Published online Apr 21, 2008. doi: 10.3748/wjg.14.2343
Revised: February 29, 2008
Published online: April 21, 2008
AIM: To investigate the effect of Chaiqinchengqi decoction (CQCQD) on sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) mRNA expression of pancreatic tissues in acute pancreatitis (AP) rats.
METHODS: Thirty Sprague-Dawley (SD) rats were randomized into control group, AP group and CQCQD group (n = 3 × 10). The rats in the CQCQD group were intragastrically administered with CQCQD (10 mL/kg every 2 h) after induction of AP by intraperitoneal injection of caerulein (50 &mgr;g/kg.h × 5) within 4 h. At 6 h after the induction of AP model, pancreatic tissues were collected for the pathological observation, mRNA extraction for determination of SERCA1 and SERCA2 mRNA expression or pancreatic acinar cell isolation for measurement of fluorescence intensity (FI) of intracellular calcium ion concentration [Ca2+]i.
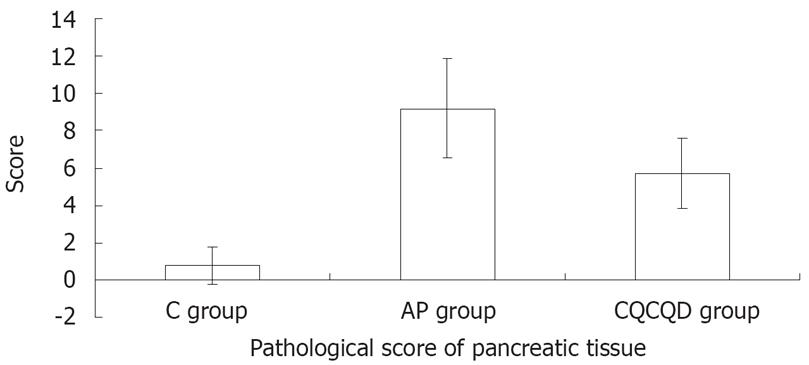
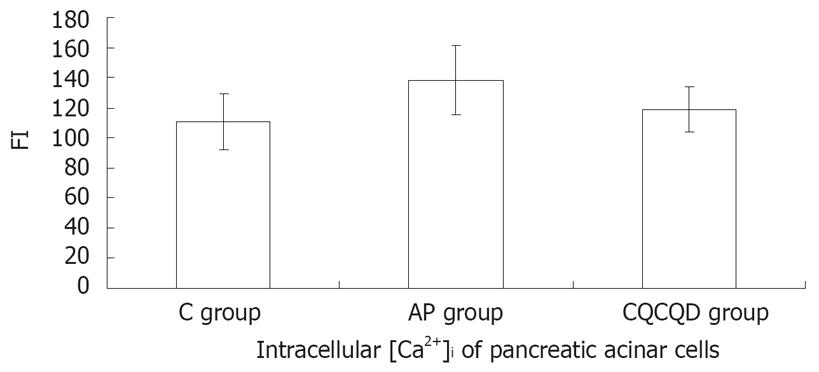
RESULTS: There was no expression of pancreatic SERCA1 mRNA in the control group and the AP group. The expression of pancreatic SERCA2 mRNA in the AP group was down-regulated (expression ratio = 0.536; P = 0.001) compared with the control group, while that in the CQCQD group was up-regulated (expression ratio = 2.00; P = 0.012) compared with AP group. The FI of intracellular [Ca2+] of pancreatic acinar cells in the AP group (138.2 ± 23.1) was higher than the C group (111.0 ± 18.4) and the CQCQD group (118.7 ± 15.2 ) (P < 0.05) and the pancreatic pathological score in the CQCQD group was lower than that in the AP group (5.7 ± 1.9 vs 9.2 ± 2.7, P < 0.05).
CONCLUSION: CQCQD can up-regulate the expression of SERCA2 mRNA of pancreatic tissues, reduce intracellular calcium overload and relieve pancreatic tissue lesions.
