Published online Mar 28, 2008. doi: 10.3748/wjg.14.1851
Revised: January 15, 2008
Published online: March 28, 2008
AIM: To evaluate the effect of Chinese traditional medicinal prescription, JIANPI HUOXUE decoction (JHD) on cytokine secretion pathway in rat liver induced by lipopolysaccharide (LPS).
METHODS: Twenty-four male SD rats were divided into normal group (n = 4), model group (n = 10) and JHD group (n = 10) randomly. Rats in model group and JHD group were administrated with normal saline or JHD via gastrogavage respectively twice a day for 3 d. One hour after the last administration, rats were injected with LPS via tail vein, 50 &mgr;g/kg. Simultaneously, rats in normal group were injected with equivalent normal saline. After LPS stimulation for 1.5 h, serum and liver tissue were collected. Pathological change of liver tissues was observed through hematoxylin-eosin (H.E.) staining. Tumor necrosis factor alpha (TNF-α) in serum were assayed by enzyme linked immunosorbent assay (ELISA). The protein expression of TNF-α, phosphorylated inhibit-κB (p-IκB) and CD68 in liver were assayed by Western blot. The distribution of CD68 protein in liver was observed through immunohistochemical staining. The mRNA expression of TNF-α, interleukin-6 (IL-6), CD14, toll-like receptor 2 (TLR2) and TLR4 in liver were assayed by real-time RT-PCR.
RESULTS: Predominant microvesicular change, hepatocyte tumefaction and cytoplasm dilution were observed in liver tissues after LPS administration as well as obvious CD68 positive staining in hepatic sinusoidal. After LPS stimulation, serum TNF-α (31.35 ± 6.06 vs 12 225.40 ± 9007.03, P < 0.05), protein expression of CD68 (1.13 ± 0.49 vs 3.36 ± 1.69, P < 0.05), p-IκB (0.01 ± 0.01 vs 2.07 ± 0.83, P < 0.01) and TNF-α (0.27 ± 0.13 vs 1.29 ± 0.37, P < 0.01) in liver and mRNA expression of TNF-α (1.96 ± 2.23 vs 21.45 ± 6.00, P < 0.01), IL-6 (4.80 ± 6.42 vs 193.50 ± 36.36, P < 0.01) and TLR2 (1.44 ± 0.62 vs 4.16 ± 0.08, P < 0.01) in liver were also increased significantly. These pathological changes were all improved in JHD group. On the other hand, TLR4 mRNA (1.22 ± 0.30 vs 0.50 ± 0.15, P < 0.05) was down-regulated and CD14 mRNA increased but not significantly after LPS stimulation.
CONCLUSION: JHD can inhibit cytokine secretion pathway induced by LPS in rat liver, which is probably associated with its regulation on CD68, p-IκB and endotoxin receptor TLR2.
- Citation: Peng JH, Hu YY, Cheng Y, Han C, Xu LL, Feng Q, Chen SD, Tao Q, Li HS, Li XM. Effect of JIANPI HUOXUE decoction on inflammatory cytokine secretion pathway in rat liver with lipopolysaccharide challenge. World J Gastroenterol 2008; 14(12): 1851-1857
- URL: https://www.wjgnet.com/1007-9327/full/v14/i12/1851.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1851
When activated under physiologically challenging conditions, such as endotoxemia or immune reactions, macrophages release large amounts of cytokines, interleukins and prostanoids, which may result in organ damage. Tumor necrosis factor alpha (TNF-α) is a potent inflammatory cytokine which can exert a variety of effects on cells ranging from mitochondrial damage and oncotic or apoptotic necrosis to cell proliferation[1]. TNF-α may also prompt the accumulation of neutrophils (PMNs) by activating endothelial cells[12]. It can indirectly promote toxicity by priming PMNs to release reactive oxygen and nitrogen species and proteases that damage nearby cells[23]. An overproduction of TNF-α is associated with the development of alcoholic liver injury[4–6]. Kupffer cells, the resident macrophages in the liver, are the major producers of TNF-α following exposure to lipopolysaccharide (LPS), the bacterial endotoxin[7], and play an important role in alcohol-induced liver damage[8]. It has been demonstrated that Kupffer cell-derived cytokines induced by intestinal-derived LPS involves in the mechanism of alcoholic liver disease (ALD)[8–11].
Multiple mammalian receptors for LPS have been identified, including two glycoproteins: LPS-binding protein (LBP) and CD14. CD14 binds to the LPS-LBP complex and interact with a transmembrane toll-like receptor (TLR) responsible for signal transduction[12]. TLR4 is a specific receptor for gram-negative bacterial LPS[13]. Several observations indicate that TLR2 is also involved in LPS signaling[14–19].
JIANPI HUOXUE decoction (JHD) consists of eight Chinese herbs. Previously, JHD was found to inhibit endotoxin levels and intestine or liver injury in ALD rats induced by Lieber-DeCarli ethanol liquid diet[2021]. In the present study, we have isolated the LPS-induced cytokine secretion pathway from the complex conditions of ALD by employing the LPS challenging model described previously[22] to further explore the effects of JHD on this pathway. This might provide a new point of view on the mechanisms of JHD anti-alcoholic liver injury.
JHD consists of Altractylodes macroce phala Koidz. Salvia miltiorrhiza Bge., Citrus aurantium L., Paeonia lzctiilora pall., Pueraria lobata (willd.) Ohwi, Alisma orientalis (Sam.) Juzep, Schisandra chinensis (Turcz.) Baill. and Curuma longa L. Altractylodes macroce phala Koidz., Citrus aurantium L. and Curuma longa L. were distilled with ethanol to get the volatilizable components for three times, each lasting 1-2 h. Schisandra chinensis (Turcz.) Baill. was also extracted with ethanol twice, independently, 1-2 h for each time. The other herbs were boiled with water for three times after being marinated in water for 1 h. The final density of the water-extraction was 1.08-1.12 (80°C) and the water-extraction was purified with ethanol. Finally, the volatilized components, ethanol-extraction of Schisandra chinensis (Turcz.) Baill. and water-extraction were mixed as the JHD. The concentration of 0.9 g crude drug/mL was used in experiments.
Twenty-four female SD rats weighing 200 g were divided randomly into normal group (n = 4), model group (n = 10) and JHD group (n = 10). Rats in model group and JHD group were administrated with normal saline or JHD via gastrogavage respectively, 5 mL/kg, twice a day, for 3 d. One hour after the last administration, rats in these two groups were injected with LPS (Escherichia coli 0111:B4, Sigma-Aldrich Co., USA) via tail vein, 50 &mgr;g/kg body weight as described previously[21]. Simultaneously, rats in normal group were injected with equivalent volume of normal saline. After LPS stimulation for 1.5 h, serum and liver tissue were collected for assay.
Sections of the liver sample (4 &mgr;m thick) were stained with hematoxylin-eosin (H.E.) and examined under light microscope (Olympus Medical Systems Corp., Tokyo, Japan).
Specimens were fixed in a 40 g/L solution of formaldehyde in 0.1 mol/L phosphate-buffed saline (pH 7.4) and embedded in paraffin wax from which 4 &mgr;m thick sections were taken on a slide coated with poly-L-lysine (Dingguo Ltd., Beijing, China) for immunohistochemical assessment. Antigen retrieval was performed with 0.6% pepsin (Dingguo Ltd., Beijing, China) at 37°C for 10 min. The specimens were treated with 0.6% hydrogen peroxide-methanol, following 30 min of endogenous peroxidase blockage at 37°C. After blockage with 0.2% bovine serum albumin (BSA, Sino-American Biotechnology Company, Shanghai, China) for 20 min at room temperature, the samples were incubated at 4°C overnight, with a 1:100 dilution of anti-CD68 primary antibody (monoclonal anti-rat CD68, AbD Serotec, NC, USA). Following the processing of the samples incubated with a 1:250 dilution of horseradish peroxidase (HRP)-linked goat anti-mouse IgG (sc-2031, Santa Cruz Biotechnology Inc. Santa Cruz, CA) for 1 h at 37°C, diamino benzidine (DAB) was applied as a chromogen and hematoxylin was used for floor staining.
Serum levels of TNF-α were determined using a commercially available enzyme linked immunosorbent assay (ELISA) kit (Biosource International Inc., Camarillo, CA) according to the manufacturer’s instruction. TNF-α was determined from a standard curve for the combination of these cytokines. The concentrations were expressed as pg/mL.
As described previously[23], total protein was extracted from liver tissue and analyzed with bicinchoninic acid (BCA) protein concentration assay kit (Beyotime Inst. Biotechnology, Jiangsu, China). Sample protein was separated by electrophoresis in 10% SDS-PAGE separating gel with Bio-Rad electrophoresis system (Bio-Rad Laboratories, Hercules, CA, USA). The primary antibodies (mouse anti-rat glyceraldehydes-3-phosphate dehydrogenase, GAPDH antibody, 1:5000 dilution, KANGCHEN Bio-Tech Inc., Shanghai, China; mouse anti-rat CD68 antibody, 1:100 dilution, AbD Serotec, NC, USA; mouse anti-rat p-IκB antibody, 1:500 dilution, Cell Signaling Technology Inc., Boston, USA; goat anti-rat TNF-α antibody, 1:500 dilution, R&D Systems Inc., Minneapolis, USA) were incubated at 4°C overnight. The corresponding horseradish peroxidase-conjugated secondary antibodies (goat anti-mouse IgG, peroxidase-linked antibody, 1:5000 dilution, Santa Cruz Biotechnology Inc., Santa Cruz, CA, rabbit anti goat-IgG, peroxidase-linked antibody, 1:5000 dilution, Jackson ImmunoResearch Laboratories Inc., PA, USA) were incubated at room temperature. The ECL kit (Pierce Biotechnology Inc., Rockford, USA) and the Furi FR-980 image analysis system (Shanghai Furi Co., Shanghai, China) were employed for revealing and quantitative analysis of the blots. GAPDH protein was used as the internal control.
Total RNA was extracted from liver tissues of each group with the tissue/cell total RNA isolation kit (Watson Biotechnologies Inc., Shanghai, China) according to the manufacturer’s protocol. The quantity and purity of RNA were detected by determining absorbance at 260/280 nm using a spectrophotometer (Unico Co., USA). Total RNA was reversely transcribed into complementary DNA (cDNA) using the cDNA synthesis kit (Fermentas Life Sciences Inc., Maryland, USA) according to the manufacturer’s protocol. The Rotor Gene-3000 PCR machine (Gene Co., Hong Kong) and real-time PCR kit (SYBR®Premix Ex TaqTM, TaKaRa Bio Inc., Japan) were employed based on the manufacturer’s instruction. The specific primers for the target genes and β-actin (synthesized by Shanghai Shenggong Co.) used are described in Table 1.
| Target gene | Primer | Target fragment length (bp) |
| β-actin | 5’-TGACGAGGCCCAGAGCAAGA-3’(F) | 331 |
| 5’-ATGGGCACAGTGTGGGTGAC-3’(R) | ||
| TNF-α | 5’-GGCAGCCTTGTCCCTTGAAGAG-3’(F) | 171 |
| 5’-GTAGCCCACGTCGTAGCAAACC-3’(R) | ||
| IL-6 | 5’-CCACTTCACAAGTCGGAGGCTTA-3’(F) | 108 |
| 5’-GTGCATCATCGCTGTTCATACAATC-3’(R) | ||
| TLR4 | 5’-CTCACAACTTCAGTGGCTGGATTTA -3’(F) | 178 |
| 5’-TGTCTCCACAGCCACCAGATTC-3’(R) | ||
| TLR2 | 5’-GGCCACAGGACTCAAGAGCA-3’(F) | 102 |
| 5’-AGAGGCCTATCACAGCCATCAAG-3'(R) | ||
| CD14 | 5’- GAATCCCAGTCGGAGGCGTA-3’(F) | 94 |
| 5’-GGAGCAAAGCCAAAGTTCCTGA-3’(R) |
Two-step PCR procedure was recommended as follows: pre-denaturation for 10 s at 95°C, 1 cycle; 95°C for 5 s and 59°C for 20 s, 40 cycles. The final products were identified by electrophoresis in 1.5% agarose gel and melt curve analysis. Two-standard curve method was employed in relative quantification analysis. Briefly, after the target gene products were emendated with internal control β-actin, the relative fluorescence values of target products in normal group were analysed and compared with other groups. The final results were described with the relative values. The calculation and analysis were performed by the software in the Rotor-Gene RG-3000 (Gene Co., Hong Kong).
All values were expressed as mean ± SD. Comparisons were analyzed by one-way ANOVA using the SPSS 10.0 statistical package. Differences were considered statistically significant if the P < 0.05.
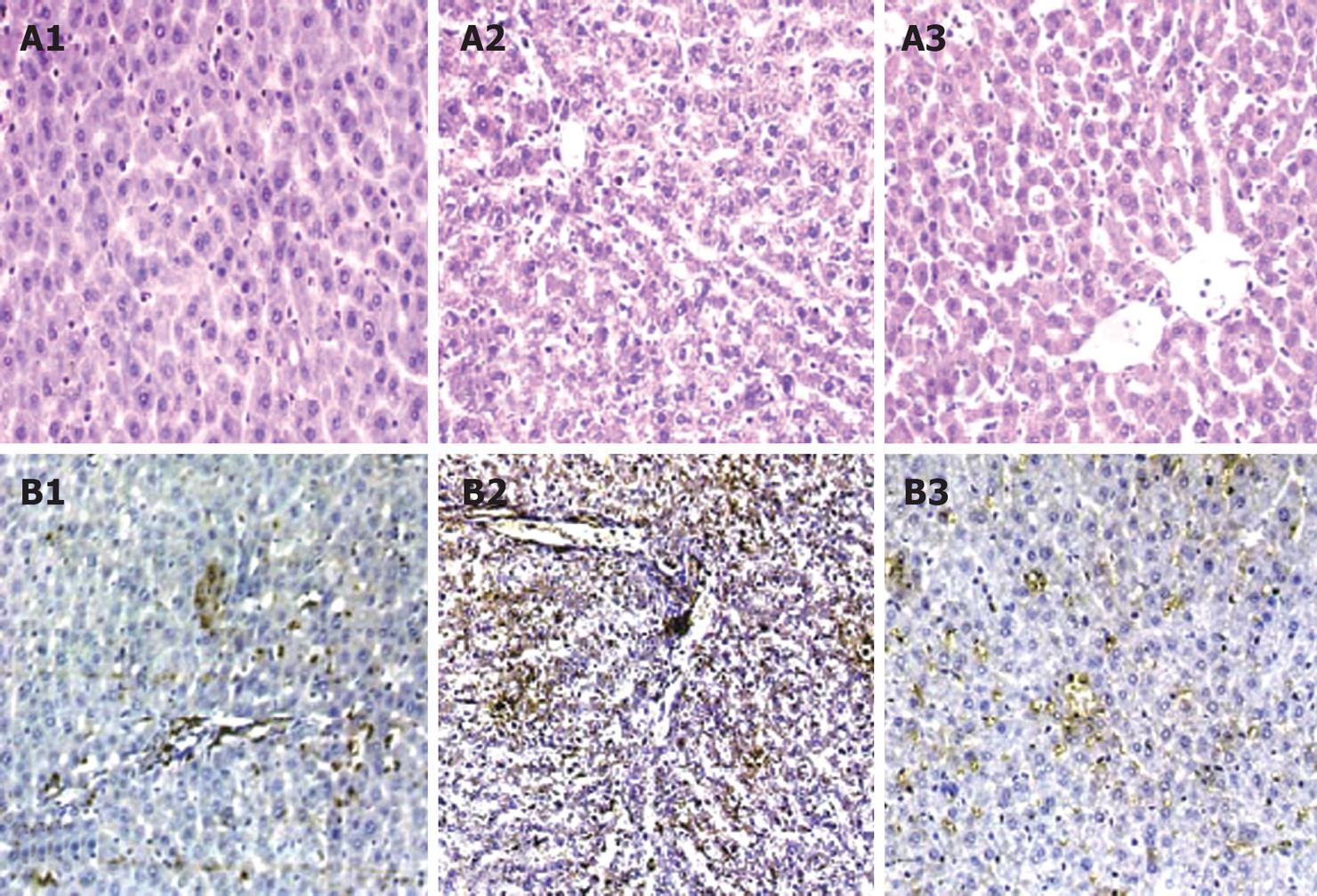
H.E. staining showed predominant microvesicular change, hepatocyte tumefaction and cytoplasm dilution after LPS stimulation in the rat liver tissues. After JHD administration, those pathological changes of liver tissues were ameliorated obviously (Figure 1A1-A3).
CD68 immunohistochemical staining indicated that a spot of positive staining existed in hepatic sinusoidal of normal rats and obvious positive staining in the sinusoidal where hepatic microvesicular change was predominant after LPS stimulation. After JHD administration, the CD68 positive staining in the sinusoidal was lightened (Figure 1B1-B3).
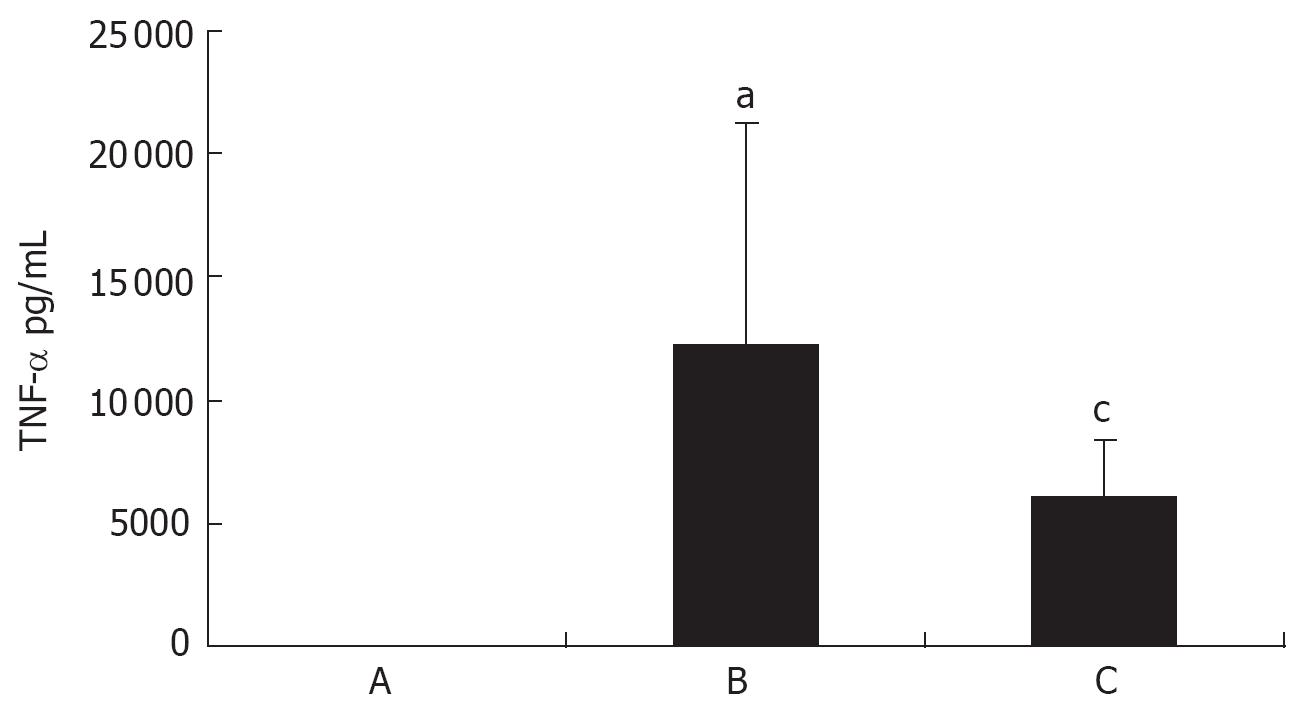
After stimulation with LPS for 1.5 h, serum TNF-α increased significantly (31.35 ± 6.06 vs 12 225.40 ± 9007.03, P < 0.05) and decreased obviously in JHD group (12 225.40 ± 9 007.03 vs 6031.70 ± 2296.56, P < 0.05) (Figure 2).
After stimulation with LPS for 1.5 h, protein expression of CD68, p-IκB and TNF-α in liver tissues were increased significantly (CD68: 1.13 ± 0.49 vs 3.36 ± 1.69, P < 0.05; p-IκB: 0.01 ± 0.01 vs 2.07 ± 0.83, P < 0.01; TNF-α: 0.27 ± 0.13 vs 1.29 ± 0.37, P < 0.01) and decreased significantly (CD68: 3.36 ± 1.69 vs 0.76 ± 0.45, P < 0.05; p-IκB: 2.07 ± 0.83 vs 0.87 ± 0.83, P < 0.01; TNF-α: 1.29 ± 0.37 vs 0.67 ± 0.36, P < 0.01) in JHD group (Figure 3).
