Published online Feb 14, 2007. doi: 10.3748/wjg.v13.i6.939
Revised: November 11, 2006
Accepted: December 21, 2006
Published online: February 14, 2007
AIM: To construct a recombinant live attenuated Salm-onella typhimurium DNA vaccine encoding H pylori ureB gene and mouse IL-2 gene and to detect its immunogenicity in vitro and in vivo.
METHODS: H pylori ureB and mouse IL-2 gene fragments were amplified by polymerase chain reaction (PCR) and cloned into pUCmT vector. DNA sequence of the amplified ureB and IL-2 genes was assayed, then cloned into the eukaryotic expression vector pIRES through enzyme digestion and ligation reactions resulting in pIRES-ureB and pIRES-ureB-IL-2. The recombinant plasmids were used to transform competent E. coli DH5α, and the positive clones were screened by PCR and restriction enzyme digestion. Then, the recombinant pIRES-ureB and pIRES-ureB-IL-2 were used to transform LB5000 and the recombinant plasmids extracted from LB5000 were finally introduced into the final host SL7207. After that, recombinant strains were grown in vitro repeatedly. In order to detect the immunogenicity of the vaccine in vitro, pIRES-ureB and pIRES-ureB-IL-2 were transfected to COS-7 cells using LipofectamineTM2000, the immunogenicity of expressed UreB and IL-2 proteins was assayed with SDS-PAGE and Western blot. C57BL/6 mice were orally immunized with 1 × 108 recombinant attenuated Salmonella typhimurium DNA vaccine. Four weeks after vaccination, mice were challenged with 1 × 107 CFU of live H pylori SS1. Mice were sacrificed and the stomach was isolated for examination of H pylori 4 wk post-challenge.
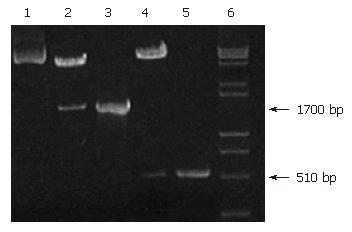
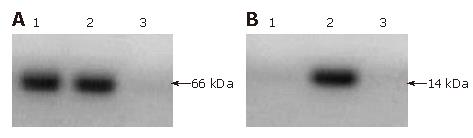
RESULTS: The 1700 base pair ureB gene fragment amplified from the genomic DNA was consistent with the sequence of H pylori ureB by sequence analysis. The amplified 510 base pair fragment was consistent with the sequence of mouse IL-2 in gene bank. It was confirmed by PCR and restriction enzyme digestion that H pylori ureB and mouse IL-2 genes were inserted into the eukaryotic expression vector pIRES. The experiments in vitro showed that stable recombinant live attenuated Salmonella typhimurium DNA vaccine carrying ureB and IL-2 genes was successfully constructed and the specific strips of UreB and IL-2 expressed by recombinant plasmids were detected through Western blot. Study in vivo showed that the positive rate of rapid urease test of the immunized group including ureB and ureB-IL-2 was 37.5% and 12.5% respectively, and was significantly lower than that (100%) in the control group (P < 0.01).
CONCLUSION: Recombinant attenuated Salmonella typhimurium DNA vaccine expressing UreB protein and IL-2 protein with immunogenicity can be constructed. It can protect mice against H pylori infection, which may help the development of a human-use H pylori DNA vaccine.
-
Citation: Xu C, Li ZS, Du YQ, Gong YF, Yang H, Sun B, Jin J. Construction of recombinant attenuated Salmonella typhimurium DNA vaccine expressing
H pylori ureB and IL-2. World J Gastroenterol 2007; 13(6): 939-944 - URL: https://www.wjgnet.com/1007-9327/full/v13/i6/939.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i6.939
H pylori infection can lead to chronic gastritis, peptic ulcer disease, and is also a risk factor for gastric adenocarcinoma and mucosa-associated lymphoid tissue (MALT) lymphoma[1-4]. More than 50% of the human population worldwide are infected with H pylori. About 10%-20% of all the patients have severe diseases such as gastric or duodenal ulcer and gastric cancer. The current therapy, based on the use of proton-pump inhibitor and antibiotics[5-7], is efficacious but faces potential problems like patient compliance, increasingly reported antibiotic resistance, and side effects such as abdominal pain, nausea, diarrhea[8]. Vaccination of humans against H pylori infection may be an effective and economic approach to its control.
