Published online Apr 14, 2008. doi: 10.3748/wjg.14.2194
Revised: December 31, 2007
Published online: April 14, 2008
AIM: To test the effect of a standardized red wine polyphenolic extract (RWPE) on the phenotype of human liver myofibroblasts in culture.
METHODS: Human myofibroblasts grown from liver explants were used in this study. Cell proliferation was measured with the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay. Signaling events were analyzed by western blot with phospho-specific antibodies. Matrix-metalloproteinase activity was measured with gel zymography.
RESULTS: We found that cell proliferation was dose-dependently decreased by up to 90% by RWPE while cell viability was not affected. Exposure to RWPE also greatly decreased the phosphorylation of ERK1/ERK2 and Akt in response to stimulation by the mitogenic factor platelet-derived growth factor BB (PDGF-BB). Finally, RWPE affected extracellular matrix remodeling by decreasing the secretion by myofibroblasts of matrix-metalloproteinase-2 and of tissue inhibitor of matrix-metalloproteinases-1.
CONCLUSION: Altogether, RWPE decreases the activation state of liver myofibroblasts. The identification of the active compounds in RWPE could offer new therapeutic strategies against liver fibrosis.
- Citation: Neaud V, Rosenbaum J. A red wine polyphenolic extract reduces the activation phenotype of cultured human liver myofibroblasts. World J Gastroenterol 2008; 14(14): 2194-2199
- URL: https://www.wjgnet.com/1007-9327/full/v14/i14/2194.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2194
Liver fibrosis is a serious health problem worldwide. It is a complication of most chronic liver diseases whether due to excessive alcohol consumption, chronic viral hepatitis B or C, non alcoholic steatohepatitis, hemochromatosis or others. The pathophysiology of liver fibrosis has been extensively studied (recently reviewed in[12]). Whatever the initial insult, the abundant extracellular matrix (ECM) characteristic of liver fibrosis is synthesized by myofibroblastic cells. Myofibroblasts are mostly absent from the normal liver but at least two types of resident liver cells can be differentiated into myofibroblasts during liver disease: hepatic stellate cells, and portal fibroblasts[3]. Myofibroblastic differentiation is characterized by a high rate of cell proliferation and of ECM synthesis and by cytoskeletal changes, notably expression of alpha smooth muscle actin (ASMA) that confers contractile properties to the cells[4]. In addition, degradation of the normal liver ECM results from an increased secretion of the enzyme matrix metalloproteinase-2 (MMP-2) by myofibroblasts, while the proteolytic degradation of the abnormal ECM is inhibited due to a high level synthesis of a MMP inhibitor, tissue inhibitor of MMP-1 (TIMP-1)[12]. In the recent years, a series of natural products were shown to be of potential benefit against liver fibrosis[56]. For instance, we and others found that a polyphenolic component of red wine, trans-resveratrol, was able to strongly deactivate liver fibrogenic cells[78], while a related molecule, trans-piceid, was uneffective[8]. However, red wine contains many other polyphenolic substances, and red wine polyphenolic extracts (RWPE) showed many interesting biological effects in other settings, notably in the prevention of experimental atherosclerosis[9]. One of the mechanisms postulated in this context is related to the inhibitory effect of RWPE on the proliferation of vascular smooth muscle cells[10]. Since vascular smooth muscle cells share many characteristics with myofibroblasts, we tested the hypothesis that RWPE would affect the phenotypic characteristics of human liver myofibroblasts, especially those related to their pro-fibrogenic activity.
