside phosphorylase and thymidine kinase in human hepatocellular carcinoma cells in vitro. Hepatology. 2001;34:511-518. |
| 20. | Beltinger C, Fulda S, Kammertoens T, Uckert W, Debatin KM. Mitochondrial amplification of death signals determines thymidine kinase/ganciclovir-triggered activation of apoptosis. Cancer Res. 2000;60:3212-3217. |
| 21. | Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244-2245. |
| 22. | Yamashita Y, Shimada M, Tachibana K, Harimoto N, Tsujita E, Shirabe K, Miyazaki J, Sugimachi K. In vivo gene transfer into muscle via electro-sonoporation. Hum Gene Ther. 2002;13:2079-2084. |
| 23. | Christiansen JP, French BA, Klibanov AL, Kaul S, Lindner JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol. 2003;29:1759-1767. |
| 24. | Koch S, Pohl P, Cobet U, Rainov NG. Ultrasound enhancement of liposome-mediated cell transfection is caused by cavitation effects. Ultrasound Med Biol. 2000;26:897-903. |
| 25. | Lawrie A, Brisken AF, Francis SE, Wyllie D, Kiss-Toth E, Qwarnstrom EE, Dower SK, Crossman DC, Newman CM. Ultrasound-enhanced transgene expression in vascular cells is not dependent upon cavitation-induced free radicals. Ultrasound Med Biol. 2003;29:1453-1461. |
| 26. | Guo DP, Li XY, Sun P, Wang ZG, Chen XY, Chen Q, Fan LM, Zhang B, Shao LZ, Li XR. Ultrasound/microbubble enhances foreign gene expression in ECV304 cells and murine myocardium. Acta Biochim Biophys Sin (Shanghai). 2004;36:824-831. |