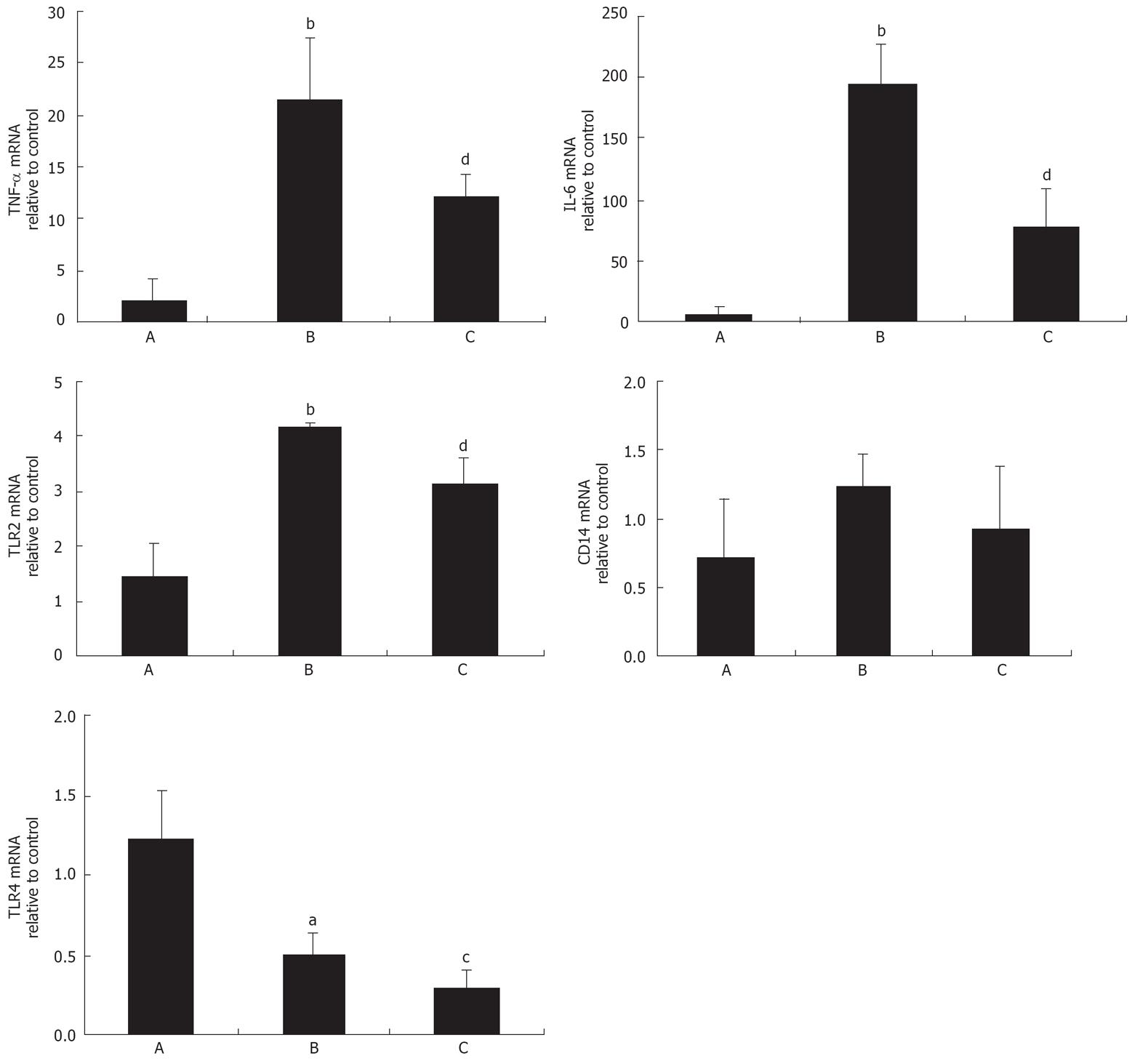
After stimulation with LPS for 1.5 h, mRNA expression of TNF-α, IL-6 and TLR2 in liver were increased significantly (TNF-α: 1.96 ± 2.23 vs 21.45 ± 6.00, P < 0.01; IL-6: 4.80 ± 6.42 vs 193.50 ± 36.36, P < 0.01; TLR2: 1.44 ± 0.62 vs 4.16 ± 0.08, P < 0.01) and decreased obviously in JHD group (TNF-α: 21.45 ± 6.00 vs 11.99 ± 2.28, P < 0.01; IL-6: 193.50 ± 36.36 vs 76.12 ± 32.16, P < 0.01; TLR2: 4.16 ± 0.08 vs 3.11 ± 0.53, P < 0.01).
On the other hand, TLR4 mRNA expression was down-regulated (1.22 ± 0.30 vs 0.50 ± 0.15, P < 0.05) and mRNA expression of CD14 was increased but not significantly after LPS stimulation for 1.5 h (Figure 4).
Gut-derived LPS is the primary endogenous endotoxin of gram-negative bacteria[9]. Extended ethanol exposure can lead to gut leakage[26] and the gut-derived LPS consequently enter the circulation. Previous researches have confirmed that LPS promoted the phosphorylation of inhibit-κB (IκB), transferred the NF-κB into nuclear, consequently promoted cytokines production in Kupffer cells, which was involved in the pathogenesis of ALD[9–122425].
In the present research, the predominant microvesicular change, hepatocyte tumefaction and cytoplasm dilution were observed in liver tissues after LPS stimulation, and simultaneously, CD68, the specific antigen-molecule on the microphage, was identified predominantly