Published online Feb 14, 2007. doi: 10.3748/wjg.v13.i6.945
Revised: November 15, 2006
Accepted: December 30, 2006
Published online: February 14, 2007
AIM: To prepare a complex of hyaluronic acid (HA) and phospholipids (PL), and study the improvement effect of PL on the oral absorption of HA.
METHODS: The complex of HA-PL (named Haplex) was prepared by film dispersion and sonication method, its physico-chemical properties were identified by infrared spectra and differential scanning calorimetry (DSC). The oral absorption of Haplex was studied. Thirty-two healthy rats were divided into 4 groups randomly: (1) a normal saline (NS) control group; (2) an HA group; (3) a mixture group and (4) a Haplex group. After intragastric administration, the concentration of HA in serum was determined.
RESULTS: The physico-chemical properties of Haplex were different from HA or PL or their mixture. After Haplex was administered to rats orally, the serum concentration of HA was increased when compared with the mixture or HA control groups from 4 h to 10 h (P < 0.05). The ∆AUC0-12 h of Haplex was also greater than that of the other three groups (P < 0.05).
CONCLUSION: The method of film dispersion and sonication can prepare HA and PL complex, and PL can enhance the oral absorption of exogenous HA.
- Citation: Huang SL, Ling PX, Zhang TM. Oral absorption of hyaluronic acid and phospholipids complexes in rats. World J Gastroenterol 2007; 13(6): 945-949
- URL: https://www.wjgnet.com/1007-9327/full/v13/i6/945.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i6.945
Hyaluronic acid (HA) is an acidic linear mucopoly-saccharide, having regularly alternating units of N-acetylglucosamine and D-glucuronic acid linked by 1, 4- or 1, 3-β-glycosidic bonds. HA distributes widely in connective tissues, vitreous body, and joint fluid of vertebrates. Its relative molecular mass (Mr) is 2 × 105-7 × 106, it possesses an important function of water keeping[1]. As is well known, the unique viscoelastic and lubricating properties of HA along with its biocompatibility and nonimmunogenicity have led to its wide uses in cosmetic, health care, medical, pharmaceutical applications, and so on.
It has been shown that HA can be absorbed by rats after oral administration in our previous experiments. As skin care and healthy food, HA can increase the precurosor which synthesizes HA in vivo. It can play a systemic role by increasing the concentration of endogenous HA from dermis to epidermis, therefore delaying aging is possible[2,3]. However, several limitations exist and influence the gastrointestinal absorption of exogenous HA when it is administered orally, such as high Mr and poor liposolubility of HA.
Phospholipids (PL) contains glycerol with a phosphate ester and two fatty acid ester side chains. They are the major building blocks of biological membranes and are tissue compatible[4]. PL is a series of amphiprotic materials, which can improve the gastrointestinal absorption of drugs. Since the 1980s, drugs especially plants extracts, complex with PL began to be a common trial to increase their oral bioavailability. The results indicate that PL complex play an important role in increasing the drug therapeutic effects, and the technique is easy to practise[5,6]. Consequently, in our experiment, PL was used as an absorption enhancer and the complex of HA and PL was prepared, which was named Haplex. The physico-chemical properties of Haplex were identified by infrared spectrum and differential scanning calorimetry. Haplex was administered to rats orally to prove if PL can enhance the oral absorption of HA.
HA was obtained from Shandong Freda Biochemical Limited Company, China. Egg lecithin was obtained from Lipoid Company, Germany. HA radioimmunoassay kit (HA RIA Kit) was obtained from Shanghai Naval Medical Research Institute, China. The other agents were all analytically pure.
Wistar rats (female, 260-300 g in weight) were obtained from the Animal Resource Center of Shandong University. Animals were housed at a constant temperature of 22 ± 2°C, and fed on standard pellet chow and water ad libitum. All experiments were carried out in accordance with the China Animal Welfare Legislation and were approved by Shandong UniversityEthics Committee of the Care and Use of Laboratory Animals.
The Haplex was prepared by film dispersion and sonication method[7,8]. The mixture of HA and PL was obtained by grinding in mortar.
We detected the position changes of main functional groups of Haplex by attenuated total reflectance infrared spectrum. Samples were spread on the KBr crystal glass plate and impacted. The spectral resolution was 8.0 cm, scanning range was 4000-500 cm-1 and the result was the average value of 200 times.
The thermochemical property changes of Haplex were detected by DSC. Its operational conditions were as follows: flow rate of N2 50 mL/min, scanning range of temperature 0-400°C and raising rate 5°C/min.
Some diseases could increase the concentration of HA in blood; therefore, the healthy animals should be screened before the experiment. The serum concentration range of HA was lower than plasma in rodent animals. The concentration range of HA in the plasma of healthy rats was 46-260 mg/L[9]. In this experiment, we found that the serum concentration of HA in most rats was lower than 180 mg/L, so these rats were selected for the following experiments.
Thirty-two healthy rats were divided into 4 groups with 8 rats in each group. The 4 groups were: a normal saline (NS) control group; an HA group; a mixture group and a Haplex group. All selected rats were fasted (but free drinking) for 12 h before experiment, and were anesthetized by intraperitoneal injection of Pentobarbital before drawing blood. The rats were intragastrically administrated with HA dosage of 60 mg/kg body weight using an intragastric injector. The injector was inserted into the stomach of rats at a depth of 4-6 cm, and the solution was pushed out.
The blood was drawn from subclavian vein at 0, 1, 2, 4, 7, 10 and 12 h after administration, and then was centrifuged after the serum separation. The serum was collected and stored in refrigerator at -80°C. After that, the concentration of HA in serum was detected using HA RIA Kit.
The data were presented as mean ± SD, and t test was performed between groups. P < 0.05 was considered statistically significant.
Because HA was inherent ingredient of serum, the concentration of HA in blank blood should be precluded when we analyze the data. The absorption effect was evaluated by the net increasing value of area under HA concentration-time curve (∆AUC0-t). In our experiment, the time of drawing blood was 0-12 h after administration. The value of ∆AUC0-12 h was calculated by trapezoidal rule, and the formula was expressed as follows:
∆ AUC0-12 h = SUM{(ti+1-ti)[(Ci- C0 ) + (Ci+1- C0)]/2}
In this formula, ti and Ci represent the time of drawing blood and the serum concentration of HA at ti. C0 indicates the serum concentration of HA at 0 h after administration.
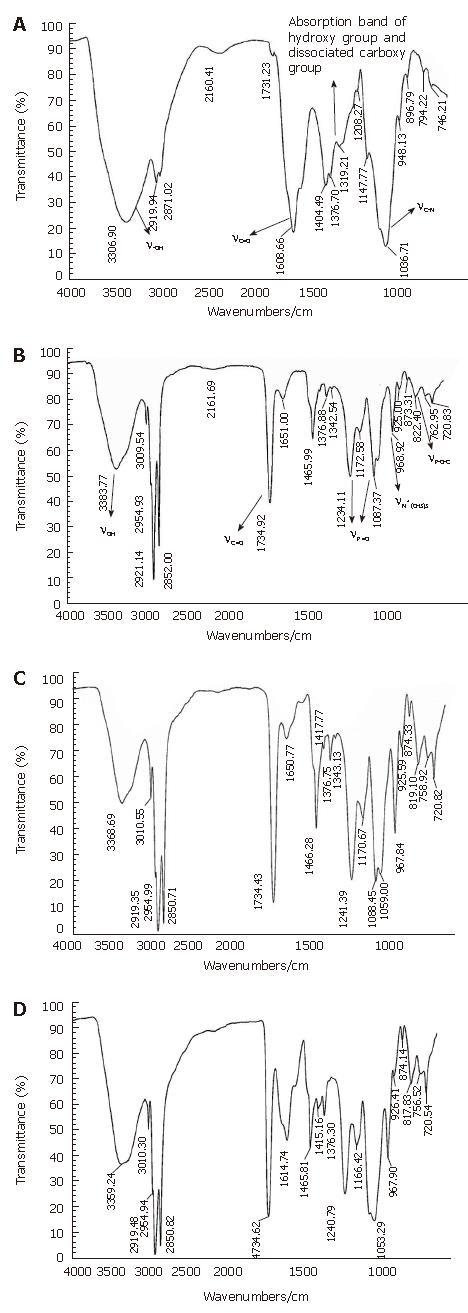
The results of IR are shown in Figure 1. As shown in Figure 1, the absorption peak of C=O and C-N of HA in Haplex shifted to superior position, the dissociated carboxy group of HA was also changed. The stretching vibration peak of P=O and P-O-C of PL in Haplex was budged too (υP = O moved to superior position, υP-O-C moved to the opposition). These changes indicated that the interactions of HA and PL in Haplex happened between the carboxyl group and amide group of HA and the polar head of PL, which belonged to ionic bond interaction and hydrophobic interaction[10-12].
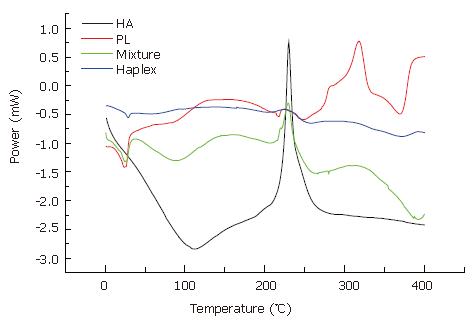
The DSC curves (Figure 2) showed that the endothermic phase transition peak and the exothermic oxygenolysis peak of PL in Haplex both disappeared. But the thermochemical property of HA had no obvious difference. The results revealed that as a mucopolysaccharide, HA could protect PL and weaken the sensibility of PL to temperature[13].
In a word, IR and DSC indicated that the physico-chemical properties of Haplex were different from HA, PL and their mixture. The results proved that the complex formed between HA and PL.
The basic principle of HA RIA Kit was the competitive binding reaction between 125I-HA in kit, non-marked HA in serum and HA binding protein (HABP). The amount of 125I-HA-HABP complex was determined[14]. The rat serum samples were collected. After setting the standard curve, the concentrations of HA in serum samples were determined.
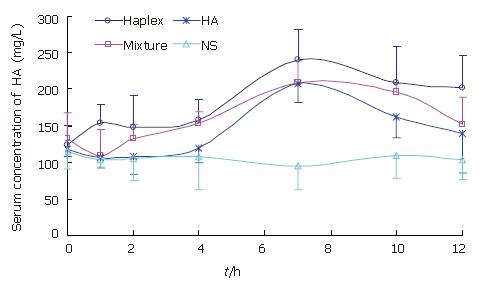
The drug concentration-time curves are shown in Figure 3. Compared with NS group, an evident absorption peak appeared in 4-12 h after administration in the other three groups (P < 0.01). From 0 h to 12 h after administration, the serum concentration of HA in Haplex group was higher than in HA group (P < 0.05 in 4-10 h after administration) and the mixture group. However, the serum concentration of HA in mixture group was higher than that in HA group at some certain time points only. The calculation results of ∆AUC0-12 h (Table 1) were coincident with Figure 3.
| Groups | ∆AUC0-12 h (ng·h/mL) |
| Haplex | 777.9 ± 318.3a |
| Mixture | 451.6 ± 401.3 |
| HA | 381.8 ± 340.8 |
Therefore, the oral absorption experiment demonstrated that: (1) exogenous HA was absorbed into blood after oral administration; (2) after the formation of the complex, PL in Haplex could promote the oral absorption of exogenous HA, increase the HA concentration in serum and prolong the time of HA absorption. However, during the experiment, no absorption peak appeared in the NS control group. It indicated that oral administration of NS could not increase the HA concentration in serum, and not influence the absorption of HA. And it is thus rational to use NS to dissolve HA and Haplex.
The results of animal experiments indicated that PL could enhance the oral absorption of HA after the formation of Haplex. The mechanism of the enhancement effect might be related to the following elements.
PL is an important component of cell membrane and the exogenous PL has strong affinity to cell surfaces. In the Haplex, HA and PL combined together, PL could facilitate HA binding to the cells. On the other hand, in the presence of excess water, PL could form lipid spheres. The polar head groups are oriented to the water; the fatty acyl chains could form a palisade structure, with their ends abutting in the center of the membrane. Similarly, Haplex might form the structure of liposome when it entered into the gastrointestinal fluid. Therefore, when Haplex got close to the gastrointestinal mucous membrane cells, fusion, pinocytosis and phagocytosis might occur due to liposome’s double molecular constitution. These contributions could improve the absorption of HA into gastrointestinal mucous membrane. Moreover, due to the smaller particle diameter of PL in Haplex, it could increase the contact area and the contact time between Haplex and intestine wall. All these effects benefit the absorption of HA[5,15-17].
The synovial fluid of normal joints contains several molecular species, the most important constitute of which is HA. HA plays critical role in the physiology of joint function, including lubrication of the synovial surfaces. In addition, synovial fluids contains phospholipids. The presence of HA and PL complexes could provide excellent protection of the cartilage surface from enzymes and free radicals in the synovial fluid, so it could be used as an oral protectant of joint function[18].
HA is a slow-release carrier due to its bioadhesion and biocompatibility properties[19]. After the formation of complex, HA could improve the absorption of PL because of the three dimensions network of HA, and enhance the health care effect of PL.
Haplex could be made in different types of oral praeparatum, including liquid and solid preparation. The nutrition additives and other active components could be added into Haplex if required. The increased effectiveness of active components might attribute to both the bioadhension property of HA and the absorption facilitation property of PL. Haplex thus may be used in skin care and joint disease prevention.
Hyaluronic acid(HA) is an endogenous glycosaminoglycan, it possesses unique viscoelastic and lubricating properties, and is widely used in cosmetic, health care, medical, pharmaceutical applications, and so on. When exogenous HA is administrated orally, it can increase the concentration of endogenous HA from dermis to epidermis, thereby, it can play systemic roles to delay aging and prevent joints diseases. Phospholipids(PL) are a series of amphiprotic materials, they can improve the gastrointestinal absorption and reduce side effects of drugs.
In this study, the authors prepared the complexes and mixture of HA and PL. The oral absorption of HA both contained in complex and mixture were studied in rats after intragastric administration.
Because of the high relative molecular mass and poor liposolubility of HA, its gastrointestinal absorption was limited when it is administered orally. In this study, the authors proved that the serum concentration of HA increased when Haplex was administrated orally. So PL could improve the oral absorption of HA after they formed complexes.
This study provided an evidence for the development of oral praeparatum containing HA. The complex could be used in the fields of healthy food development and joint function protection.
This is a descriptive study that shows the improvement effect of phospholipids (PL) on the oral absorption of hyaluronic acid (HA) using a complex between both named Haplex. The manuscript is easy to understand and in general terms well written.
S- Editor Liu Y L- Editor Ma JY E- Editor Ma WH
| 1. | Kaufmann J, Mohle K, Hofmann HJ, Arnold K. Molecular dynamics study of hyaluronic acid in water. J Mol Struct (Theochem). 1998;422:109-121. [RCA] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Ling PX. Hyaluronan. Beijing: Zhongguo Qinggongye Chubanshe 2000; 122-134. |
| 3. | Yamamoto H. Antiaging and cosmetic effects of dietary hyaluronic acid (cxtracellular matrix extract). New Food Ind. 1998;40:33-41. |
| 4. | Cao D, Qiu AY, Wang XG. The structure, nature, function and researching situation of phospholipids. Liangshi yu Youzhi. 2004;5:3-7. |
| 5. | Zhai GX, Lou HX, Zou LJ, Bi DZ. Advancement of drugs and phospholipids complex. Zhongguo Yaoxue Zazhi. 2001;36:801-803. |
| 6. | Wu JM, Chen DW, Sun B, Li SM, Zhang RH. Review of natrual principles complex with phospholipid in pharmacy. Zhongguo Yaoxue Zazhi. 1998;33:9-11. |
| 7. | Pasquali-Ronchetti I, Quaglino D, Mori G, Bacchelli B, Ghosh P. Hyaluronan-phospholipid interactions. J Struct Biol. 1997;120:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Ghosh P, Hutadilok N, Adam N, Lentini A. Interactions of hyaluronan (hyaluronic acid) with phospholipids as determined by gel permeation chromatography, multi-angle laser-light-scattering photometry and 1H-NMR spectroscopy. Int J Biol Macromol. 1994;16:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Reed RK, Lilja K, Laurent TC. Hyaluronan in the rat with special reference to the skin. Acta Physiol Scand. 1988;134:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 119] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Tian F, Zeng GM, Wang DM, Xi SQ. FTIR study on molecular mechanism of the interactions between rare earth ions and dipalmitoylphosphatidylcholine cast-dry film. Wuli Huaxue Xuebao. 1995;8:134-138. |
| 11. | Wang QH, Feng YY, Yang SL, Chen LF. Study on the phospha-tidycholine by means of infrared and ultraviolet spectroscopy. Nanjing Shida Xuebao (Natural Sci). 1994;17:51-53. |
| 12. | Zhang XL, Liu LY, Wu GZ. Preparation and quality analysis of the hyaluronic acid from human umbilical cord. Zhongguo Yaofang. 1999;10:10-11. |
| 13. | Ohtake S, Schebor C, de Pablo JJ. Effects of trehalose on the phase behavior of DPPC-cholesterol unilamellar vesicles. Biochim Biophys Acta. 2006;1758:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Li SL. Nuclear Medicine. 6th ed. Beijing: Renmin Weisheng Chubanshe 2004; 33-35. |
| 15. | Li L. Medicinal value of phospholipids. Liangshi yu Youzhi. 2001;8:34-36. |
| 16. | Desai MP, Labhasetwar V, Amidon GL, Levy RJ. Gastrointestinal uptake of biodegradable microparticles: effect of particle size. Pharm Res. 1996;13:1838-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 633] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 17. | Ling PX, Tang X, Wang FS, Zhu MH, Zhang TM. Current status in research on complex of drug with phospholipid. Zhongguo Yaoxue Zazhi. 2005;40:401-402. |
| 18. | Kawano T, Miura H, Mawatari T, Moro-Oka T, Nakanishi Y, Higaki H, Iwamoto Y. Mechanical effects of the intraarticular administration of high molecular weight hyaluronic acid plus phospholipid on synovial joint lubrication and prevention of articular cartilage degeneration in experimental osteoarthritis. Arthritis Rheum. 2003;48:1923-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Yerushalmi N, Arad A, Margalit R. Molecular and cellular studies of hyaluronic acid-modified liposomes as bioadhesive carriers for topical drug delivery in wound healing. Arch Biochem Biophys. 1994;313:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |