Published online Feb 28, 2007. doi: 10.3748/wjg.v13.i8.1252
Revised: December 19, 2006
Accepted: January 23, 2007
Published online: February 28, 2007
AIM: To compare the gadolinium-enhanced multiphase dynamic magnetic resonance imaging (MRI) and multiphase multirow-detector helical CT (MDCT) scanning for detection of small hepatocellular carcinoma (HCC).
METHODS: MDCT scanning and baseline MRI with SE T1-WI and T2-WI sequence combined with FMPSPGR sequence were performed in 37 patients with 43 small HCCs. Receiver operating characteristic (ROC) curves were plotted to analyze the results for modality.
RESULTS: The areas below ROC curve (Az) were calculated. There was no statistical difference in dynamic enhancement MDCT and MRI. The detection rate of small HCC was 97.5%-97.6% on multiphase MDCT scanning and 90.7%-94.7% on MRI, respectively. The sensitivity of detection for small HCC on MDCT scanning was higher than that on dynamic enhancement MRI. The sensitivity of detection for minute HCC (tumor diameter ≤ 1 cm) was 90.0%-95.0% on MDCT scanning and 70.0%-85.0% on MRI, respectively.
CONCLUSION: MDCT scanning should be performed for early detection and effective treatment of small HCC in patients with chronic hepatitis and cirrhosis during follow-up.
- Citation: Zhao H, Yao JL, Wang Y, Zhou KR. Detection of small hepatocellular carcinoma: Comparison of dynamic enhancement magnetic resonance imaging and multiphase multirow-detector helical CT scanning. World J Gastroenterol 2007; 13(8): 1252-1256
- URL: https://www.wjgnet.com/1007-9327/full/v13/i8/1252.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i8.1252
Hepatocellular carcinoma (HCC) is the most common primary malignancy tumor of the liver. Dual-phase CT scanning is a sensitive method for the detection of HCC. The developments in rapid magnetic resonance imaging (MRI) in combination with gadolinium-enhanced multiphase multirow-detector helical CT (MDCT) scanning can obviously improve the detection of small HCC. However, MRI and MDCT have a lower sensitivity for detecting small HCC, especially minute HCC. MDCT scanning has a higher sensitivity for detection of small HCC[1-5]. The purpose of this study was to compare the dynamic enhancement MRI and MDCT for the detection of small HCC in patients with chronic liver disease and cirrhosis and to value the clinical role of new imaging technology in detection of small HCC.
Between October 2002 and December 2004, 37 patients (29 men, 8 women, mean age: 56 years, rang: 29-70 years) with chronic hepatic disease and cirrhosis who were suspected of having HCC during postoperative follow-up were included in this study. All the patients underwent multiphase contrast enhanced dynamic MDCT and gadolinium-enhanced dynamic MRI at 7 d intervals.
Small HCC was confirmed in 24 of 37 patients. Fifteen of the 24 patients with 43 small HCCs underwent surgery and pathologic examination. Of the 15 patients undergoing surgery, small HCC was found in 2 by needle biopsy, in 5 by digital subtraction angiography (DSA), and in 2 by iodized oil CT and elevated serum α-fetoprotein level (> 400 ng/mL) and ultrasound examination. Two out of 37 patients undergoing multiphase dynamic contrast enhanced MDCT and dynamic gadolinium enhancement MRI respectively, were suspected of having small HCC. Their serum α-fetoprotein level was decreased. Ultrasound examination one month after follow-up and multiphase dynamic contrast enhanced MDCT examination three months after follow-up showed that the lesions remained unchanged. Five out of 37 patients with liver cirrhosis undergoing gadolinium enhancement dynamic MRI scanning and multiphase dynamic contrast enhanced MDCT imaging were suspected of having small HCC. However, small HCC could not be found. Small HCC was not found in 3 of 5 patients who were followed up for 2-3 mo by contrast-enhanced MDCT, and in 2 of 5 patients who were followed up for 3 mouths by enhancement dynamic MRI. Multiphase dynamic contrast-enhanced MDCT and MRI examination displayed no new liver lesions in another 6 patients who were not diagnosed having small HCC during the tree-month follow-up period. The size of small HCC was ≤ 1 cm in 20, and > 1 cm or ≤ 3 cm in 23 of 43 small HCCs, respectively.