Studies of H pylori vaccine have focused on the individual H pylori proteins or the whole bacterial cell sonicates as antigens which need mucosal adjuvants like cholera toxin or E. coli labile toxin to elicit effective protection[9-11]. However, their use in humans is hampered by extremely high toxicity of mucosal adjuvants[12,13]. Recently, DNA vaccine without such mucosal adjuvants has been demonstrated to induce both humoral and cellular immunity and is becoming a promising treatment for viral, bacterial and parasitic pathogens[14,15]. Protective immunity against HIV, influenza virus, rabies virus, malaria and tuberculosis has been shown in animal models[16-19]. DNA vaccine of H pylori is seldom reported.
It was reported that coexpression of cytokine genes together with antigen-encoding genes in DNA vaccination vectors can increase both humoral and cellular immune response[20,21]. No information is available about the effects of cytokine-derived adjuvant in combination with H pylori vaccine.
In the present study we constructed a recombinant live attenuated Salmonella typhimurium DNA vaccine carrying H pylori ureB gene and mouse IL-2 gene, and identified its immunogenicity in transfected COS-7 cells in vitro and its prophylactic immunization in vivo.
Attenuated Salmonella typhimura LB5000 and SL7207 kindly provided by Professor Bruce Stocker of Stanford University, USA, were cultured in Amp (-) LB medium. E. coli DH5α was grown in LB medium containing 50mg ampicillin per liter. H pylori strain CCUG17874 (NCTC11638) kindly provided by the Italian IRIS Research Center was cultured on H pylori selective agar plates with 10% defibrillated sheep blood and antibiotics (Merck Company, Germany) at 37°C under microaerophilic conditions with 5% O2, 10% CO2 and 85% N2. COS-7 cell line and recombinant plasmid pCIneo-IL-2 were provided by the Department of Immunology, Secondary Military Medical University of China. The mammalian expression vector pIRES was purchased from Clontech, USA.
Genomic DNA of H pylori was extracted as previously described using CTAB[22]. According to the complete DNA sequence of H pylori published and multiple clone sites of pIRES, the primers to amplify ureB containing Nhe I site in P1 and Xho I site in P2 respectively were designed (P1: 5’ GCTAGCCACCATGAAAAAGATTAGCAGAAAAG 3’, P2: 5’ CTCGAGCTAGAAAATGCTAAAGAGTTGCGCC 3’). The primers to amplify IL-2 containing Sal I site in P3 and Not I site in P4 were designed (P3: 5’ GTCGACCACCATGTACAGCATGCAGCTCG3’, P4: 5’GCGGCCGCTTATTGAGGGCTTGTTGAGATG 3’). Amplication conditions for ureB were as follows: at 95°C for 5 min, then 35 cycles at 95°C for 30 s, at 55°C for 50 s and at 72°C for 90 s, followed by 10 min at 72°C. Amplication conditions for IL-2 were at 95°C for 5 min, then 30 cycles at 95°C for 30 s, at 55°C for 40 s and at 72°C for 1 min, followed by 5 min at 72°C. The amplified products were analyzed on 1.2% agarose gels stained with ethidium bromide.
The PCR products were separated using a QIAquick gel extraction kit (QIAGEN, CA, USA). Purified ureB and IL-2 fragments were each subcloned into TA cloning vector pUCmT (Takara, Dalian, China) resulting in pUCmT-ureB and pUCmT-IL-2, then the nucleotide sequences of ureB and IL-2 were analyzed using an automatic sequencer.
Fragments of Nhe I and Xho I-digested pUCmT-ureB were inserted into the Nhe I/Xho I site of eukaryotic expression vector pIRES, through a serial of enzyme digestion and ligation reactions resulting in pIRES-ureB. Fragments of Sal I and Not I-digested pUCmT-IL-2 were inserted into the Sal I/Not I site of pIRES-ureB. The recombinant pIRES-ureB and pIRES-ureB-IL-2 were all confirmed by PCR and restriction enzyme digestion.
Recombinant plasmids of pIRES-ureB and pIRES-ureB-IL-2 were transfected into COS-7 cells respectively in order to detect the proteins expressed by pIRES-ureB and pIRES-ureB-IL-2. COS-7 cell line was cultured at 37°C, in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS (Gibco-BRL, UK), 100 U/mL penicillin and 100 μg/mL streptomycin, 15 mmol/L HEPES, 2 mmol/L L-glutamine. The mixture of pIRES-ureB and Lipofectamine TM2000 (Invitrogen, USA) or pIRES-ureB-IL-2 and LipofectamineTM2000 were added to the COS-7 cells. Forty-eight hours after transfection, the cells were washed with PBS and protein extraction reagent was added. The lysate was collected and centrifuged at 12 000 ×g for 5 min at 4°C.
Supernatant containing proteins was electrophoretically analyzed in a 10% polyacrylamide gel, subsequently electrotransferred onto nitrocellulose membranes. Nonspecific binding sites were blocked with 2% bovine serum albumin (BSA), then rabbit anti-H pylori or rabbit anti-IL-2 antibodies and peroxidase-labeled anti-rabbit immunoglobulin G (IgG) were added. The antigens were visualized by chemiluminescence (Bio-Rad, Germany) according to the manufacturer’s instructions.
Recombinant pIRES-ureB and pIRES-ureB-IL-2 were used to transform attenuated Salmonella typhimurium LB5000 for methylation decoration using calcium chloride, then the recombinant plasmids extracted were transformed into the final host strain SL7207 by electroporation. The attenuated Salmonella typhimurium SL7207 carrying ureB gene and SL7207 carrying both ureB and IL-2 genes were cultured in LB medium to 80 generations. The recombinant plasmids in transformed SL7207 were isolated from every 10 generations and identified by restriction enzymes and PCR.
Prior to immunization, mice were left overnight without solid food and 4 h without water. One hundred μL of 3% sodium bicarbonate was given orally using a stainless steel catheter tube to neutralize the stomach pH. Immediately after that, mice in the vaccination group were orally given 5 × 108 live attenuated salmonella typhimurium DNA vaccine ureB or ureB-IL-2 in a total volume of 200 μL, mice in the control group were given 200 μL PBS. Mice had free access to water and food after immunization.
Four weeks after immunization, all mice were challenged with 200 μL H pylori SS1 (1 × 107 CFU). Before challenge, all mice were prepared as described above.
Four weeks after challenge, all mice were sacrificed and the stomach was carefully removed from each mouse under aseptic conditions. The stomach was washed 3 times in sterile PBS to remove food residues. A part of the antral region was used for rapid urease test to measure the urease activity.
Differences in H pylori strain-induced urease activity in stomach of immunized and non- immunized mice were evaluated using a two-tailed Fisher’s exact test.
Sequence analysis of ureB and IL-2 showed that the sequences of amplified fragments were consistent with those of H pylori ureB and mouse IL-2 published in the gene bank respectively.
After pUCmT-ureB and pIRES were digested by both Nhe I and Xho I, a 1700 bp fragment of ureB was directly cloned into Nhe I/Xho I site of pIRES, resulting in a recombinant plasmid pIRES-ureB. IL-2 was inserted into the Sal I/Not I site of pIRES-ureB to get pIRES-ureB-IL-2. Both PCR and restriction enzyme digestion demonstrated that recombinant plasmids contained the ureB and IL-2 genes. PCR and restriction enzyme digestion products were analyzed on agarose gel (Figure 1).
Identification of pIRES-ureB and pIRES-ureB-IL-2 in vitro expression was carried out. The lysate of COS-7 cells transfected by pIRES-ureB and pIRES-ureB-IL-2 was analyzed by Western blot (Figure 2A and B). It revealed the band about 66 000 in relative molecular weight corresponded to UreB protein and 14 000 to IL-2 protein, but the control transfected with pIRES had no specific band.
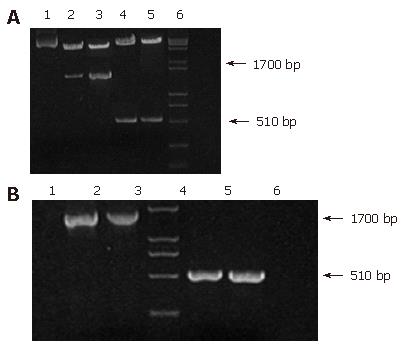
After transformed by pIRES-ureB-IIL-2, recombinant plasmid extracted from LB5000 was used to transform SL7207. Plasmid stability is essential to assure the stable expression of antigens encoded by genes which were cloned into the plasmids. Therefore, SL7207 carrying pIRES-ureB-IIL-2 was grown in vitro up to 80 generations to examine the plasmid stability. The objective fragments (1.7 kb and 510 bp ) could be seen on the map of agarose gel of PCR products and those of restriction enzyme digested recombinant plasmid isolated from transformed SL7207 (Figure 3A and B).
All mice in the control PBS group were infected with H pylori. In contrast, 62.5% and 87.5% of mice immunized with ureB and ureB-IL-2 were resistant to H pylori (P < 0.01). The negative rate of rapid urease test in ureB-IL-2 group was significantly higher than that in ureB group (P < 0.05, Table 1).
| Group | Positive (n) | Negative (n) | Total (n) | Negative rate (%) |
| PBS | 10 | 0 | 10 | 0 |
| ureB | 9 | 15 | 24 | 62.5b |
| ureB-IL-2 | 3 | 21 | 24 | 87.5a,b |
DNA vaccine or genetic vaccine can induce immune response to DNA-encoded proteins after naked DNA is injected into a host. DNA vaccines have been widely used in laboratory animals to elicit comprehensive humoral and cellular immune responses. Clinical trials have shown that DNA vaccines are safe and well tolerated[15,23]. Moreover, some reports have demonstrated that DNA vaccine could produce long-lasting immunity[24-25]. The vaccine itself is a recombinant plasmid with heat stability and does not need to be purified. It can be used to protect against and treat tumors[26-29].
However, this approach has not been fully explored in H pylori vaccine. The available H pylori vaccine is a protein vaccine, including H pylori whole cells or one of the recombinant proteins of H pylori as an antigen of the vaccine in combination with mucosal adjuvants such as cholera toxin or heat-labile toxin of enterotoxigenic E. coli [11,30,31]. The manufacture of such vaccines is complicated, and some mucosal adjuvants have gastrointestinal toxicity. It was reported that mucosal immunization with Helicobacter heilmannii urease B or H pylori urease, given nasally with cholera toxin, could protect BALB/c mice against H heilmannii infection and significantly reduce the preexisting infection[32]. However, immunization aggravates gastric corpus atrophy .
Urease is an enzyme consisting of six UreA subunits and six UreB subunits. Urease negative H pylori strains are unable to colonize the stomach of gnotobiotic piglets, demonstrating its role in the colonization. Urease may intervene directly or indirectly in induction of tissue damage in the stomach. Moreover, it may play an additional role in adhesion and stimulation of inflammatory cells. Urease is a highly conserved protein among gastric H pylori species and a potential antigen for prophylactic and therapeutic vaccines against H pylori infection among different animals[33-35], suggesting that it is an ideal antigen candidate for H pylori vaccine.
It has been shown that live attenuated bacteria carrier including attenuated strains of Salmonella in vitro could deliver DNA vaccines to human cells, thus allowing vaccination via mucosal surfaces and specific targeting of professional antigen presenting cells in mucosa-associated lymphoid tissue tumors[36,37].
Several studies have shown that co-administration of cytokine proteins or of cytokine-expressing plasmids can modulate the immune response to DNA vaccination[40,41]. Enhancement of antigen-specific antibody response and T cell response has been demonstrated by vaccine co-expressing DNA and immune adjuvants like IL-2 or granulocyte macrophage colony stimulating factor (GM-CSF) gene[40,41].
In this study, we constructed a live recombinant attenuated Salmonella typhimurium DNA vaccine strain expressing UreB protein and mouse IL-2 protein. The complete ureB gene fragment and mouse IL-2 gene were amplified and then sequence analysis was performed after they were cloned respectively into the TA cloning vector pUCmT. Purified ureB and IL-2 were cloned into eukaryotic expression vector pIRES. Both the enzyme digestion and PCR confirmed the successful construction of recombinant plasmid pIRES-ureB and pIRES-ureB-IL-2. Recombinant attenuated Salmonella typhimurium carrying H pylori ureB gene and mouse IL-2 was successfully constructed after recombinant plasmid was used to transform LB5000 and SL7207. Since the stability of the protective antigen is very important for a vaccine, we assayed the stability of the recombinant plasmid in vitro. PCR and restriction enzyme digestion confirmed the presence of pIRES-ureB-IL-2 in all transformed strains of SL7207 up to the 80th generation, demonstrating the stability of the recombinant plasmid in SL7207.
It was also shown in vitro in our present study that COS-7 cellls transfected by pIRES-ureB-IL-2 could express 66 000 UreB and 14 000 IL-2, but COS-7 cells transfected by pIRES could not express them. The pIRES-ureB-IL-2 DNA vaccine we constructed could express UreB protein which can react with anti-H pylori protein and IL-2 which can react with anti-mouse IL-2 protein.
We also demonstrated that a single dose of ureB or both ureB and adjuvant IL-2 delivered by the live attenuated Salmonella typhimurium SL7207 strain could protect C57BL/6 mice against H pylori colonization in the stomach. No protection was observed in the control group. Compared with ureB group, ureB-IL-2 group appeared to induce a better protection against H pylori infection with a significantly higher negative rate of rapid urease test, suggesting that adjuvant IL-2 may improve the protective immunization level.
In summary, recombinant attenuated Salmonella typhimurium DNA vaccine encoding H pylori ureB gene and mouse IL-2 gene can express UreB and IL-2 with immunogenicity. DNA vaccine can protect against H pylori challenge in animal models. The therapeutic effect of DNA vaccine on H pylori infection and its mechanism should be further studied.
Studies of H pylori vaccine have focused on the individual H pylori proteins or the whole bacterial cell sonicates as antigens which need mucosal adjuvants like cholera toxin or Escherichia coli labile toxin to elicit effective protection. However, clinical trials have shown the intestinal toxicity of mucosal adjuvants. Recently, DNA vaccine without such mucosal adjuvants has been demonstrated to induce humoral and cellular immunity and is becoming a promising treatment for viral, bacterial and parasitic pathogens. Protective immunity against HIV, influenza virus, rabies virus, malaria and tuberculosis has been shown in animal models. DNA vaccine against H pylori is seldom reported.
It was reported that co-expression of cytokine genes and antigen-encoding genes in DNA vaccination vectors can increase humoral and cellular immune response. No information is available about the effects of cytokine-derived adjuvant in combination with H pylori vaccine.
Recombinant attenuated Salmonella typhimurium DNA vaccine encoding H pylori ureB gene and mouse IL-2 gene can express UreB and IL-2 with immunogenicity. DNA vaccine against H pylori and adjuvant IL-2 may improve the protective immunization level.
H pylori vaccine can prevent H pylori infection in human beings.
The manuscript by Dr. Xu et al described an interesting study on the construction of a recombinant attenuated S. typhimurium DNA vaccine for Helicobacter pylori infection. The authors have coexpressed H pylori ureB and cytokine IL-2 which can improve the potential immunogenicity and protective efficacies of the vaccine.
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH
| 1. | Isakov V, Malfertheiner P. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2003;8 Suppl 1:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Miwa H, Sakaki N, Sugano K, Sekine H, Higuchi K, Uemura N, Kato M, Murakami K, Kato C, Shiotani A. Recurrent peptic ulcers in patients following successful Helicobacter pylori eradication: a multicenter study of 4940 patients. Helicobacter. 2004;9:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Takahashi S. Long-term Helicobacter pylori infection and the development of atrophic gastritis and gastric cancer in Japan. J Gastroenterol. 2002;37 Suppl 13:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Al-Akwaa AM, Siddiqui N, Al-Mofleh IA. Primary gastric lymphoma. World J Gastroenterol. 2004;10:5-11. [PubMed] |
| 5. | Suzuki H, Masaoka T, Nomura S, Hoshino Y, Kurabayashi K, Minegishi Y, Suzuki M, Ishii H. Current consensus on the diagnosis and treatment of H. pylori-associated gastroduodenal disease. Keio J Med. 2003;52:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Perri F, Qasim A, Marras L, O'Morain C. Treatment of Helicobacter pylori infection. Helicobacter. 2003;8 Suppl 1:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Anagnostopoulos GK, Kostopoulos P, Margantinis G, Tsiakos S, Arvanitidis D. Omeprazole plus azithromycin and either amoxicillin or tinidazole for eradication of Helicobacter pylori infection. J Clin Gastroenterol. 2003;36:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Cameron EA, Powell KU, Baldwin L, Jones P, Bell GD, Williams SG. Helicobacter pylori: antibiotic resistance and eradication rates in Suffolk, UK, 1991-2001. J Med Microbiol. 2004;53:535-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Kotloff KL, Sztein MB, Wasserman SS, Losonsky GA, DiLorenzo SC, Walker RI. Safety and immunogenicity of oral inactivated whole-cell Helicobacter pylori vaccine with adjuvant among volunteers with or without subclinical infection. Infect Immun. 2001;69:3581-3590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Jeremy AH, Du Y, Dixon MF, Robinson PA, Crabtree JE. Protection against Helicobacter pylori infection in the Mongolian gerbil after prophylactic vaccination. Microbes Infect. 2006;8:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Durrani Z, Rijpkema S. Orogastric vaccination of guinea pigs with Helicobacter pylori sonicate and a high dose of cholera toxin lowers the burden of infection. FEMS Immunol Med Microbiol. 2003;36:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Holmgren J, Adamsson J, Anjuère F, Clemens J, Czerkinsky C, Eriksson K, Flach CF, George-Chandy A, Harandi AM, Lebens M. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol Lett. 2005;97:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Pizza M, Giuliani MM, Fontana MR, Monaci E, Douce G, Dougan G, Mills KH, Rappuoli R, Del Giudice G. Mucosal vaccines: non toxic derivatives of LT and CT as mucosal adjuvants. Vaccine. 2001;19:2534-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 227] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 14. | Martin JE, Sullivan NJ, Enama ME, Gordon IJ, Roederer M, Koup RA, Bailer RT, Chakrabarti BK, Bailey MA, Gomez PL. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol. 2006;13:1267-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 186] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Schalk JA, Mooi FR, Berbers GA, van Aerts LA, Ovelgönne H, Kimman TG. Preclinical and clinical safety studies on DNA vaccines. Hum Vaccin. 2006;2:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Muthumani K, Zhang D, Dayes NS, Hwang DS, Calarota SA, Choo AY, Boyer JD, Weiner DB. Novel engineered HIV-1 East African Clade-A gp160 plasmid construct induces strong humoral and cell-mediated immune responses in vivo. Virology. 2003;314:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Soboll G, Horohov DW, Aldridge BM, Olsen CW, McGregor MW, Drape RJ, Macklin MD, Swain WF, Lunn DP. Regional antibody and cellular immune responses to equine influenza virus infection, and particle mediated DNA vaccination. Vet Immunol Immunopathol. 2003;94:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Carvalho LJ, Daniel-Ribeiro CT, Goto H. Malaria vaccine: candidate antigens, mechanisms, constraints and prospects. Scand J Immunol. 2002;56:327-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Ha SJ, Jeon BY, Kim SC, Kim DJ, Song MK, Sung YC, Cho SN. Therapeutic effect of DNA vaccines combined with chemotherapy in a latent infection model after aerosol infection of mice with Mycobacterium tuberculosis. Gene Ther. 2003;10:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Chang SY, Lee KC, Ko SY, Ko HJ, Kang CY. Enhanced efficacy of DNA vaccination against Her-2/neu tumor antigen by genetic adjuvants. Int J Cancer. 