- Citation: Xue P, Deng LH, Zhang ZD, Yang XN, Xia Q, Xiang DK, Huang L, Wan MH. Effect of Chaiqinchengqi decoction on sarco/endoplasmic reticulum Ca2+-ATPase mRNA expression of pancreatic tissues in acute pancreatitis rats. World J Gastroenterol 2008; 14(15): 2343-2348
- URL: https://www.wjgnet.com/1007-9327/full/v14/i15/2343.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2343
Acute pancreatitis (AP) is a potentially lethal disorder with no specific therapeutic options[1–5]. The major obstacle to the development of therapies is our limited understanding of the pathogenesis of AP. Despite a number of theories have been proposed to explain the pathogenesis from various aspects, but there are still controversies about the mechanism of the disorder. Since Ward[6] firstly proposed that calcium overload in pancreatic acinar cells should be a “trigger point” of AP, intracellular calcium overload has been generally accepted to play a crucial role in the occurrence and deterioration of AP[7].
The increased intracellular free calcium ion concentration ([Ca2+]i) is believed to originate from the influx of extracellular Ca2+ and the release of Ca2+ of intracellular Ca2+ stores[8]. In AP, the quantity and/or activity of plasma membrane Ca2+-ATPase (PMCA) and/or sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) were decreased, the intracellular Ca2+ was not pumped out of cell or back into Ca2+ stores in time[9]. But the exact mechanism of intracellular calcium overload in AP remains unclear. Therefore, a better elucidation would improve the therapeutic strategies of this disease.
Chaiqinchengqi decoction (CQCQD) has been proved to be effective in the treatment of AP[10]. Our previous experiments found that CQCQD inhibited the elevation of [Ca2+] i and had a protective effect on pancreatic acinar cells in AP rats[11]. In the present study, we aim to explore the mechanism of intracellular calcium overload by determining the SERCA mRNA expression changes of pancreatic tissues in AP rats and further to find out the therapeutic mechanisms of CQCQD in AP rats.
Thirty male Sprague-Dawley (SD) rats weighing 250-300 g were purchased from the Experimental Animal Center of West China Center of Medical Sciences of Sichuan University. All animals were kept at constant room temperature in a 12-h light/dark cycle with free access to standard chow and water. All animals were adjusted to laboratory conditions for 1 wk and a 12-h fast with free access to drinking water before the experiments. This study was approved by and conducted under the guidelines of Animal Use and the Committee of Scientific Research of West China Hospital of Sichuan University.
Caerulein was purchased from Sigma Co. (USA), trizol and diethyl pyrocarbonate (DEPC) were from Invitrogen Company (USA), hexanucleotide random primer and Superscript II RNase H-reverse transcriptase (RNase H-RT) was from Life Technologies, dNTPs and the RNAase inhibitor were from Takara Co. (Japan), and Taq DNA polymerase was from Promega (USA). Primers for SERCA1 and SERCA2 gene were designed according to the method reported previously. Primers for SERCA1, SERCA2 and GAPDH genes were synthesized by Shanghai Biotechnology Co. Ltd. (Table 1). Dulbecco’s modified Eagle’s medium (DMEM) is a product of GIBCO BRL and preoxygenated for 30 min before use. Collagenase P was purchased from Roche Applied Science. Fluo-3/AM was a product of Molecular Probes Co, USA. All other reagents were of the highest purity available and were purchased from Sigma unless indicated otherwise.
| Genes | Probes | Upstream primers | Downstream primers | Product (bp) |
| SERCA1 | 5’-CGATGTCCCGAGCCTTGATCC-3’ | 5’-GGTTTGGCAGGAACGGAAT-3’ | 5’-GGTGGATTTGATGGAGAGGAT-3’ | 199 |
| SERCA2 | 5’-CACACTCTTTCTGTCCTGTCG-3’ | 5’-ATGAACCTGAAATGGGCAAG-3’ | 5’-GGAACTTTGTCACCAACAGCA-3’ | 115 |
| GAPDH | 5’-FAM-ACCACAGTCCATGCCATCAC-TAMRA-3’ | 5’-CCTCAAGATTGTCAGCAAT-3’ | 5’-CCATCCACAGTCTTCTGAGT-3’ | 141 |
The Chinese medicinal herbs in CQCQD provided by Chinese Herbs Pharmacy of West Chinese Hospital of Sichuan University included Chaihu (Radix Bupleuri) 15 g, Huangqin (Radix Scutellariae) 15 g, Houpo (Cortex Magnoliae Officinalis) 15 g, Zhishi (Fructus Aurantii Immaturus) 15 g, Yinchen (Herba Artemisiae Scopariae) 15 g, Zhizi (Fructus Gardeniae) 20 g, Dahuang (Radix et Rhizoma Rhei) 15 g and Mangxiao (Natrii Sulfas) 10 g. The decoction was made into 200 mL juice and then into lyophilized powder. Before experiment, the lyophilized powder of CQCQD was prepared to a concentration of 2 g/mL of crude herbs.
SD rats were randomized into control group (C group, n = 10), AP group (n = 10) and CQCQD group (n = 10). AP model was induced by intraperitoneal injection of caerulein (50 &mgr;g/kg.h × 5) within 4 h[12]. Rats in the C group were administered with the same volume of physiological saline. After the induction of AP, the rats in the CQCQD group were injected orally into the stomach with CQCQD (10 mL/kg.2 h), and other two groups were intragastrically administered with the same volume of physiological saline as CQCQD group. At 6 h after the induction of AP, the rats were anesthetized with intraperitoneal sodium pentobarbital (40 mg/kg), the abdomen was opened and the pancreatic tissues were rapidly collected for pathological examination, mRNA extraction or cell isolation for measurement of [Ca2+]i. The rats were sacrificed by exsanguinations after experiments.
After removal of the pancreatic tissues, the sections of samples were fixed in 4% neutral buffer formaldehyde, embedded with paraffin wax, cut into slices, and stained with Hematoxylin-Eosin (HE) and observed under light microscopy. Pathological grading and scoring criteria are shown in Table 2[13–15]. For each pathological section, 10 visual fields under a high-power microscope (HE, × 400) were randomly selected and scored by one pathologist. The mean score of the 10 visual fields of one pathological section was calculated as the pathological score.
| Pathological changes | Scores |
| Edema | |
| Focal expansion of interlobular septa | 1 |
| Same as 1 + diffuse expansion of interlobar; | 2 |
| Septa/diffuse expansion of interlobar septa | |
| Same as 2 + expansion of interacinar septa | 3 |
| Same as 3 + expansion of intercellular spaces | 4 |
| Inflammation and perivascular infltrate | |
| 2-10 intralobular or perivascular leukocytes/HPF | 1 |
| 11-20 intralobular or perivascular leukocytes/HPF | 2 |
| 21-30 intralobular or perivascular leukocytes/HPF | 3 |
| > 30 leukocytes/HPF or confluent microabscesses | 4 |
| Acinar necrosis | |
| Diffuse occurrence of 1-4 necrotic cells/HPF | 1 |
| Diffuse occurrence of 5-10 necrotic cells/HPF | 2 |
| Diffuse occurrence of 11-16 necrotic cells/HPF (foci of confluent necrosis) | 3 |
| > 16 necrotic cells/HPF (Extensive confluent necrosis) | 4 |
| Hemorrhage and fat necrosis | |
| 1-2 focus | 1 |
| 3-4 focus | 2 |
| 5-6 focus | 3 |
| > 7 focus | 4 |
Pancreatic tissue was quickly removed and immediately transferred to iced DMEM. Collagenase P (0.3 g/L) in 2.5 mL DMEM containing 2 g/L bovine serum albumin (BSA) and 0.1 g/L soybean trypsin inhibitor (SBTI) was infiltrated into the tissue with a 5 mL syringe. The tissue was minced into small fragments and digested in Collagenase P solution at 37°C in a shaking water bath for 3 × 15 min (120 cycles/min), dispersed with a plastic pipette, filtered through a nylon mesh (150 meshes), and layered into DMEM containing 4% BSA. The acinar cells were centrifuged at 400 r/min for 3 min for three times in fresh DMEM medium containing 2 g/L BSA and 0.1 g/L SBTI and resuspended before use.
According to the previously reported methods[16], the pancreatic acinar cells were loaded with 5 &mgr;mol/L Fluo-3/AM and 0.01% Pluronic F-127 in darkness for 30 min at 37°C and observed under Leica TCS-sp2 laser scanning confocal microscopy (LSCM). Twenty different cells in each visual field were randomly selected. The cells loaded with Fluo-3/AM were excited at a wavelength of 488 nm and the emitted fluorescence was detected at 488 nm and 560 nm. The relative fluorescence was analyzed using fluorescence quantitative analysis software and the intracellular calcium fluorescence intensity (FI) was presented as intracellular [Ca2+]i.
Pancreatic tissues in each group was quickly removed and immediately transferred into liquid nitrogen. Total RNA of pancreatic tissue was extracted using Trizol reagent. The integrity of the total RNA was confirmed by agarose gel electrophoresis. Quantity and purity of the total RNA were determined by an ultraviolet spectrophotometer, and RNA concentration of the sample was calculated.
The total RNA was reverse-transcribed using hexa-nucleotide random primers with RNase H-RT. The cDNA was amplified as a template for subsequent PCR using Taq DNA polymerase in a Perkin Elmer Cetus DNA thermocycler. PCR reaction system included 5 &mgr;L cDNA-RT product, 3 &mgr;L 10 × PCR buffer, 3 &mgr;L MgCl2 of 25 mmol/L, 0.36 &mgr;L dNTP of 25 mmol/L, 1 &mgr;L upstream primer of 10 &mgr;mol/L, 1 &mgr;L downstream primer of 10 &mgr;mol/L, 0.6 &mgr;L probe of 10 &mgr;mol/L, 0.3 &mgr;L Taq polymerase of 5 U/&mgr;L, and 15.74 &mgr;L deionized double-distilled water. PCR cycling conditions were as follows: initial denaturation at 94°C for 2 min, followed by 45 cycles of 94°C for 20 s, 56°C for 30 s and 60°C for 40 s.
In this study, a negative control with the same volume of deionized double-distilled water instead of cDNA was used and amplified to control DNA contamination, and a positive control with the same volume of the cDNA of skeletal muscle of rat instead of cDNA of pancreatic tissues. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as internal housekeeping gene. The amplified DNAs were resolved by agarose gel electrophoresis and stained with ethidium bromide. The bands were visualized and photographed under ultraviolet light.
Data were expressed as mean ± SD. Data in normal distribution were analyzed using analysis of variance; data in abnormal distribution was analyzed using Wilcoxon rank sum test. The Relative Expression Software Tool was employed for statistical analysis of gene expression[1718]. P < 0.05 was considered statistically significant.
The pathological score of pancreatic tissue in the AP group was the highest (9.2 ± 2.7) and followed by the CQCQD group (5.7 ± 1.9) and the C group (0.8 ± 1.0) (P < 0.05), (Figure 1).
The FI of [Ca2+]i pancreatic acinar cells in the AP group was higher than that in the C group (138.2 ± 23.1 vs 111.0 ± 18.4) or CQCQD group (138.2 ± 23.1 vs 118.7 ± 15.2 ), (P < 0.05), but there was no statistical difference between the C group and CQCQD group in [Ca2+]i (P > 0.05), (Figure 2).
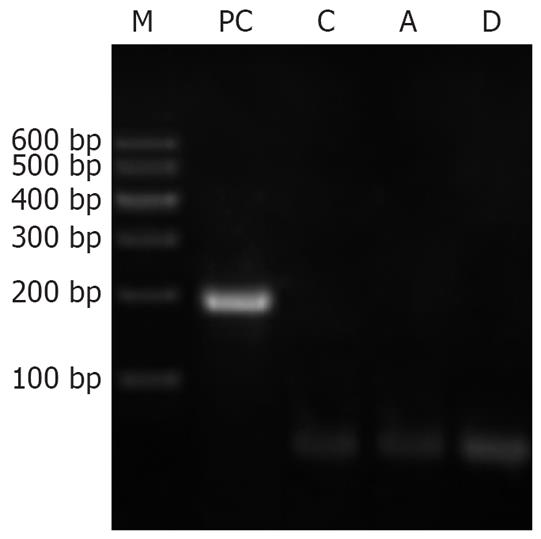
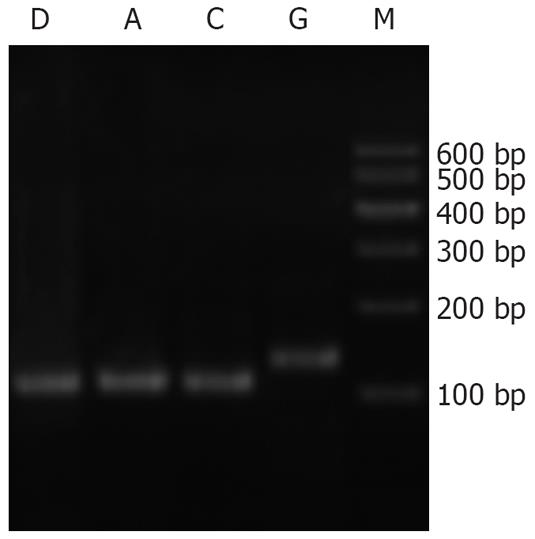
Neither the pancreatic tissues of normal rats nor that of AP rats expressed SERCA1 mRNA. Skeletal muscle, however, expressed SERCA1 mRNA as the positive control, shown by the electrophoresis of PCR products in Figure 3.
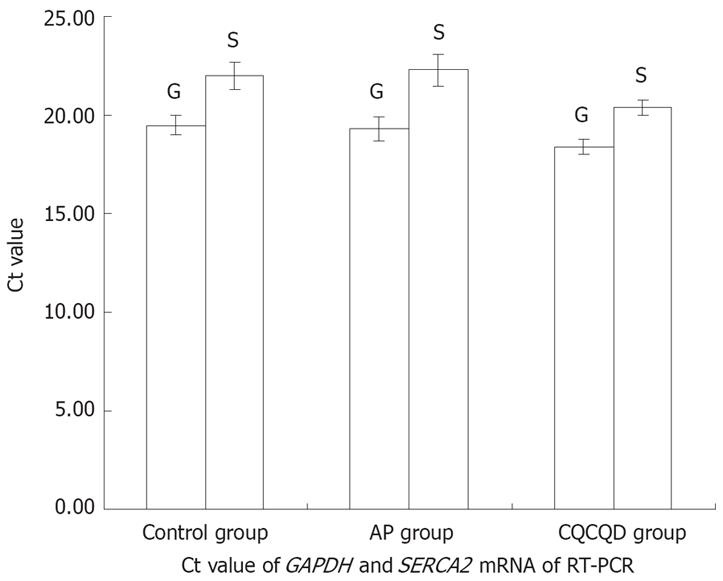
Cycle threshold (Ct) value of GAPDH and SERCA2 mRNA in the three groups is shown in Figure 4 and the agar gel electrophoresis photograph of PCR products of SERCA2 mRNA is shown in Figure 5. The expression of SERCA2 mRNA was highest in the C group, and lowest in the AP group, and moderate in the CQCQD group. Compared with the C group, the expression of SERCA2 mRNA in the AP group was decreased with an expression ratio of 0.536 (P = 0.001). Compared with the AP group, the expression of SERCA2 mRNA in the CQCQD group was increased with an expression ratio of 2.000 (P = 0.012).
Intracellular calcium overload of pancreatic acinar cells has been confirmed in experimental acute pancreatitis[19–23] and extensively accepted to be an important triggering and exacerbating mechanism of AP[672425]. Ca2+-ATPase plays a key role in the mechanism of intracellular calcium overload of pancreatic acinar cells. Qiu Y et al[9] found that the activity of intracellular Ca2+-Mg2+-ATPase was decreased in AP rats.
As it is known, Ca2+ in cytoplasma of pancreatic acinar cells during calcium overload mainly originates from the release of intracellular calcium stores[2627]. In pancreatic acinar cells, Ca2+ released from the store is mainly resequestered into the stores by SERCA and only partly expelled into the extracellular space by PMCA[28]. There are three isoforms of SERCA, including SERCA1, SERCA2 and SERCA3 in rats, in which the expressions of SERCA1 and SERCA2 mRNA were found in pancreatic tissues. Therefore, intracellular Ca2+-ATPase activity was also affected by the change of mRNA expression of the isoforms.
With the consideration that SERCA is the major contributor to maintenance of Ca2+ homeostasis and it may be affected by the isoforms of SERCA, a decreased quantity or activity of SERCA isoform would be expected to induce intracellular calcium overload and subsequently cause AP[29–31] and the rising [Ca2+]i of pancreatic acinar cells positively correlated to the severity of pancreatic pathology[3233]. To explore the mechanism of intracellular calcium overload in AP rats, we studied the change of SERCA1 and SERCA2 mRNA expressions by real-time RT-PCR. We found that the SERCA1 mRNA did not express in pancreatic tissues of normal rats as well as AP rats with the positive control of rat skeletal muscle; the SERCA2 mRNA expression of pancreatic tissues was down-regulated with remarkable elevation of intracellular [Ca2+]i and pathological score of pancreatic tissues in AP rats. The results suggested that down-regulated SERCA2 mRNA expression might take part in the pathogenesis of AP by decreasing the activity of SERCA and increasing [Ca2+]i of pancreatic acinar cells in AP rats.
Chinese medicine CQCQD, modified from Dachengqi decoction, is an effective compound for the treatment of AP[10]. It has been proved to improve systemic inflammatory response syndrome in acute necrotizing pancreatitis through the cholinergic anti-inflammatory pathway[34]. We have also found that the protective effect of CQCQD on pancreatic acinar cells was related to the inhibited exocrine of digestive enzymes and reduced elevation of [Ca2+]i[11]. Chinese herbs in CQCQD including Herba Artemisiae Scopariae, Gardenia and rhubarb may have the activity of calcium channel blockers. Gardenia was found to inhibit the decline of activity of Na+-K+-ATPase and PCMA in AP[9]. Our study showed that in AP rats after treatment with CQCQD, SERCA2 mRNA expression in pancreatic tissues was up-regulated, and intracellular [Ca2+]i of pancreatic acinar cells and pathological score of the pancreatic tissues were decreased. The result indicates that CQCQD might protect pancreatic tissues from injuries by increasing SERCA activity, reducing calcium overload in pancreatic acinar cells and relieving the pathological severity.
In conclusion, the down-regulated expression of pancreatic SERCA2 mRNA may be involved in the pathogenesis of intracellular calcium overload in AP. CQCQD has a protective effect on pancreatic tissues by increasing the expression of SERCA2 mRNA and relieving intracellular calcium overload.
The pathogenesis of acute pancreatitis (AP) has not been fully elucidated up to date, whereas intracellular calcium overload of pancreatic acinar cells has been accepted as the pivot in triggering and deterioration of AP. The increased intracellular free calcium ion concentration ([Ca2+]i) is believed to originate from the influx of extracellular Ca2+ and the release of Ca2+ of intracellular Ca2+ stores. Despite of those theories proposed, the exact mechanism of intracellular calcium overload still remains obscure.
This pilot animal experiment has found Chaiqinchengqi decoction (CQCQD), which is an effective herbal prescription in the treatment of AP, has a protective effect on pancreatic acinar cells in AP rats by inhibiting the elevation of [Ca2+]i. Advanced researches are needed to explore its mechanism.
Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) has been proved to play a key role in the mechanism of intracellular calcium overload of pancreatic acinar cells, but the effect of CQCQD is not available in published studies. The authors innovatively studied the effect of CQCQD on SERCA mRNA expression of pancreatic tissue in AP rats. The result reveals for the first time that CQCQD up-regulated SERCA mRNA expression and relieved intracellular calcium overload to protect pancreatic tissues. Thus, this study elucidated the mechanism of CQCQD in the treatment of AP from genetic point of view.
As the efficacy of CQCQD has been proved in clinical trails, the present study intensively ratified the protective effect on pancreatic tissues in animal experiment. Moreover, the study in genetic mechanism of CQCQD in inhibiting intracellular calcium overload may light up advanced studies on calcium overload in the treatment of AP.
Acute pancreatitis (AP) is a common acute abdominal disorder, caused by the unregulated activation of enzyme within pancreatic acinar cells and the autodigestion of the gland leading to inflammation of pancreas. Calcium overload is intracellular [Ca2+]i abnormally increases which may subsequently lead to cell and tissue damages. Chaiqinchengqi decoction (CQCQD) is a herbal prescription comprising 8 Chinese herbals of Chaihu, Huangqin, Houpo, Zhishi, Zhizi and Mangxiao with the main therapeutic effect of clearing heat to detoxification and purging the bowel to restore the motility, which is a basic compound formula commonly used in the treatment of AP. Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) is an enzyme in arco/endoplasmic reticulum which in physiological conditions utilizes ATP as the energy to pump Ca2+ back to intracellular calcium stores to maintain intracellular calcium concentration in a normal range.
This is a pilot research that focuses on the value of herbal medicine in reducing the severity of AP and exploring the mechanism on relieving intracellular calcium overload. The results of the study extend our understanding in the treatment mechanism of Chinese herbal prescription for AP and have an edificatory value to the studies in this field. Further researches such as protein expression are needed to explain its mechanism.
| 1. | Mifkovic A, Pindak D, Daniel I, Pechan J. Septic complications of acute pancreatitis. Bratisl Lek Listy. 2006;107:296-313. |
| 2. | Liu XB, Jiang JM, Huang ZW, Tian BL, Hu WM, Xia Q, Chen GY, Li QS, Yuan CX, Luo CX. Clinical study on the treatment of severe acute pancreatitis by integrated traditional Chinese medicine and Western medicine. Sichuan Da Xue Xue Bao Yi Xue Ban. 2004;35:204-208. |
| 3. | Mofidi R, Madhavan KK, Garden OJ, Parks RW. An audit of the management of patients with acute pancreatitis against national standards of practice. Br J Surg. 2007;94:844-848. |
| 4. | Beckingham IJ, Bornman PC. ABC of diseases of liver, pancreas, and biliary system. Acute pancreatitis. BMJ. 2001;322:595-598. |
| 5. | Mann DV, Hershman MJ, Hittinger R, Glazer G. Multicentre audit of death from acute pancreatitis. Br J Surg. 1994;81:890-893. |
| 6. | Ward JB, Petersen OH, Jenkins SA, Sutton R. Is an elevated concentration of acinar cytosolic free ionised calcium the trigger for acute pancreatitis? Lancet. 1995;346:1016-1019. |
| 7. | Rattner DW, Napolitano LM, Corsetti J, Compton C, Stanford GG, Warshaw AL, Chernow B. Hypocalcemia in experimental pancreatitis occurs independently of changes in serum nonesterified fatty acid levels. Int J Pancreatol. 1990;6:249-262. |
| 8. | Waterford SD, Kolodecik TR, Thrower EC, Gorelick FS. Vacuolar ATPase regulates zymogen activation in pancreatic acini. J Biol Chem. 2005;280:5430-5434. |
| 9. | Qiu Y, Li YY, Li SG, Song BG, Zhao GF. Effect of Qingyitang on activity of intracellular Ca2+-Mg2+-ATPase in rats with acute pancreatitis. World J Gastroenterol. 2004;10:100-104. |
| 10. | Xue P, Huang ZW, Guo J, Zhao JL, Li YH, Wang ZC. Clinical study of Chaiqin Chengqi Decoction in treating severe acute biliary pancreatitis. Zhongxiyi Jiehe Xuebao. 2005;3:263-265. |
| 11. | Deng LH, Yang XN, Xia Q. Protective effects of Chaiqin Chengqi Decoction on isolated pancreatic acinar cells in acute pancreatitis rats and the mechanisms. Zhongxiyi Jiehe Xuebao. 2008;6:176-179. |
| 12. | Warzecha Z, Dembinski A, Ceranowicz P, Dembinski M, Cieszkowski J, Kusnierz-Cabala B, Naskalski JW, Jaworek J, Konturek SJ, Pawlik WW. Influence of ischemic preconditioning on blood coagulation, fibrinolytic activity and pancreatic repair in the course of caerulein-induced acute pancreatitis in rats. J Physiol Pharmacol. 2007;58:303-319. |
| 13. | Zhang XP, Tian H, Lai YH, Chen L, Zhang L, Cheng QH, Yan W, Li Y, Li QY, He Q. Protective effects and mechanisms of Baicalin and octreotide on renal injury of rats with severe acute pancreatitis. World J Gastroenterol. 2007;13:5079-5089. |
| 14. | Schmidt J, Rattner DW, Lewandrowski K, Compton CC, Mandavilli U, Knoefel WT, Warshaw AL. A better model of acute pancreatitis for evaluating therapy. Ann Surg. 1992;215:44-56. |
| 15. | Chen CC, Wang SS, Tsay SH, Lee FY, Wu SL, Lu RH, Lee SD. A model of experimental acute necrotizing pancreatitis. Zhonghua Yi Xue Za Zhi (Taipei). 1995;56:373-379. |
| 16. | Zhang H, Li YY, Wang SN, Zhang KH, Wu XZ. Effects of lipopolysaccharides on calcium homeostasis in isolated pancreatic acinar cells of rat. Acta Pharmacol Sin. 2003;24:790-795. |
| 17. | Mu SM, Ji XH, Ma B, Yu HM, Li XJ. Differential protein analysis in rat renal proximal tubule epithelial cells in response to acetazolamide and its relation with the inhibition of AQP1. Yao Xue Xue Bao. 2003;38:169-172. |
| 18. | Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. |
| 19. | Mithofer K, Fernandez-del Castillo C, Frick TW, Lewandrowski KB, Rattner DW, Warshaw AL. Acute hypercalcemia causes acute pancreatitis and ectopic trypsinogen activation in the rat. Gastroenterology. 1995;109:239-246. |
| 20. | Shen J, Wu ZP, Xiao H, Pu QF, Song YH, Liu M, Yan XL, Huang JM, Yan LN. The role of cell calcium overload in the conversion of edematous to necrotizing pancreatitis: Effects of verapamil on cytosolic free calcium of rat pancreatic acini. Zhonghua Shiyan Waike Zazhi. 1997;14:201-202. |
| 21. | Kim JY, Kim KH, Lee JA, Namkung W, Sun AQ, Ananthanarayanan M, Suchy FJ, Shin DM, Muallem S, Lee MG. Transporter-mediated bile acid uptake causes Ca2+-dependent cell death in rat pancreatic acinar cells. Gastroenterology. 2002;122:1941-1953. |
| 22. | Voronina S, Longbottom R, Sutton R, Petersen OH, Tepikin A. Bile acids induce calcium signals in mouse pancreatic acinar cells: implications for bile-induced pancreatic pathology. J Physiol. 2002;540:49-55. |
| 23. | Mooren FCh, Hlouschek V, Finkes T, Turi S, Weber IA, Singh J, Domschke W, Schnekenburger J, Kruger B, Lerch MM. Early changes in pancreatic acinar cell calcium signaling after pancreatic duct obstruction. J Biol Chem. 2003;278:9361-9369. |
| 24. | Criddle DN, Gerasimenko JV, Baumgartner HK, Jaffar M, Voronina S, Sutton R, Petersen OH, Gerasimenko OV. Calcium signalling and pancreatic cell death: apoptosis or necrosis? Cell Death Differ. 2007;14:1285-1294. |
| 25. | Wang L, Ma Q, Chen X, Sha H, Ma Z. Effects of resveratrol on calcium regulation in rats with severe acute pancreatitis. Eur J Pharmacol. 2008;580:271-276. |
| 26. | Voronina SG, Barrow SL, Gerasimenko OV, Petersen OH, Tepikin AV. Effects of secretagogues and bile acids on mitochondrial membrane potential of pancreatic acinar cells: comparison of different modes of evaluating DeltaPsim. J Biol Chem. 2004;279:27327-27338. |
| 27. | Fischer L, Gukovskaya AS, Young SH, Gukovsky I, Lugea A, Buechler P, Penninger JM, Friess H, Pandol SJ. Phosphatidylinositol 3-kinase regulates Ca2+ signaling in pancreatic acinar cells through inhibition of sarco(endo)plasmic reticulum Ca2+-ATPase. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1200-G1212. |
| 28. | Che Y, Simon Potocnik, Li CG, Wang ZG. Pharmacological and molecular biological characteristics of store-operated calcium ciannels. Zhongguo Yaolixue Tongbao. 2002;18:365-369. |
| 29. | Pandol SJ. Acute pancreatitis. Curr Opin Gastroenterol. 2005;21:538-543. |
| 30. | Pu QF, Yan LN, Shen J, Liu ZP, Tan Js, Zuo FQ, Wu ZF. Effects of calcium overload in the conversion of acute edematous pancreatitis to necrotizing pancreatitis in rats. Zhonghua Yixue Zazhi. 1999;79:143-145. |
| 31. | Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci USA. 2005;102:14386-14391. |
| 32. | Zhang XP, Li ZJ, Liu DR. Progress in research into the mechanism of Radix salviae miltiorrhizae in treatment of acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2006;5:501-504. |
| 33. | Xue P, Huang ZW, Zhang HY, Xia Q, Li YH, Wang ZC, You Z, Guo J. Impact of Chai Qin Cheng Qi Decoction on cholinergic anti-inflammatory pathway in rats with severe acute pancreatitis. Sichuan Da Xue Xue Bao Yi Xue Ban. 2006;37:66-68. |
| 34. | Jia YJ, Jiang MN, Pei DK, Ji XP, Yu GJ. Effects of gardenia jasminoides ellis on the membranous functions of pancreatic cell in acute pancreatitis. Zhongguo Zhongxiyi Jiehe Waike Zazhi. 1996;2:176-178. |