All CT examinations were performed using a commercially available multidetector CT scanner (Marconi Mx 8000) with 0.5-0.75 s gantry rotation speed, 23.3 mm/s table speed, 5.0 mm-thick section, reconstruction interval 2.5 mm, 120 Kv and 200-250 mA.
Entire pre-contrast hepatic scanning was followed by a nonionic contrast enhancement (Omnipaque 300 mg I/mL) with 1.5 mL/kg and injection rate of 3 mL/s via an antecubital vein. Multiphase acquisitions were performed. The scanning delay set for early arterial phase (EAP), late arterial phase (LAP) and portal venous phase (PVP) was 21 s, 34 s and 85 s, respectively. Each of the entire liver scanning in cephalad-caudal orientation was completed in 4-8 s with patient's breath held.
MRI was performed with a GE Signa 1.5 T MR imaging system. A standard whole-body coil was used as the receiver coil for examinations. All the 37 patients underwent baseline MR imaging, including breath-hold spin-echo T1- weighted imaging(TR 500-700 ms, TE 14-20 ms), fat-suppressed fast spin-echo T2-weighted imaging (TR 2000-4000 ms, TE 80-120 ms) and gadolinium-enhanced triphasic dynamic gradient-recalled echo imaging with a fast multiplanar spoiled-gradient-recalled echo breath-hold imaging (FMPSPGR TR/TE/Flip Angle = 100-150 ms/4.6 ms/60-90°), matrix (256 × 128), 7 mm-thick section, and 1 acquisition. MR imaging was performed before and after gadolinium-enhanced dynamic gradient-recalled echo imaging (Gd-DTPA, Magnevist, Germany, NJ). Dynamic imaging was performed with a fast multiplanar spoiled-gradient-recalled echo breath-hold imaging. The contrast material was 0.2 mL/kg of body weight administered as a rapid IV bolus. After un-enhanced imaging, arterial phase image was obtained during 20-25 s. The second and third sets of images were obtained after approximately 60-90 s and 3 min, respectively.
All MRI and MDCT images were reviewed by two experienced radiologists, who knew that patients with liver cirrhosis were at risk of developing HCC, but were unaware of the presence and location of liver lesions and the result of other imaging examinations. The size and number of lesions were analyzed for the various multiphase dynamic contrast-enhanced MR sequences and MDCT imagines (early arterial, later arterial, portal phases) of the 37 patients.
The readers scored each imagine for the presence or absence of focal hepatic lesions, and assigned confidence levels to their observation: 1 = definite presence, 2 = probable presence, 3 = equivocal, 4 = probable absence, 5 = definite absence[6].