2004;111:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Buonocore F, Mazzini M, Forlenza M, Randelli E, Secombes CJ, Zou J, Scapigliati G. Expression in Escherchia coli and purification of sea bass (Dicentrarchus labrax) interleukin 1beta, a possible immunoadjuvant in aquaculture. Mar Biotechnol (NY). 2004;6:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Xu C, Li ZS, Tu ZX, Xu GM, Gong YF, Man XH. Distribution of cagG gene in Helicobacter pylori isolates from Chinese patients with different gastroduodenal diseases and its clinical and pathological significance. World J Gastroenterol. 2003;9:2258-2260. [PubMed] |
| 23. | Liu MA, Wahren B, Karlsson Hedestam GB. DNA vaccines: recent developments and future possibilities. Hum Gene Ther. 2006;17:1051-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Medjitna TD, Stadler C, Bruckner L, Griot C, Ottiger HP. DNA vaccines: safety aspect assessment and regulation. Dev Biol (Basel). 2006;126:261-270; discussion 327. [PubMed] |
| 25. | Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40:86-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Henke A. DNA immunization--a new chance in vaccine research? Med Microbiol Immunol. 2002;191:187-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Abdelnoor AM. Plasmid DNA vaccines. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Donnelly J, Berry K, Ulmer JB. Technical and regulatory hurdles for DNA vaccines. Int J Parasitol. 2003;33:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Reisfeld RA, Niethammer AG, Luo Y, Xiang R. DNA vaccines designed to inhibit tumor growth by suppression of angiogenesis. Int Arch Allergy Immunol. 2004;133:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Guy B, Hessler C, Fourage S, Haensler J, Vialon-Lafay E, Rokbi B, Millet MJ. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 96] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Keenan JI, Rijpkema SG, Durrani Z, Roake JA. Differences in immunogenicity and protection in mice and guinea pigs following intranasal immunization with Helicobacter pylori outer membrane antigens. FEMS Immunol Med Microbiol. 2003;36:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Dieterich C, Bouzourène H, Blum AL, Corthésy-Theulaz IE. Urease-based mucosal immunization against Helicobacter heilmannii infection induces corpus atrophy in mice. Infect Immun. 1999;67:6206-6209. [PubMed] |
| 33. | Dunn BE, Phadnis SH. Structure, function and localization of Helicobacter pylori urease. Yale J Biol Med. 1998;71:63-73. [PubMed] |
| 34. | Sougioultzis S, Lee CK, Alsahli M, Banerjee S, Cadoz M, Schrader R, Guy B, Bedford P, Monath TP, Kelly CP. Safety and efficacy of E coli enterotoxin adjuvant for urease-based rectal immunization against Helicobacter pylori. Vaccine. 2002;21:194-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Hocking D, Webb E, Radcliff F, Rothel L, Taylor S, Pinczower G, Kapouleas C, Braley H, Lee A, Doidge C. Isolation of recombinant protective Helicobacter pylori antigens. Infect Immun. 1999;67:4713-4719. [PubMed] |
| 36. | Thole JE, van Dalen PJ, Havenith CE, Pouwels PH, Seegers JF, Tielen FD, van der Zee MD, Zegers ND, Shaw M. Live bacterial delivery systems for development of mucosal vaccines. Curr Opin Mol Ther. 2000;2:94-99. [PubMed] |
| 37. | Dietrich G, Spreng S, Favre D, Viret JF, Guzman CA. Live attenuated bacteria as vectors to deliver plasmid DNA vaccines. Curr Opin Mol Ther. 2003;5:10-19. [PubMed] |
| 38. | Sirard JC, Niedergang F, Kraehenbuhl JP. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol Rev. 1999;171:5-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Nobiron I, Thompson I, Brownlie J, Collins ME. Cytokine adjuvancy of BVDV DNA vaccine enhances both humoral and cellular immune responses in mice. Vaccine. 2001;19:4226-4235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | McGettigan JP, Koser ML, McKenna PM, Smith ME, Marvin JM, Eisenlohr LC, Dietzschold B, Schnell MJ. Enhanced humoral HIV-1-specific immune responses generated from recombinant rhabdoviral-based vaccine vectors co-expressing HIV-1 proteins and IL-2. Virology. 2006;344:363-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Niethammer AG, Xiang R, Ruehlmann JM, Lode HN, Dolman CS, Gillies SD, Reisfeld RA. Targeted interleukin 2 therapy enhances protective immunity induced by an autologous oral DNA vaccine against murine melanoma. Cancer Res. 2001;61:6178-6184. [PubMed] |