in the sinusoidal. The protein and/or gene expression of inflammatory factors, such as TNF-α and IL-6, and the protein expression of CD68 and p-IκB were also increased by LPS stimulation. On the contrary, JHD inhibited the pathological changes significantly.
The endotoxin receptors are necessary for LPS signal transferring. CD14, TLR4 and TLR2 have been identified as the main endotoxin receptors[12–19].
Soluble CD14 (sCD14) exists in blood and membrane CD14 (mCD14) anchors on the membrane of peripheral or liver resident microphage (Kupffer cell) through glycosylphosphatidylinositol (GPI). LPS-LBP complex combines with mCD14 and activated cells through TLR-4[27]. CD14 has also been found to bind lipoteichoic acid (LTA) and peptidoglycan (PGN) from the cell wall of gram-positive bacteria and activate cells through TLR2[27]. The expression of CD14 stimulated by LPS was reported as a dynamic process. C Fearns et al[28] found that 1 h after intraperitoneal injection of LPS, CD14 mRNA was induced in the liver of mice but not significantly, and peaked at 8-16 h. By 24 h, the level of CD14 mRNA returned to the basal level. In the present study, we also found that 1.5 h after injection of LPS via tail vein, the level of CD14 mRNA increased but not significantly, which might suggest that at this time point, CD14 gene level did not reach the peak in this model.
TLR4 has been proved to be the specific receptor for the LPS of gram-negative bacteria[1129]. The reports on TLR4 mRNA expression stimulated by LPS were not consistent. Matsumura T et al[18] found when mice were administered LPS, TLR4 mRNA was decreased in the brain, increased in the heart and lung and not affected in the liver, kidney, and spleen. In the study by Choda Y et al[30], TLR4 mRNA level was increased 0.5 h after intraportal LPS administration, but decreased thereafter. Liu XW et al[31] found that 3 h after intraperitoneal injection LPS in mice, TLR4 mRNA was down-regulated in liver and failed to be detected by 6-12 h, but resumed to the basal level by 24 h. In our research, significant decrease of TLR4 mRNA expression was observed in liver 1.5 h after LPS administration, which was consistent with the report by Y Choda.
The primary ligands of TLR2 are gram-positive bacteria-derived lipoteichoic acid (LTA), peptidoglycan (PGN) and mycobacterial lipoarabinomannan[32]. Previous reports suggested that neither human nor murine TLR2 plays a role in LPS signaling[1533]. But the subsequent research[34] found that phenol repurificated LPS did not activate cells from TLR2-mediated signaling, but the commercially prepared LPS contained low concentrations of highly bioactive contaminants described previously as endotoxin protein activated TLR2-mediated signaling. On the other hand, myeloid differentiated protein-2 (MD-2) which is necessary for TLR4-mediated LPS signaling was also proved to enable TLR2 to respond to endotoxin protein-free LPS and enhance TLR2-mediated responses to both gram-negative bacteria and their LPS[16]. Furthermore, the cytokines induced by LPS, such as interleukin-1 beta (IL-1β) or TNF-α, also up-regulate TLR2 mRNA of rat hepatocyte in vivo and in vitro[17]. As a result, LPS from gram-negative bacteria does not induce TLR2 expression directly, but induced the commercial LPS containing endotoxin protein indirectly. LPS-derived cytokine and MD-2 can induce TLR2 expression greatly. Our results also suggested that TLR2 mRNA was up-regulated significantly in liver after LPS administration. And as expected, TLR2 mRNA in JHD group decreased significantly.
In conclusion, the present study confirmed that the inhibitory effects of JHD on cytokines (TNF-α, IL-6) protein or gene expression induced by LPS in liver is associated with its inhibition on the LPS-activated Kupffer cell signal pathway, including CD68 and p-IκB protein expression and TLR2 mRNA expression.
The “two-hits” theory brought about great advancement in pathogenesis research of alcoholic liver disease (ALD). Attention has been attracted in the mechanism of endotoxin or lipopolysaccharides (LPS) signal pathway involved in ALD. The resident macrophage in liver, Kupffer cells, is an important target cell of LPS and secreting cytokines. The endotoxin receptors, identified as CD14, toll-like receptor (TLR) 4, are essential for LPS signaling.
In this study, the expression of endotoxin receptors, CD14, TLR4 and TLR2 was observed after LPS or LPS + JHD administration. The difference of endotoxin receptors expression under similar challenge conditions is interesting, especially, TLR4 and CD14.
This study confirmed one of the possible mechanisms of JHD, a Chinese herbs decoction, on anti-alcoholic liver injury, inhibiting some targets in LPS-activated cytokine secretion pathway, such as CD68, the specific molecular marker of macrophage, phosphorylated inhibit-κB (p-IκB) and TLR2, the endotoxin receptor.
The present study provides a new experimental evidence of JHD inhibition alcoholic liver injury. To further evaluate the effects of JHD on Kupffer cell activation and endotoxin receptors expression induced by LPS, the multiple-time point observation and isolated Kupffer cells should be employed in the subsequent experiments.
Lipopolysaccharide (LPS), the major composition of gram-negative bacterial wall, can excite intensive inflammation reaction.
This is an interesting paper, describing the effects of a traditional Chinese medicine on the LPS activated Kupffer cells of murine liver. Methodology used is sound, results are well presented and analyzed, and conclusions are documented by the findings of the study.
| 1. | Bradham CA, Plumpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387-G392. |
| 2. | Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411-452. |
| 3. | Nagaki M, Muto Y, Ohnishi H, Moriwaki H. Significance of tumor necrosis factor (TNF) and interleukin-1 (IL-1) in the pathogenesis of fulminant hepatitis: possible involvement of serine protease in TNF-mediated liver injury. Gastroenterol Jpn. 1991;26:448-455. |
| 4. | McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349-351. |
| 5. | Nanji AA, Zhao S, Sadrzadeh SM, Waxman DJ. Use of reverse transcription-polymerase chain reaction to evaluate in vivo cytokine gene expression in rats fed ethanol for long periods. Hepatology. 1994;19:1483-1487. |
| 6. | Kamimura S, Tsukamoto H. Cytokine gene expression by Kupffer cells in experimental alcoholic liver disease. Hepatology. 1995;22:1304-1309. |
| 7. | Decker K. Biologically active products of stimulated liver macrophages (Kupffer cells). Eur J Biochem. 1990;192:245-261. |
| 8. | Cubero FJ, Nieto N. Kupffer cells and alcoholic liver disease. Rev Esp Enferm Dig. 2006;98:460-472. |
| 9. | Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881-G884. |
| 10. | Hanck C, Rossol S, Bocker U, Tokus M, Singer MV. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol Alcohol. 1998;33:606-608. |
| 11. | Su GL. Lipopolysaccharides in liver injury: molecular mechanisms of Kupffer cell activation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G256-G265. |
| 13. | Lorenz E. TLR2 and TLR4 expression during bacterial infections. Curr Pharm Des. 2006;12:4185-4193. |
| 14. | Kirschning CJ, Wesche H, Merrill Ayres T, Rothe M. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J Exp Med. 1998;188:2091-2097. |
| 15. | Heine H, Kirschning CJ, Lien E, Monks BG, Rothe M, Golenbock DT. Cutting edge: cells that carry A null allele for toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971-6975. |
| 16. | Dziarski R, Wang Q, Miyake K, Kirschning CJ, Gupta D. MD-2 enables Toll-like receptor 2 (TLR2)-mediated responses to lipopolysaccharide and enhances TLR2-mediated responses to Gram-positive and Gram-negative bacteria and their cell wall components. J Immunol. 2001;166:1938-1944. |
| 17. | Liu S, Salyapongse AN, Geller DA, Vodovotz Y, Billiar TR. Hepatocyte toll-like receptor 2 expression in vivo and in vitro: role of cytokines in induction of rat TLR2 gene expression by lipopolysaccharide. Shock. 2000;14:361-365. |
| 18. | Matsumura T, Ito A, Takii T, Hayashi H, Onozaki K. Endotoxin and cytokine regulation of toll-like receptor (TLR) 2 and TLR4 gene expression in murine liver and hepatocytes. J Interferon Cytokine Res. 2000;20:915-921. |
| 19. | Frost RA, Nystrom G, Burrows PV, Lang CH. Temporal differences in the ability of ethanol to modulate endotoxin-induced increases in inflammatory cytokines in muscle under in vivo conditions. Alcohol Clin Exp Res. 2005;29:1247-1256. |
| 20. | Fang ZH, Hu YY, Cui JW. Relationship between alcoholic liver injury and endotoxin leakage from gut and intervention effect of jianpi liqi huoxue decoction. Zhongguo Zhongxiyi Jiehe Zazhi. 2006;26:813-817. |
| 21. | Peng JH, Fang ZH, Cui JW, Feng Q, Xu LL, Gu HT, Hu YY. Effects of Jianpi Huoxue Decoction on Kupffer cell signal pathway activation in rats with liver injury induced by Lieber-Decarli liquid diet and lipopolysaccharide. Zhongxiyi Jiehe Xuebao. 2007;5:302-306. |
| 22. | Ponnappa BC, Israel Y, Aini M, Zhou F, Russ R, Cao QN, Hu Y, Rubin R. Inhibition of tumor necrosis factor alpha secretion and prevention of liver injury in ethanol-fed rats by antisense oligonucleotides. Biochem Pharmacol. 2005;69:569-577. |
| 23. | Cheng Y, Ping J, Xu LM. Effects of curcumin on peroxisome proliferator-activated receptor gamma expression and nuclear translocation/redistribution in culture-activated rat hepatic stellate cells. Chin Med J (Engl). 2007;120:794-801. |
| 24. | Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605-G611. |
| 25. | Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218-224. |
| 26. | Hansen J, Cherwitz DL, Allen JI. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20:461-474. |
| 27. | Triantafilou K, Triantafilou M, Dedrick RL. Interactions of bacterial lipopolysaccharide and peptidoglycan with a 70 kDa and an 80 kDa protein on the cell surface of CD14+ and CD14- cells. Hum Immunol. 2001;62:50-63. |
| 28. | Fearns C, Kravchenko VV, Ulevitch RJ, Loskutoff DJ. Murine CD14 gene expression in vivo: extramyeloid synthesis and regulation by lipopolysaccharide. J Exp Med. 1995;181:857-866. |
| 29. | Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101-108. |
| 30. | Choda Y, Morimoto Y, Miyaso H, Shinoura S, Saito S, Yagi T, Iwagaki H, Tanaka N. Failure of the gut barrier system enhances liver injury in rats: protection of hepatocytes by gut-derived hepatocyte growth factor. Eur J Gastroenterol Hepatol. 2004;16:1017-1025. |
| 31. | Liu XW, You Y, Lu FG. TLR4 mRNA expression and liver injury in LPS-induced mouse. Hunan Yike Daxue Xuebao. 2003;28:217-220. |
| 32. | Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675-680. |