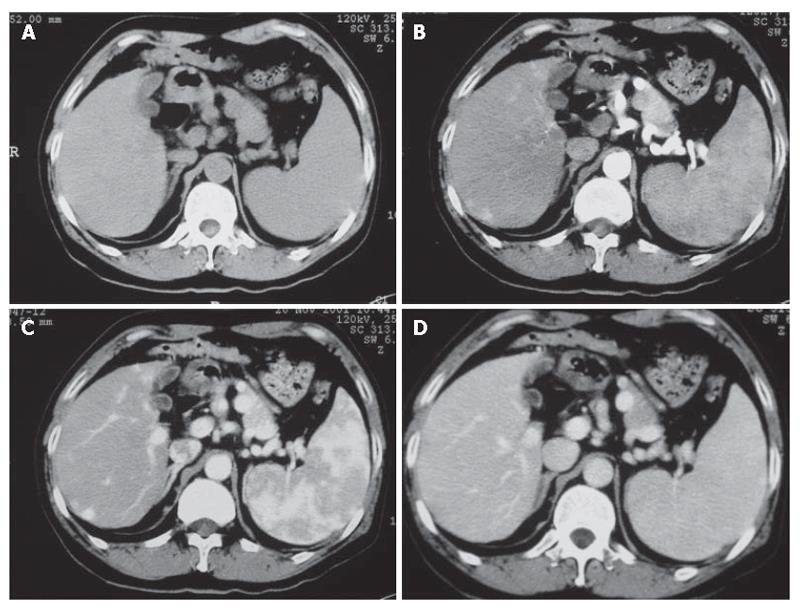
For each imaging method, a binomial receivor operationg characteristic (ROC) curve was fitted to each radiologist’s confidence rating using maximum likelihood estimation. The diagnostic accuracy of each imaging set for each observer and the composite data were calculated by measuring the area under the alternative free response ROC curve. The differences between imaging sets in terms of the mean Az value, were statistically analyzed using the two-tail Student’s t test for paired data. The sensitivity and positive predictive values for each image set were then calculated. The sensitivity of each observer was determined by detecting the number of lesions assigned a confidence level of 1 or 2 from 42 HCCs. The degree of inter-observer agreement was calculated with chance-corrected kappa statistics. In general, a kappa statistic value greater than 0.75 is considered excellent agreement, 0.4-0.75 good agreement, and less than 0.4 poor agreement[6-8]. Statistical analyses were performed using the SPSS Statistical Programs, version 10.0. P < 0.05 was considered statistically different (Figure 1).
The kappa values were excellent between observers 1 and 2 for multiphase dynamic contrast-enhanced MDCT (κ value = 0.883) and MRI (κ value = 0.812). The Az values calculated by each observer with multiphase dynamic contrast-enhanced MDCT and MRI for 42 lesions are shown in Table 1. For detection of lesions, two observers achieved a slightly higher diagnostic performance with MDCT than with MRI, but the difference in the mean Az values of both imagine sets was not significant. The chance-corrected kappa values indicated the confidence levels for the imagine interpretation of the ROC analysis between the two observers.
| Observer 1 | Observer 2 | |||
| Imaging techniques | Az value | 95% CI | Az value | 95% CI |
| MDCT | 0.983 | 0.950-1.013 | 0.99 | 0.968-1.012 |
| MRI | 0.951 | 0.901-1.001 | 0.94 | 0.882-0.999 |
| t value | -0.425 | -0.956 | ||
| P value | 0.672 | 0.348 | ||
The sensitivity and positive predictive values obtained with dynamic contrast-enhanced MDCT and MRI are shown in Table 2. For less than or equal to 1 cm minute HCC, the sensitivity of MRI was 85% and 70% , respectively for the two observers, but the sensitivity of MDCT for the two observers was higher than that of MRI (95% and 90%, respectively). The positive predictive value for MDCT was 97.5%-97.6% for MDCT and 90.7%-94.7% for MRI, respectively. There were less false-positive findings on MDCT.
| Sensitivity(%) | Positivepredictivevalue (%) | ||||
| ImagingTechnique | ≤1 cm | > 1 cm ≤3 cm | Total | ||
| SHCC | SHCC | SHCC | |||
| n = 20 | n = 23 | n = 43 | n = 43 | ||
| Observer | MDCT | 19 (95.0) | 22 (95.7) | 41 (95.31) | 97.6 |
| 1 | MRI | 17 (85.0) | 22 (95.72) | 39 (90.7) | 90.7 |
| Observer | MDCT | 18 (90.0) | 21 (91.31) | 39 (90.79) | 97.5 |
| 2 | MRI | 14 (70.0) | 22 (95.7) | 36 (83.7) | 94.7 |
Most HCCs occur in cirrhotic liver. Oka et al[9] in a 6-year follow-up study of 140 patients with cirrhosis, Oka et al[9] found that the cumulative incidence of HCC is 39% and its per year occurrence rate is 5.3%-8.8%. Small HCC results from cirrhosis. Early detection and diagnosis of small HCC are important in its effective treatment. Lim et al[10] have analyzed the sensitivity and specificity of double phase SCT in detecting small HCC. It was reported that imaging technology should be used in detecting small HCC[9,11].