Preparation and characterization of the polyphenolic extract (RWPE) from a red French wine (Corbières, A. O. C.) was as described previously[1112]. One liter of red wine produced 2.9 g of extract, which contained 471 mg/g total phenolic compounds expressed as gallic acid. Phenolic levels in the extract were obtained according to HPLC analysis procedure. In particular, the extract contained 8.6 mg/g catechin, 8.7 mg/g epicatechin, dimers (B1: 6.9 mg/g; B2: 8.0 mg/g; B3: 20.7 mg/g; and B4: 0.7 mg/g), anthocyanins (malvidin-3-glucoside: 11.7 mg/g; peonidin-3-glucoside: 0.66 mg/g; and cyanidin-3-glucoside: 0.06 mg/g), and phenolic acids (gallic acid: 5.0 mg/g; caffeic acid 2.5 mg/g; and caftaric acid: 12.5 mg/g). Stock solutions (1 mg/mL) were prepared in distilled water containing 1% ethanol and further diluted in culture medium. All dilutions were adjusted so as to contain 0.1% ethanol, and medium with 0.1% ethanol was used as a control.
Fetal calf serum (FCS) was from Dutscher (Brumath, France) and human serum from Etablissement Français du Sang (Bordeaux, France). Epidermal growth factor (EGF) was from Peprotech (Tebu, Le Perray en Yvelines, France) and recombinant platelet-derived growth factor BB (PDGF-BB) was from Eurobio (les Ulis, France). Phospho Thr308 AKT-1 and AKT-1 antibodies, phospho-ERK1/ERK2 and total ERK antibodies were from Cell Signaling Technology (Ozyme, Saint Quentin Yvelines, France). ASMA and vimentin antibodies were from Dako (Trappes, France). The TIMP antibody was from Santa Cruz Biotechnologies (Santa Cruz, CA). Beta-actin antibody was from Sigma (Saint Quentin Fallavier, France). IRDye 680 and IRDye 800 conjugated secondary antibodies; Odyssey Blocker and Odyssey infrared imaging system were from LI-COR Biosciences (ScienceTec, Les Ulis, France).
Human hepatic myofibroblasts were obtained from explants of non-tumor liver resected during partial hepatectomy and characterized as described previously[1314]. Specifically, the procedure, based on the selective growth advantage of myofibroblasts in the culture conditions used, allowed for 100% pure myofibroblasts population, as shown by positive staining for ASMA and vimentin, and negative staining for CD68 (a Kupffer cell marker), von Willebrand factor (an endothelial cell marker) or cytokeratin (an epithelial cell marker). This procedure is in accordance with INSERM ethical regulation imposed by French legislation. Myofibroblasts were used between the 3rd and the 6th passage, and were grown in DMEM containing 5% FCS, 5% pooled human serum and 5 ng/mL EGF. EGF was removed from the medium at least 3 d before experiments.
Cells were seeded at a density of 104/well in 24-well plates. On the following day, the medium was replaced by DMEM with 5% FCS and RWPE dilutions to be tested. After three or seven days, the medium was removed and the cells incubated with 1 mg/mL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide] for 2 h at 37°C[15]. The crystals were then dissolved with DMSO and the optical density was recorded at 540 nm. Results were expressed as proliferation index = (B-A)/(C-A) where A is the optical density recorded at d 0, B is the optical density with the test compound and C is the optical density obtained in the control wells.
Cells were grown to confluence in 24-well plates, serum-starved for 24 h, then incubated with RWPE. After 24 h, the cells were lysed with NaH2PO4/Na2HPO4 50 mmol/L, pH 7.4, NaCl 2 mol/L, EDTA 2 mmol/L, and DNA content was measured as described[16].
ERK and Akt phosphorylation was measured essentially as described previously[17]. Briefly, cells were grown to confluency and serum-starved for three days. They were then pre-incubated with RWPE in serum-free Waymouth medium for 1 h, and then exposed to 20 ng/mL PDGF-BB for 10 min. At the end of the incubation, cell lysates were prepared in the presence of proteases and phosphatases inhibitors as described[18]. Proteins were measured with a Bio-Rad assay. Equivalent amounts of proteins were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and analyzed by two color Western blotting with antibodies to total-ERK and phospho-ERK, or phospho-Akt-1 and β-actin. Blots were blocked in Odyssey Blocker and incubated simultaneously with both primary antibodies, followed by both IR-labeled secondary antibodies. Signals were detected and quantified using the Odyssey infrared imaging system.
The expression of ASMA and of vimentin was measured in cell extracts from cells exposed for seven days to RWPE using Western blot as described[8].
The concentration of TIMP-1 in conditioned medium was also measured by Western blot. Confluent cells were incubated for two days with RWPE, the medium was collected, centrifuged and aliquots, normalized for DNA content of the cell layers, were analyzed by Western blot.
The detection of MMP-2 in conditioned medium was performed by gelatin zymography[19], essentially as previously described[820]. Briefly, cells were grown to confluence in 24-well plates, serum-starved for 24 h, then incubated with RWPE. The medium was collected, centrifuged and aliquots, normalized for DNA content of the cell layers, were analyzed on 8% SDS-PAGE gels containing 1 mg/mL gelatin. Following staining with Coomassie blue, MMP activity is detected as a white zone on a blue background.
Cells were seeded at a density of 104/well on glass coverslips in 24-well plates. On the following day, the medium was replaced by DMEM with 5% FCS and RWPE dilutions to be tested. After three days, cells were fixed with methanol at -20°C, then incubated sequentially with an anti-ASMA monoclonal antibody and a Texas Red-conjugated secondary antibody. The slides were examined with a Zeiss Axioplan fluorescence microscope.
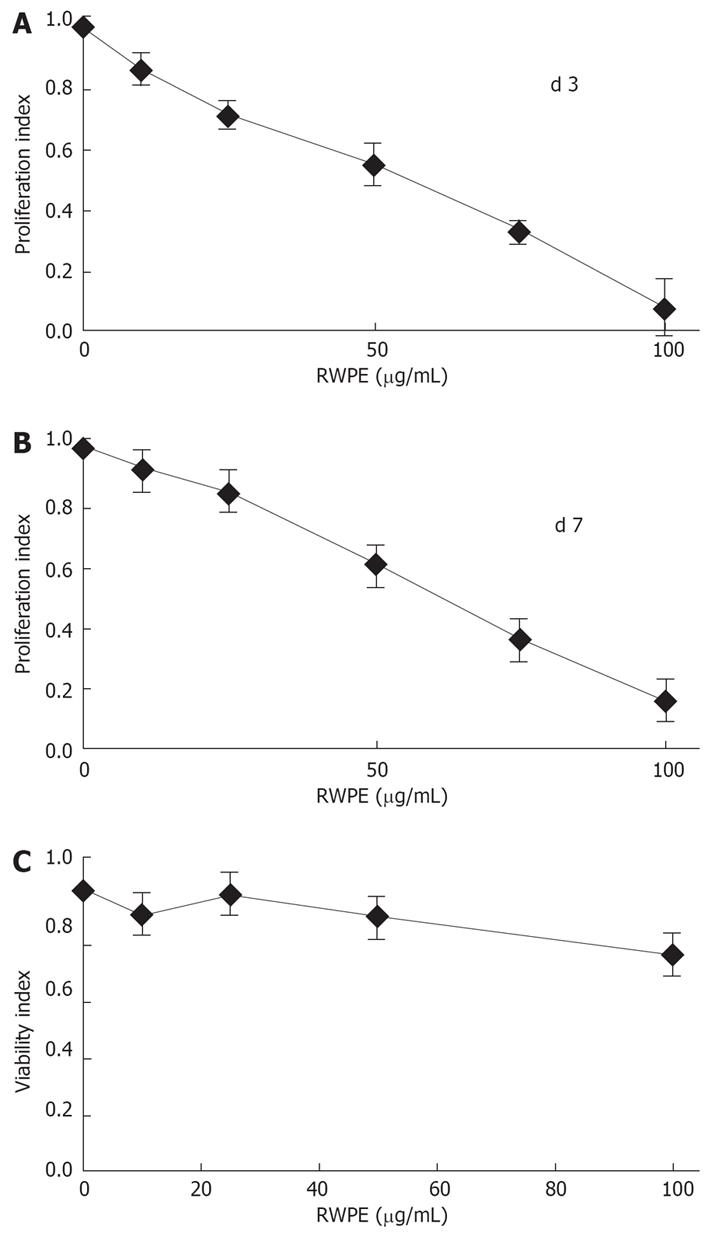
The RWPE dose-dependently inhibited the proliferation of myofibroblasts down to 7.8% ± 4.5% of control at d 3 and 15.8 ± 8.0 at d 7 (Figure 1A and B). The concentration that reduced growth by 50% was 50 &mgr;g/mL. A toxic effect of RWPE on cells could be ruled out because no morphological signs of toxicity nor cell detachment were observed (Figure 2); furthermore, when confluent cells were exposed to a dose range of RWPE, there was no decrease in DNA content of the cell layers even at the highest concentrations (Figure 1C).
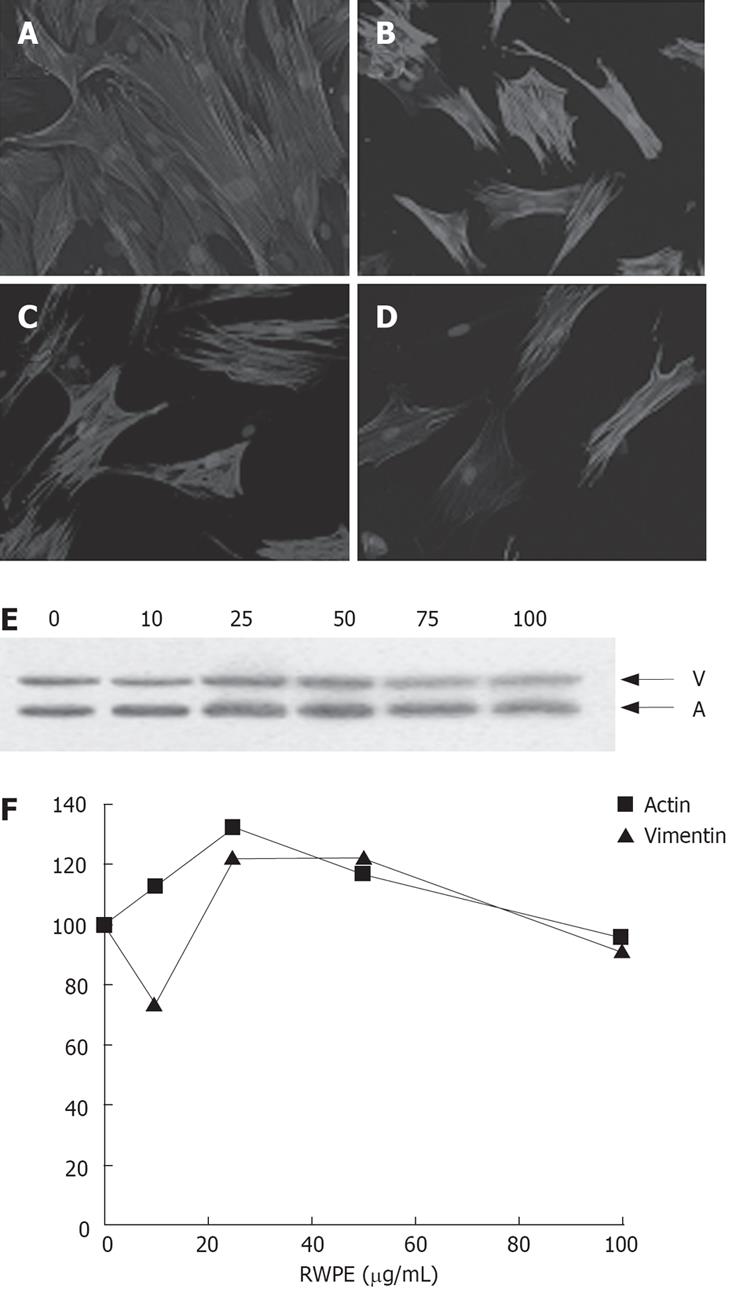
Expression of the cytoskeletal protein ASMA is hallmark of activated liver fibrogenic cells. We found that long term (up to seven days) exposure of liver myofibroblasts to RWPE did not affect ASMA expression. This was shown both by immunofluorescence and by Western blot (Figure 2). In addition, the expression of another cytoskeletal protein, vimentin, which expression is independent of fibrogenic cell activation, was also unaffected by RWPE treatment (Figure 2C).
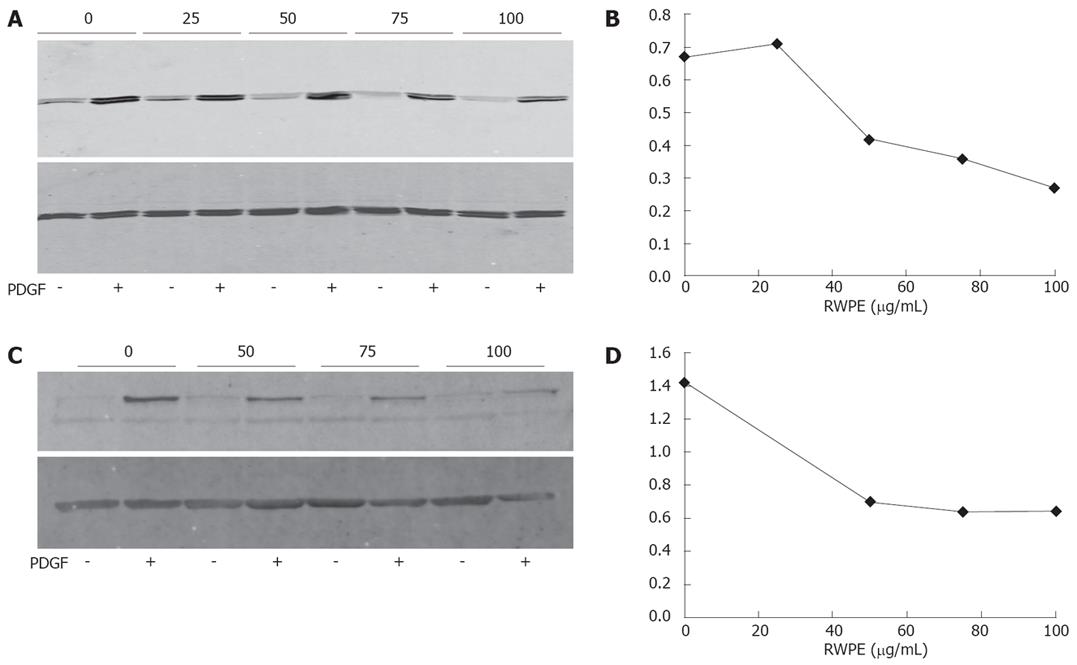
In order to delineate the mitogenic pathways affected by RWPE, myofibroblasts were briefly exposed to PDGF-BB. PDGF-BB is major mediator of liver fibrogenic cell activation, as it stimulates notably their proliferation[1321] and migration[2223], and is abundant in serum. We then examined the effect of RWPE on signalization pathways elicited by PDGF-BB. As expected, treatment with PDGF-BB induced a major increase in the phosphorylation of ERK1/ERK2 and of Akt. Exposure to RWPE greatly decreased the effect of PDGF-BB on the phosphorylation of both ERK1/ERK2 and Akt (Figure 3).
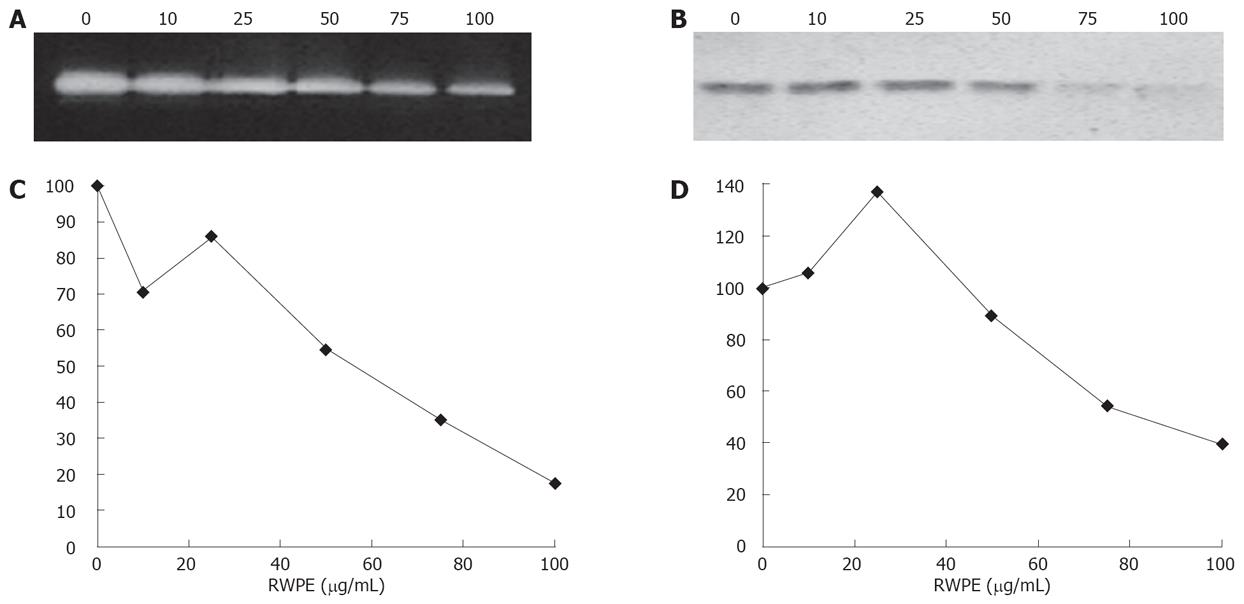
A high level expression of the matrix remodeling enzyme MMP-2[24], and of the inhibitor of extracellular matrix degradation, TIMP-1[25], is characteristic of activated liver fibrogenic cells. Gelatin zymography showed a gelatinolytic band migrating at an apparent molecular weight of 72 kDa characteristic of MMP-2. The RWPE strikingly and dose-dependently decreased the secretion of MMP-2 (Figure 4A). It also greatly decreased TIMP-1 secretion, as assessed by Western blot (Figure 4B).
We show here that a standardized RWPE has striking effects on the phenotype of human liver myofibroblasts. This is shown by a decreased proliferation rate together with decreased secretion of MMP-2 and of TIMP-1. These effects are not the consequence of a direct toxicity of the extract on cells as shown by morphological examination and DNA content measurement. We investigated further the mechanism of the decreased proliferation rate and found that RWPE treatment largely abolished the phosphorylation of ERK1/ERK2 and of Akt induced by the mitogenic factor PDGF-BB. Previously, Iijima et al showed that a RWPE decreased Akt but not ERK activation in vascular smooth muscle cells stimulated with PDGF[26]. The differences may be due to the fact that different RWPEs were used, or to a true cell specificity. For instance, despite the fact that myofibroblasts and smooth muscle cells are related cells, we found that they had a differential response to trans-resveratrol[8]. Some of the effects of the RWPE are reminiscent of those of trans-resveratrol, raising the possibility that RWPE effects may be due to this compound. However, resveratrol is present only at low concentration in wine (reviewed in[27]) and is unlikely to explain the effects of RWPE. In addition, whereas it does indeed decrease myofibroblasts proliferation, MMP-2 secretion[8] and Akt activation[28], it does not affect ERK activation in response to PDGF[28]. Furthermore, contrary to RWPE, it does decrease ASMA expression[8].
The RWPE effects observed in the present study are potentially of benefit against liver fibrosis, if they held true in the in vivo situation. This seems obvious for the reduced cell proliferation that will decrease the number of ECM-producing cells. Notably, the drastic effect on TIMP-1 secretion may have a major implication since TIMP-1 overexpression is considered one of the main determinants of liver fibrosis through the inhibition of ECM-degrading enzymes activity[2930].
Thus, although excessive wine consumption is one of the major causes of chronic liver diseases, wine itself may unexpectedly contain potent anti-fibrotic compounds. The identification of the active compounds in RWPE could offer new therapeutic strategies against liver fibrosis.
Liver fibrosis is a worldwide problem, as it complicates all chronic liver diseases. There is no established treatment for liver fibrosis.
A series of natural products have shown beneficial effects on liver fibrosis in cell culture and animal models. In addition, red wine polyphenols were shown to reduce the proliferation of vascular smooth muscle cells, a cell type closely related to liver fibrogenic cells.
We found that a standardized red wine polyphenolic extract greatly decreased the proliferation of human liver fibrogenic cells. It also reduced their synthesis of matrix-metalloproteinase-2 and of the tissue inhibitor of matrix metalloproteinase-1, thus suggesting that it could affect the cell ability to remodel the extracellular matrix.
The identification of the active compound(s) in the extract could lead to in vivo testing of its anti-fibrotic activity in liver disease.
Liver fibrosis: a common complication of most chronic liver diseases, where an excess of extracellular matrix components are deposited in the liver.
This is a very nice communication and provides mechanistic data, is well-written, and ends with a thorough discussion. The results of this study are novel and potentially lay the ground work for the understanding of the effect of wine/wine extracts on human hepatic fibrosis.
| 1. | Lotersztajn S, Julien B, Teixeira-Clerc F, Grenard P, Mallat A. Hepatic fibrosis: molecular mechanisms and drug targets. Annu Rev Pharmacol Toxicol. 2005;45:605-628. |
| 2. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. |
| 3. | Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265-278. |
| 4. | Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730-2741. |
| 5. | Gebhardt R. Oxidative stress, plant-derived antioxidants and liver fibrosis. Planta Med. 2002;68:289-296. |
| 6. | Stickel F, Brinkhaus B, Krahmer N, Seitz HK, Hahn EG, Schuppan D. Antifibrotic properties of botanicals in chronic liver disease. Hepatogastroenterology. 2002;49:1102-1108. |
| 7. | Kawada N, Seki S, Inoue M, Kuroki T. Effect of antioxidants, resveratrol, quercetin, and N-acetylcysteine, on the functions of cultured rat hepatic stellate cells and Kupffer cells. Hepatology. 1998;27:1265-1274. |
| 8. | Godichaud S, Krisa S, Couronne B, Dubuisson L, Merillon JM, Desmouliere A, Rosenbaum J. Deactivation of cultured human liver myofibroblasts by trans-resveratrol, a grapevine-derived polyphenol. Hepatology. 2000;31:922-931. |
| 9. | Waddington E, Puddey IB, Croft KD. Red wine polyphenolic compounds inhibit atherosclerosis in apolipoprotein E-deficient mice independently of effects on lipid peroxidation. Am J Clin Nutr. 2004;79:54-61. |
| 10. | Iijima K, Yoshizumi M, Ouchi Y. Effect of red wine polyphenols on vascular smooth muscle cell function--molecular mechanism of the 'French paradox'. Mech Ageing Dev. 2002;123:1033-1039. |
| 11. | Auger C, Caporiccio B, Landrault N, Teissedre PL, Laurent C, Cros G, Besancon P, Rouanet JM. Red wine phenolic compounds reduce plasma lipids and apolipoprotein B and prevent early aortic atherosclerosis in hypercholesterolemic golden Syrian hamsters (Mesocricetus auratus). J Nutr. 2002;132:1207-1213. |
| 12. | Carando S, Teissedre PL. Catechin and procyanidin levels in French wines: contribution to dietary intake. Basic Life Sci. 1999;66:725-737. |
