Published online Mar 28, 2008. doi: 10.3748/wjg.14.1958
Revised: December 8, 2007
Published online: March 28, 2008
This report describes serial observations of the growth process of a small invasive ductal carcinoma (IDC) of the pancreas from imaging studies. Histopathological studies showed IDC with macroscopic retention cysts proximal to an intraductal papillary-mucinous adenoma with mild atypia of the branch duct type in the pancreatic body, with no relation between the two lesions. IDC was demonstrated as an extremely low-echoic mass resembling a cyst with an unclear margin on the initial endoscopic ultrasonography. We misinterpreted the low-echoic mass as a benign intraductal mucinous-papillary neoplasm (IPMN) based on findings of other imaging studies, and the patient was followed-up. The mass increased from 7 mm to 13 mm in diameter over 22 mo, and remained smaller than 10 mm in diameter for about 420 d. The tumor volume doubling time was 252 d. The Ki67 labeling index was 15.9%, similar to that described in previous reports. Hence, IDC may grow slowly while remaining small.
- Citation: Hisa T, Ohkubo H, Shiozawa S, Ishigame H, Takamatsu M, Furutake M, Nobukawa B, Suda K. Growth process of small pancreatic carcinoma: A case report with imaging observation for 22 months. World J Gastroenterol 2008; 14(12): 1958-1960
- URL: https://www.wjgnet.com/1007-9327/full/v14/i12/1958.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1958
Invasive ductal carcinoma (IDC) of the pancreas has the worst prognosis of all digestive carcinomas. Its histogenesis and natural progression are unknown, and small IDCs are still difficult to detect. This is an extremely rare case of a small IDC in which the growth process was observed on imaging studies for 22 mo.
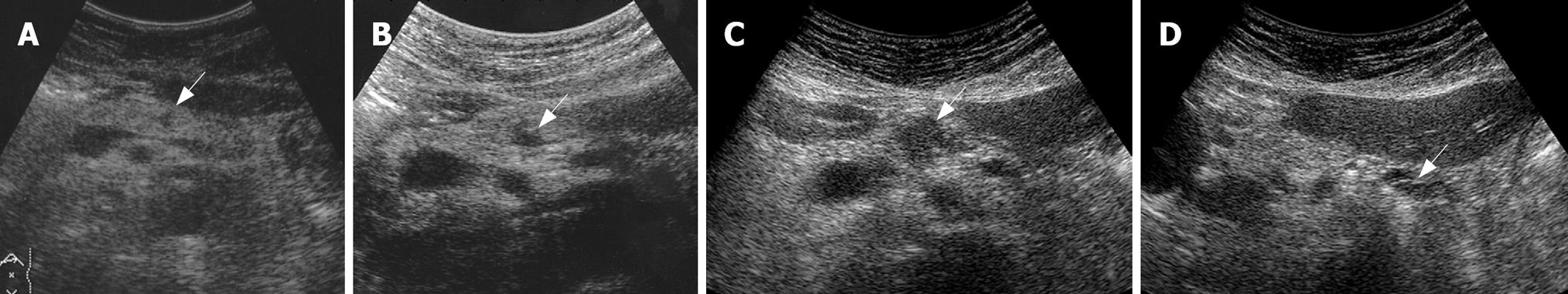
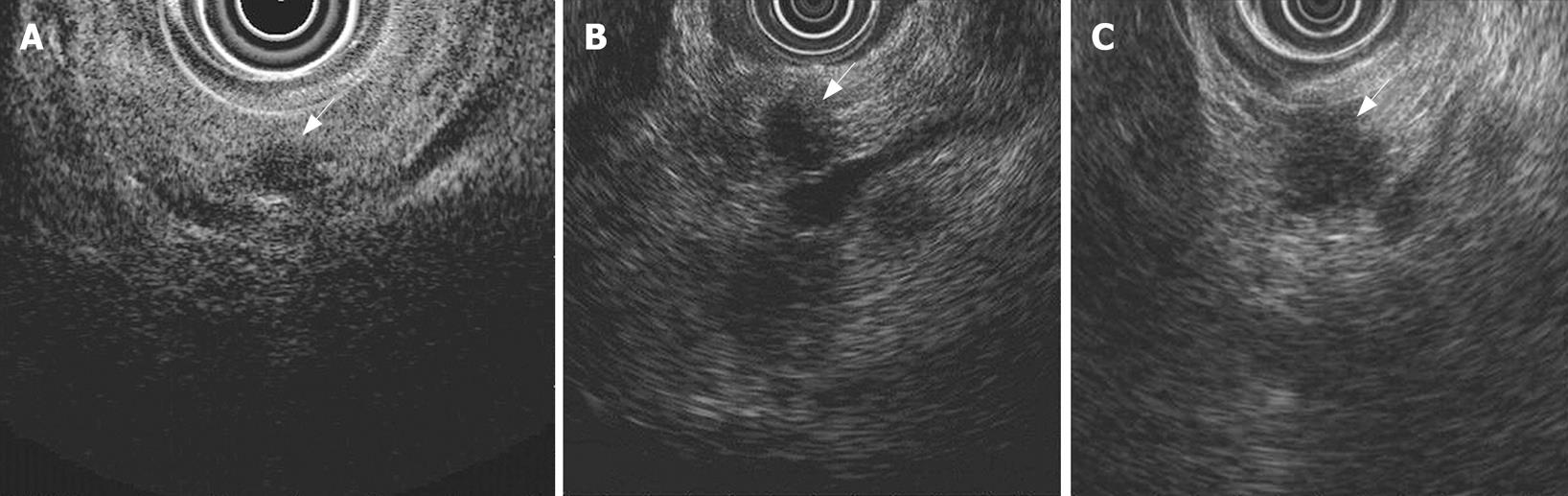
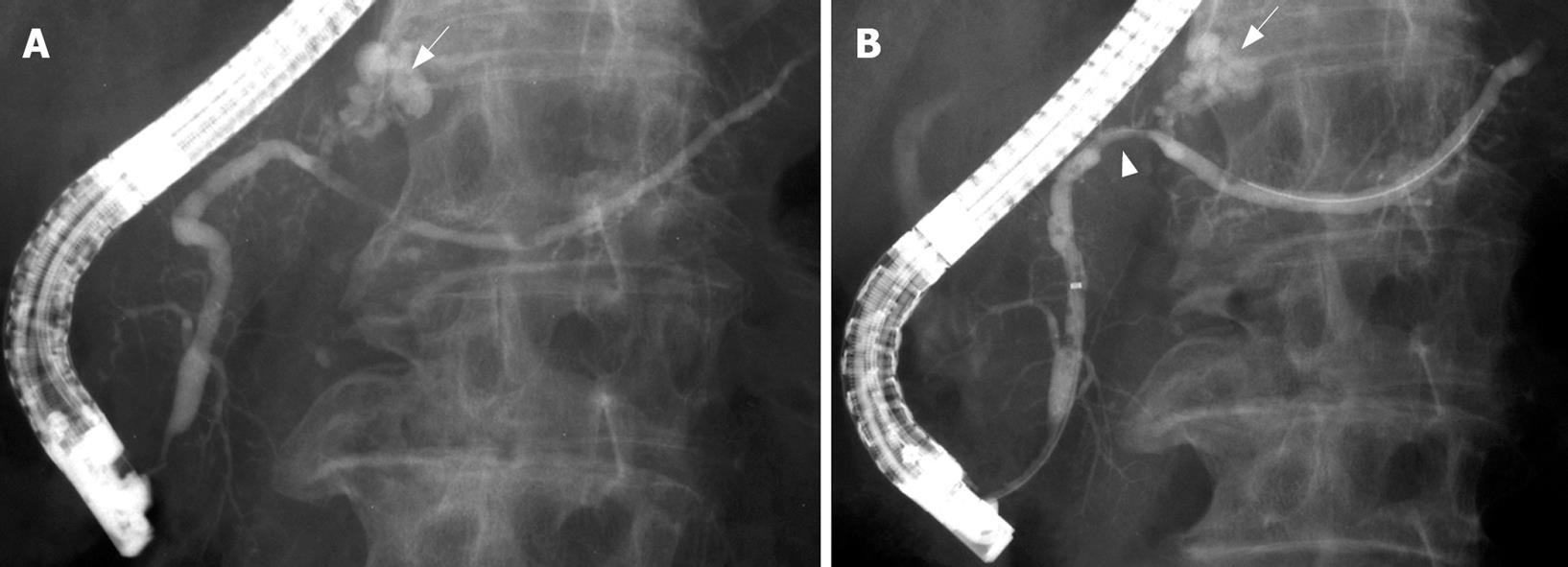
A 77-year-old man, who was being followed-up for chronic Hepatitis C infection, was referred to our department for evaluation of a pancreatic mass on screening transabdominal ultrasonography (US). US showed a low-echoic mass, 7 mm in diameter, in the pancreatic body, without distal dilatation of the main pancreatic duct (MPD) (Figure 1A). Endoscopic ultrasonography (EUS) demonstrated an extremely low-echoic mass with posterior echo enhancement, which appeared to be a cyst (Figure 2A). Contrast-enhanced computed tomography (CT) scan and magnetic resonance cholangiopancreatography (MRCP) revealed a grape-like cyst in the pancreatic body. Endoscopic retrograde pancreatography (ERP) indicated mucus in the MPD and a dilated branch pancreatic duct in the pancreatic body without mural nodules (Figure 3A). We misinterpreted the low-echoic mass in US/EUS images as a benign intraductal mucinous-papillary neoplasm (IPMN) of the branch duct type, and observed the lesion by US/EUS every 6 mo (Figures 1B and 2B).
Twenty-two months after the initial diagnosis, US/EUS showed the low-echoic mass had increased in diameter to 13 mm (Figures 1C and 2C). Then, for the first time, we detected a grape-like cyst distal to the lesion on US/EUS (Figure 1D), and recognized the low-echoic mass being followed was not identical to the initially diagnosed IPMN. A contrast-enhanced CT scan revealed a hypovascular area proximal to the grape-like cyst. On MRCP, the cyst did not show any change, but MPD in the pancreatic body became unclear. ERP demonstrated slight compression of the MPD proximal to a dilated branch duct (Figure 3B), and brush cytology did not detect any malignant cells.
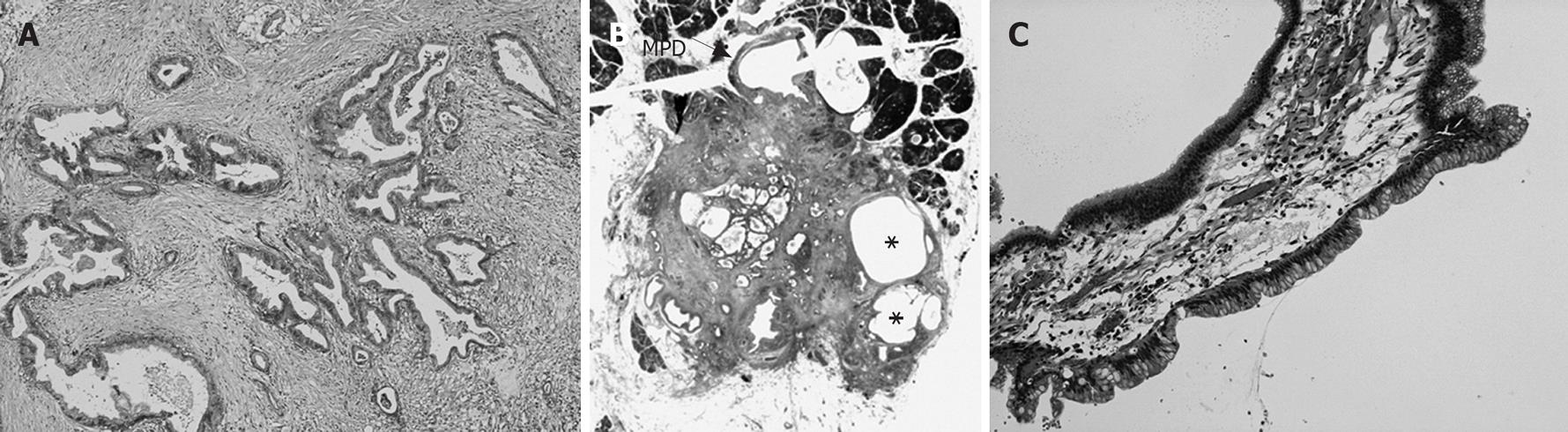
Distal pancreatectomy was performed under a diagnosis of IDC concomitant with IPMN. The cut surface of the resected specimen showed a white, irregular-shaped mass with a clear margin in the pancreatic body, and a dilated branch pancreatic duct distal to the mass. Microscopic examination showed that the 13 mm × 12 mm mass was composed of moderately differentiated tubular adenocarcinoma with desmoplastic fibrosis limited to the pancreas, and included macroscopic retention cysts (Figures 4A and B). This mass was diagnosed as an ordinary IDC, not derived from IPMN, and minimal intraductal extension of IDC was seen in the MPD compressed by the mass. A dilated branch pancreatic duct distal to the IDC was lined with low-papillary columnar cells with intracellular mucus; this was diagnosed as intraductal papillary-mucinous adenoma with mild atypia (Figure 4C). The IDC and IPMN were unrelated and were separated by normal epithelium in the MPD. After follow-up for 32 mo, our patient has shown no evidence of recurrence.
This patient demonstrated small IDC concomitant with synchronous IPMN. A branch duct type IPMN without mural nodules is a candidate for regular follow-up[1–3]. Because IPMN is sometimes superimposed on synchronous/metachronous IDC, the entire pancreas should be included in follow-up examinations for IPMN[24]. In our case, a low-echoic mass seen at the initial US/EUS was misinterpreted as being identical to a cystic dilated branch pancreatic duct seen in other imaging studies, and was clinically diagnosed as a benign IPMN. Therefore, short-term observation and repeated examination were selected.
Although a small IDC is usually depicted as a solid, low-echoic mass on EUS[5], the initial EUS in this case showed an extremely low-echoic mass resembling a cyst with an unclear margin. Histopathologically, the IDC included macroscopic retention cysts. Therefore, we considered the cyst-like mass seen at the initial EUS reflected IDC with retention cysts. It is difficult to diagnose IDC on initial imaging examination, although it is unknown whether transpapillary cytology would indicate malignant cells. However, EUS-guided fine-needle aspiration biopsy (EUS-FNAB) should be performed with caution, because cases of seeding after EUS-FNAB have been reported[67]. Retrospectively, we ought to have noticed the low-echoic mass that we were following was slowly increasing in diameter, and should have selected surgical resection sooner.
In our case, the tumor volume doubling time of the IDC on US/EUS was 252 d. Furukawa et al[8] have reported the tumor volume doubling time of IDC on CT scan was 159 ± 67 (median, 144) d, shorter than that in our patient. The reason for this difference may be that the initial diameter of IDCs in their study ranged from 13 to 47 mm, with a mean of 19 mm, and because the final diameter ranged from 15 to 47 mm with a mean of 30 mm, larger than that in our patient. More interesting is the fact the tumor remained smaller than 10 mm in diameter for about 420 d. The Ki67 labeling index in the present case was 15.9%, while that of the previous reports ranged from 14.5% to 29.3%[910]. Hence, an IDC may grow slowly while remaining small, although the accumulation of more cases is necessary.
| 1. | Maguchi H. Clinicopathological and diagnostic study of mucin producing pancreatic tumors. Nippon Shokakibyo Gakkai Zasshi. 1994;91:1003-1015. |
| 2. | Kobayashi G, Fujita N, Noda Y, Ito K, Horaguchi J, Takasawa O, Akaishi S, Tsuchiya T, Kobari M. Mode of progression of intraductal papillary-mucinous tumor of the pancreas: analysis of patients with follow-up by EUS. J Gastroenterol. 2005;40:744-751. |
| 3. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. |
| 4. | Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano K, Tanaka M. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology. 2002;2:484-490. |
| 5. | Ariyama J, Suyama M, Satoh K, Wakabayashi K. Endoscopic ultrasound and intraductal ultrasound in the diagnosis of small pancreatic tumors. Abdom Imaging. 1998;23:380-386. |
| 6. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. |
| 7. | Paquin SC, Gariepy G, Lepanto L, Bourdages R, Raymond G, Sahai AV. A first report of tumor seeding because of EUS-guided FNA of a pancreatic adenocarcinoma. Gastrointest Endosc. 2005;61:610-611. |
| 8. | Furukawa H, Iwata R, Moriyama N. Growth rate of pancreatic adenocarcinoma: initial clinical experience. Pancreas. 2001;22:366-369. |
| 9. | Terada T, Ohta T, Kitamura Y, Ashida K, Matsunaga Y. Cell proliferative activity in intraductal papillary-mucinous neoplasms and invasive ductal adenocarcinomas of the pancreas: an immunohistochemical study. Arch Pathol Lab Med. 1998;122:42-46. |
| 10. | Yamasaki S, Suda K, Nobukawa B, Sonoue H. Intraductal spread of pancreatic cancer. Clinicopathologic study of 54 pancreatectomized patients. Pancreatology. 2002;2:407-412. |