Multiphase dynamic CT using a helical scanner has become the standard technology for detection and diagnosis of small HCC. The detection of hepatic nodular lesions by CT depends on the attenuation difference in the normal parenchyma and enhancement nodule lesions. Multiphase dynamic CT scanning can show the change between the tumor and its surrounding parenchyma. This may in part be a result of the difference in uptake and secretion of contrast material by hepatocytes of the normal liver parenchyma and HCC cells. Because the arterial phase is shorter than the portal venous phase, the scanning time of hepatic lesions is not the optimal time of tumors. Therefore, the dual-phase scanning is difficult to detect small HCC[7,9,11,12].
MRI is a less invasive and more feasible technique. The spin-echo sequence and gadolinium-enhanced triphasic dynamic breath-hold imaging are more effective. The advantage of contrast enhancement MRI is to deliver a small contrast material volume over a shorter period of time, which results in a tighter bolus. MRI has several advantages over dual-phase SCT in detection and characterization of small HCC, including greater soft-tissue contrast and sensitivity to intravascular contrast agent, and more types of sequences[6,14,16].
Tang et al[17] reported that 94% of small HCC can be found in cirrhotic liver by dynamic gadolinum-enhanced MRI. Yan et al[18] reported that 94.12% of small HCC (diameter < 1 cm) can be detected by MRI. Kim et al[19] reported that 67% of small HCC (diameter < 1 cm) can be displayed by MRI.
The optimal imaging technology can detect most of small HCC. MDCT scanner recently has been introduced into clinical CT practice, and allows faster Z-axis coverage speed and hepatic imaging with thin image thickness in a very short time. MDCT can scan the entire liver during the double arterial phase. In addition, it improves the sensitivity of depicting small HCC and increases conspicuity for hypervascular lesions, and sensitivity of detecting small HCC[4,5,24].
The ROC curve analysis revealed that there was a higher validity for two imaging methods. Two observers achieved slightly higher diagnostic efficiency with multiphase contrast-enhanced MDCT than with dynamic enhanced MRI, but the difference in the areas below the ROC cure was not statistically significant (observer 1, P = 0.672; observer 2, P = 0.348). Multiphase contrast-enhanced MDCT had slightly higher predictive values than dynamic contrast enhanced MRI. The positive predictive value for MDCT and MRI by observer 1 was 97.6% and 90.7%, and 97.5% and 94.7% by observer 2, respectively. There were less false-positive findings on MDCT, but whether the diagnostic efficiency of multiphase contrast-enhanced MDCT is superior to dynamic contrast enhancement MRI needs further study with a large sample.
It was reported that the sensitivity of MRI in detection mall HCC is lower[20-22]. However, several factors can affect the visualization of primary focal hepatic lesions during contrast-enhanced or un-enhanced MRI, including the functionality of normal hepatic parenchyma, as well as the dimension, composition, and degree of visibility of the lesions, and the residual hepatic functionality of neoplastic cells themselves[23,24]. Since these factors tend to vary from patient to patient, it is often difficult to predict the behavior of a given lesion. There is a very good correlation between the blood supply and the degree of pathologic characteristics of hepatic lesions.
In our study, the sensitivity of MRI in detecting small HCC (≤ 1 cm) was 70% and 85%, respectively. Seventy percent of 20 small HCCs showed isointensity, 4 demonstrated the signal change from isointensity to hyperintensity during arterial phase, portal venous phase, and delay phase. The other lesions displayed the signal change from hyperintensity to isointensity. These different findings may be due to the following reasons. First, cirrhotic liver has homogeneous or nonhomogeneous density, the signal intensity of cirrhotic liver parenchyma is higher than normal liver parenchyma, and the high patching signal intensity on T1-weigted imagines is due to fatty deposition or hepatitis. Second, since the degree of the liver parenchyma is lower because of hemosiderin deposition and large fibrous tissue in the liver parenchyma, the impaired hepatic cells could not absorb contrast material. Third, the portal hypertension splits liver blood flow into collateral vessels, thus reducing the contrast material in the liver parenchyma. Forth, since the time window of arterial phase is narrower and the imaging time of MRI is fixed at the arterial phase 20-25 s, portal venous phase 60-90 s, and delayed phase 120 s, it is difficult to show a real arterial phase[4].