| 13. | Win KM, Charlotte F, Mallat A, Cherqui D, Martin N, Mavier P, Preaux AM, Dhumeaux D, Rosenbaum J. Mitogenic effect of transforming growth factor-beta 1 on human Ito cells in culture: evidence for mediation by endogenous platelet-derived growth factor. Hepatology. 1993;18:137-145. |
| 14. | Blazejewski S, Preaux AM, Mallat A, Brocheriou I, Mavier P, Dhumeaux D, Hartmann D, Schuppan D, Rosenbaum J. Human myofibroblastlike cells obtained by outgrowth are representative of the fibrogenic cells in the liver. Hepatology. 1995;22:788-797. |
| 15. | Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271-277. |
| 16. | Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344-352. |
| 17. | Neaud V, Duplantier JG, Mazzocco C, Kisiel W, Rosenbaum J. Thrombin up-regulates tissue factor pathway inhibitor-2 synthesis through a cyclooxygenase-2-dependent, epidermal growth factor receptor-independent mechanism. J Biol Chem. 2004;279:5200-5206. |
| 18. | Neaud V, Faouzi S, Guirouilh J, Le Bail B, Balabaud C, Bioulac-Sage P, Rosenbaum J. Human hepatic myofibroblasts increase invasiveness of hepatocellular carcinoma cells: evidence for a role of hepatocyte growth factor. Hepatology. 1997;26:1458-1466. |
| 19. | Herron GS, Banda MJ, Clark EJ, Gavrilovic J, Werb Z. Secretion of metalloproteinases by stimulated capillary endothelial cells. II. Expression of collagenase and stromelysin activities is regulated by endogenous inhibitors. J Biol Chem. 1986;261:2814-2818. |
| 20. | Taras D, Blanc JF, Rullier A, Dugot-Senant N, Laurendeau I, Bieche I, Pines M, Rosenbaum J. Halofuginone suppresses the lung metastasis of chemically induced hepatocellular carcinoma in rats through MMP inhibition. Neoplasia. 2006;8:312-318. |
| 21. | Pinzani M, Gesualdo L, Sabbah GM, Abboud HE. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989;84:1786-1793. |
| 22. | Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor's actions on hepatic stellate cells. Gastroenterology. 1997;112:1297-1306. |
| 23. | Tangkijvanich P, Santiskulvong C, Melton AC, Rozengurt E, Yee HF Jr. p38 MAP kinase mediates platelet-derived growth factor-stimulated migration of hepatic myofibroblasts. J Cell Physiol. 2002;191:351-361. |
| 24. | Arthur MJ, Stanley A, Iredale JP, Rafferty JA, Hembry RM, Friedman SL. Secretion of 72 kDa type IV collagenase/gelatinase by cultured human lipocytes. Analysis of gene expression, protein synthesis and proteinase activity. Biochem J. 1992;287:701-707. |
| 25. | Iredale JP, Murphy G, Hembry RM, Friedman SL, Arthur MJ. Human hepatic lipocytes synthesize tissue inhibitor of metalloproteinases-1. Implications for regulation of matrix degradation in liver. J Clin Invest. 1992;90:282-287. |
| 26. | Iijima K, Yoshizumi M, Hashimoto M, Akishita M, Kozaki K, Ako J, Watanabe T, Ohike Y, Son B, Yu J. Red wine polyphenols inhibit vascular smooth muscle cell migration through two distinct signaling pathways. Circulation. 2002;105:2404-2410. |
| 28. | Godichaud S, Si-Tayeb K, Auge N, Desmouliere A, Balabaud C, Payrastre B, Negre-Salvayre A, Rosenbaum J. The grape-derived polyphenol resveratrol differentially affects epidermal and platelet-derived growth factor signaling in human liver myofibroblasts. Int J Biochem Cell Biol. 2006;38:629-637. |
| 29. | Roderfeld M, Weiskirchen R, Wagner S, Berres ML, Henkel C, Gretzinger J, Gressner AM, Matern S, Roeb E. Inhibition of hepatic fibrogenesis by matrix metalloproteinase-9 mutants in mice. FASEB J. 2006;20:444-454. |
| 30. | Parsons CJ, Bradford BU, Pan CQ, Cheung E, Schauer M, Knorr A, Krebs B, Kraft S, Zahn S, Brocks B. Antifibrotic effects of a tissue inhibitor of metalloproteinase-1 antibody on established liver fibrosis in rats. Hepatology. 2004;40:1106-1115. |