In conclusion, small HCC often manifests as relatively small lesions in cirrhotic livers. There is an obviously enhanced small nodule in hepatic arterial phase whether it has homogeneous or nonhomogeneous density. The signal intensity and contrast enhancement patterns cannot be used in the final diagnosis of small HCC. It is important for patients with cirrhosis to undergo follow-up imaging more frequently. Nodule growth is highly predictive of small HCC[21,26]. Further study is needed since our study has some limitations.
S- Editor Liu Y L- Editor Wang XL E- Editor Zhou T
| 1. | Braga HJ, Choti MA, Lee VS, Paulson EK, Siegelman ES, Bluemke DA. Liver lesions: manganese-enhanced MR and dual-phase helical CT for preoperative detection and characterization comparison with receiver operating characteristic analysis. Radiology. 2002;223:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Savci G. The changing role of radiology in imaging liver tumors: an overview. Eur J Radiol. 1999;32:36-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Kawata S, Murakami T, Kim T, Hori M, Federle MP, Kumano S, Sugihara E, Makino S, Nakamura H, Kudo M. Multidetector CT: diagnostic impact of slice thickness on detection of hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 2002;179:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Foley WD, Mallisee TA, Hohenwalter MD, Wilson CR, Quiroz FA, Taylor AJ. Multiphase hepatic CT with a multirow detector CT scanner. AJR Am J Roentgenol. 2000;175:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 7.6] [Reference Citation Analysis (1)] |
| 5. | Murakami T, Kim T, Takamura M, Hori M, Takahashi S, Federle MP, Tsuda K, Osuga K, Kawata S, Nakamura H. Hypervascular hepatocellular carcinoma: detection with double arterial phase multi-detector row helical CT. Radiology. 2001;218:763-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 198] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Kondo H, Kanematsu M, Hoshi H, Murakami T, Kim T, Hori M, Matsuo M, Nakamura H. Preoperative detection of malignant hepatic tumors: comparison of combined methods of MR imaging with combined methods of CT. AJR Am J Roentgenol. 2000;174:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Yamashita Y, Mitsuzaki K, Yi T, Ogata I, Nishiharu T, Urata J, Takahashi M. Small hepatocellular carcinoma in patients with chronic liver damage: prospective comparison of detection with dynamic MR imaging and helical CT of the whole liver. Radiology. 1996;200:79-84. [PubMed] |
| 8. | Ward J, Guthrie JA, Scott DJ, Atchley J, Wilson D, Davies MH, Wyatt JI, Robinson PJ. Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology. 2000;216:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 133] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Oka H, Kurioka N, Kim K, Kanno T, Kuroki T, Mizoguchi Y, Kobayashi K. Prospective study of early detection of hepatocellular carcinoma in patients with cirrhosis. Hepatology. 1990;12:680-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 260] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 10. | Lim JH, Kim CK, Lee WJ, Park CK, Koh KC, Paik SW, Joh JW. Detection of hepatocellular carcinomas and dysplastic nodules in cirrhotic livers: accuracy of helical CT in transplant patients. AJR Am J Roentgenol. 2000;175:693-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Peterson MS, Baron RL, Murakami T. Hepatic malignancies: usefulness of acquisition of multiple arterial and portal venous phase images at dynamic gadolinium-enhanced MR imaging. Radiology. 1996;201:337-345. [PubMed] |
| 12. | Furuta A, Ito K, Fujita T, Koike S, Shimizu A, Matsunaga N. Hepatic enhancement in multiphasic contrast-enhanced MDCT: comparison of high- and low-iodine-concentration contrast medium in same patients with chronic liver disease. AJR Am J Roentgenol. 2004;183:157-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Francis IR, Cohan RH, McNulty NJ, Platt JF, Korobkin M, Gebremariam A, Ragupathi KI. Multidetector CT of the liver and hepatic neoplasms: effect of multiphasic imaging on tumor conspicuity and vascular enhancement. AJR Am J Roentgenol. 2003;180:1217-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Yu JS, Kim KW, Kim EK, Lee JT, Yoo HS. Contrast enhancement of small hepatocellular carcinoma: usefulness of three successive early image acquisitions during multiphase dynamic MR imaging. AJR Am J Roentgenol. 1999;173:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Semelka RC, Martin DR, Balci C, Lance T. Focal liver lesions: comparison of dual-phase CT and multisequence multiplanar MR imaging including dynamic gadolinium enhancement. J Magn Reson Imaging. 2001;13:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 180] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Ito K, Mitchell DG. Imaging diagnosis of cirrhosis and chronic hepatitis. Intervirology. 2004;47:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Tang Y, Yamashita Y, Arakawa A, Namimoto T, Mitsuzaki K, Abe Y, Katahira K, Takahashi M. Detection of hepatocellular carcinoma arising in cirrhotic livers: comparison of gadolinium- and ferumoxides-enhanced MR imaging. AJR Am J Roentgenol. 1999;172:1547-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 100] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Fu-Hua Y, Jun Yang, Meng-Su Z. comparison of dynamic MRI, and helical CT and ultrasonic imaging to diagnosis small hepatocellular carcinoma. Zhongguo Yixue Jisuanji Chengxiang Zazi. 1997;3:20-24. |
| 19. | Kim T, Murakami T, Oi H, Matsushita M, Kishimoto H, Igarashi H, Nakamura H, Okamura J. Detection of hypervascular hepatocellular carcinoma by dynamic MRI and dynamic spiral CT. J Comput Assist Tomogr. 1995;19:948-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Krinsky GA, Lee VS, Theise ND, Weinreb JC, Rofsky NM, Diflo T, Teperman LW. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Jeong YY, Mitchell DG, Kamishima T. Small (< 20 mm) enhancing hepatic nodules seen on arterial phase MR imaging of the cirrhotic liver: clinical implications. AJR Am J Roentgenol. 2002;178:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Kelekis NL, Semelka RC, Worawattanakul S, de Lange EE, Ascher SM, Ahn IO, Reinhold C, Remer EM, Brown JJ, Bis KG. Hepatocellular carcinoma in North America: a multiinstitutional study of appearance on T1-weighted, T2-weighted, and serial gadolinium-enhanced gradient-echo images. AJR Am J Roentgenol. 1998;170:1005-1013. [PubMed] |
| 23. | Grazioli L, Morana G, Caudana R, Benetti A, Portolani N, Talamini G, Colombari R, Pirovano G, Kirchin MA, Spinazzi A. Hepatocellular carcinoma: correlation between gadobenate dimeglumine-enhanced MRI and pathologic findings. Invest Radiol. 2000;35:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Matsui O, Kadoya M, Kameyama T, Yoshikawa J, Takashima T, Nakanuma Y, Unoura M, Kobayashi K, Izumi R, Ida M. Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology. 1991;178:493-497. [PubMed] |
| 25. | Ichikawa T, Kitamura T, Nakajima H, Sou H, Tsukamoto T, Ikenaga S, Araki T. Hypervascular hepatocellular carcinoma: can double arterial phase imaging with multidetector CT improve tumor depiction in the cirrhotic liver? AJR Am J Roentgenol. 2002;179:751-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Turnheim K, Donath R, Weissel M, Kolassa N. Myocardial glucose uptake and breakdown during adenosine-induced vasodilation. Pflugers Arch. 1976;365:197-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |