Published online Dec 21, 2007. doi: 10.3748/wjg.v13.i47.6441
Revised: October 10, 2007
Accepted: November 19, 2007
Published online: December 21, 2007
Malignant fibrous histiocytoma (MFH) is a pleomorphic mesenchynal sarcoma. It is uncommonly arises primarily from the intra-peritoneal cavity. Primary peritoneal MFH with tumor bleeding and rupture is rare. We describe the imaging features of a 70-year-old patient presenting with ruptured hemorrhagic peritoneal MFH at subhepatic area, accompanied by massive hemoperitoneum, mimicking a ruptured pedunculated hepatocellular carcinoma. Computed tomography (CT) revealed a large heterogeneous enhanced subhepatic mass with adjacent liver, gallbladder and colon invasion. Tumor hemorrhage and rupture complicated with peritoneal seeding and massive bloody ascites were also detected. Angiography showed a hypervascular tumor fed by enlarged right hepatic arteries, cystic artery and omental branches of gastroepiploic artery. The patient underwent laparotomy for tumor resection, but the tumor recurred one month after operation. To our knowledge, the CT appearance of ruptured intraperitoneal MFH complicated by hemoperitoneum has not been previously described.
- Citation: Chen HC, Chen CJ, Jeng CM, Yang CM. Malignant fibrous histiocytoma presenting as hemoperitoneum mimicking hepatocellular carcinoma rupture. World J Gastroenterol 2007; 13(47): 6441-6443
- URL: https://www.wjgnet.com/1007-9327/full/v13/i47/6441.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i47.6441
Massive hemoperitoneum is an emergent life-threatening condition requiring prompt management. Tumor-associated hemoperitoneum is commonly related to hypervascular intra-abdominal visceral organ tumor rupture, but rarely related to hemorrhage of the hypervascular tumor of primary peritoneal origin. Malignant fibrous histiocytoma (MFH) is the most common sarcoma at late adult life. About 5%-10% of MFH lesions arise from the peritoneal cavity[1], and may develop various degrees of tumor bleeding[1-3]. Herein, we present a case of spontaneously bleeding peritoneal MFH located in the subhepatic area with direct liver invasion presenting as massive hemoperitoneum, mimicking a ruptured pedunculated hepatocellular carcinoma.
A 70-year-old man was admitted to the emergency department because of intensifying abdominal pain of a 10-days’ duration with aggravation in the recent two days before admission. The patient also complained of body weight loss. Physical examination revealed a pallid face, orthopnea and a distended abdomen with a palpable tender mass in the right upper to middle quadrant of the abdomen. Pulse rate and blood pressure were normal. Mild anemia (hemoglobin of 130 g/L), leukocytosis (white blood cell count 16.83 × 109/L) and elevated C-reactive protein (247 mg/L) were also noted. Other laboratory data were unremarkable. Serological tests were negative for hepatitis B and positive for hepatitis C. The serum levels of tumor markers of alpha-fetoprotein, CEA and CA19-9 were within normal limits.
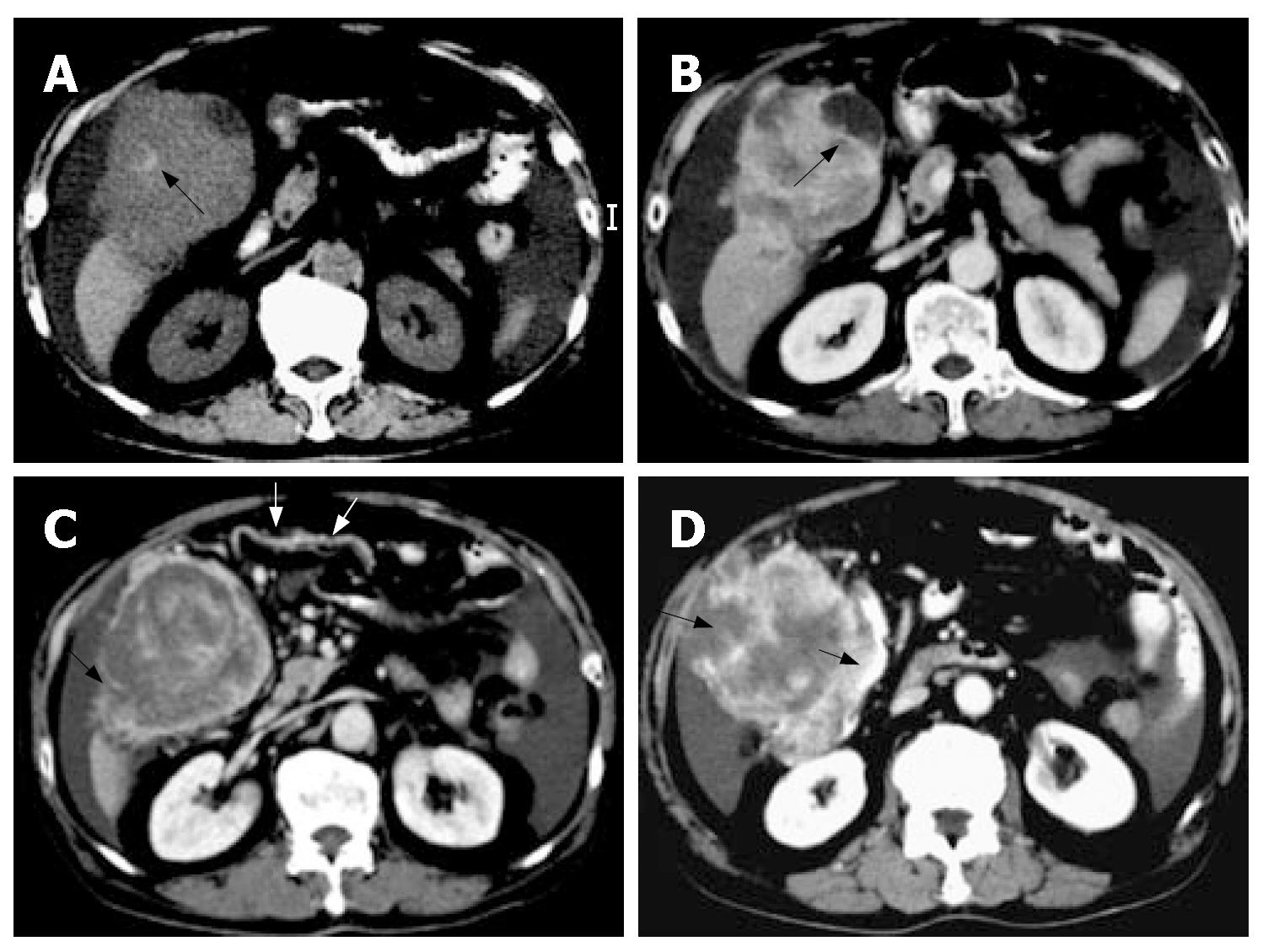
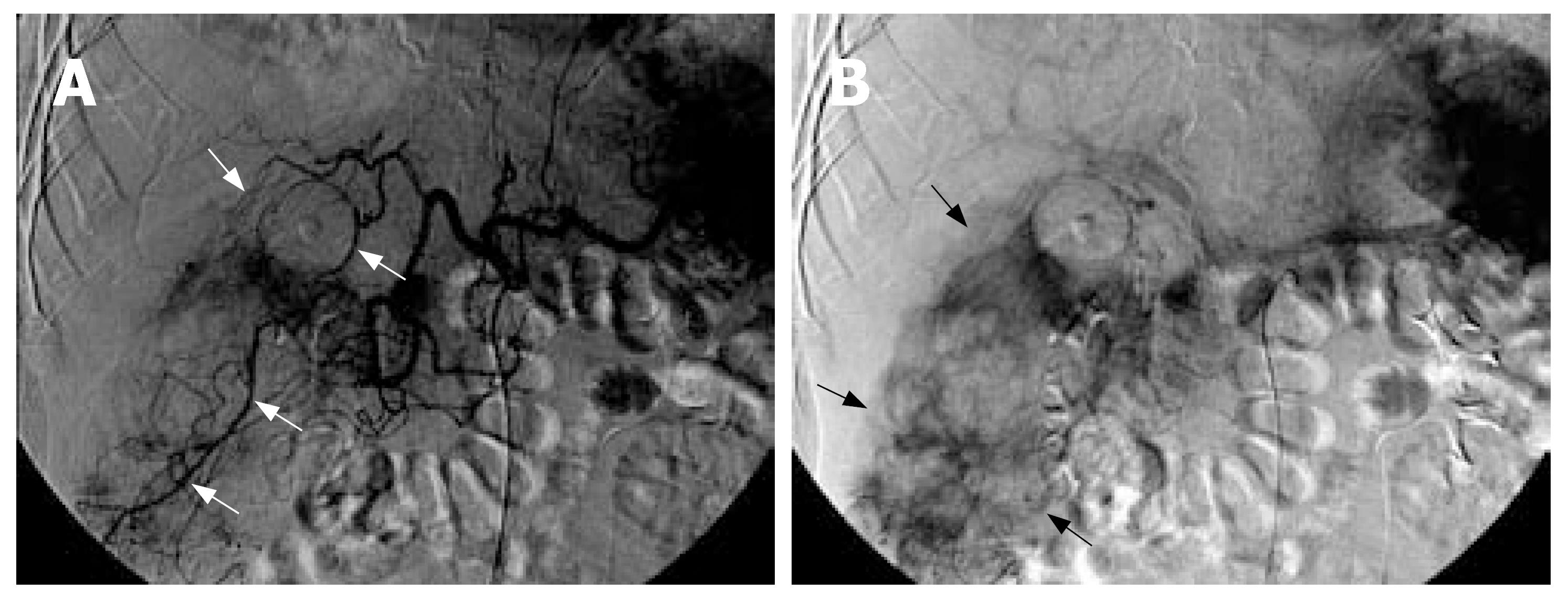
Abdominal sonography revealed a large mixed hyperechoic and hypoechoic mass at the hepatic right lower region with massive ascites. Abdominal paracentesis revealed bloody ascites, with an erythrocyte count of 100000/mm3. Computed tomography (CT) depicted a large ill-defined hemorrhagic mass (approximately 7.5 cm × 8.6 cm × 12.4 cm in size) with heterogeneous enhancement involving segments V and VI of liver with large extra-hepatic components in the peritoneal cavity accompanied with massive bloody ascites (Figure 1). The subhepatic mass also compressed and invaded the gallbladder and adjacent hepatic flexure of colon. Enlarged omental arteries were found surrounding the subhepatic mass (Figure 1). Tumor rupture with irregular tumor margin and peritoneal seeding were also present. A presumptive diagnosis of pedunculated hepatocellular carcinoma rupture was made. Therefore, emergent angiography was performed and disclosed a large hypervascular tumor located at the right subhepatic region, supplied by enlarged right inferior hepatic arteries, cystic artery, and omental branches of gastroepiploic artery (Figure 2). Transarterial embolization with gelfoam pieces was performed to achieve hemostasis for these engorged feeding arteries.
Six days later, the patient underwent laparotomy for radical tumor excision. Intraoperative examination revealed a large bleeding peritoneal subhepatic tumor rupture with contiguous omentum, liver, gallbladder and colon invasion. Peritoneal seeding and 3 L bloody ascites were also found. Pathology of the excised specimen revealed a storiform-pleomorphic malignant fibrous histiocytoma with marked hemorrhage and necrosis.
One month after the operation, the patient was readmitted to the emergency department due to massive upper gastrointestinal bleeding. CT revealed multiple hepatic and peritoneal metastases with duodenal involvement. Panendoscopy confirmed the duodenal invasion by the recurrent tumor causing internal bleeding. Despite intensive fluid supplementation and blood transfusion therapy, the patient died of hypovolemic shock 3 d later.
MFH is a pleomorphic sarcoma initially described by O’Brien and Stout[4]. It is the most common sarcoma in late adulthood, with a peak incidence in the fifth and sixth decades. Men are affected twice as frequently as women. MFH is microscopically characterized by areas of spindle cells arranged in a storiform pattern, and pleomorphic areas with haphazardly arranged sheets of fibroblasts and histiocytes. This neoplasm has a variety of histologic subtypes, including storiform-pleomorphic, myxoid, giant cell, inflammatory and angiomatoid. MFH generally affects the extremities and retroperitoneum. Uncommon locations include the head and neck region, dura mater, brain, lung, heart, aorta, pancreas, liver, spleen, breast, intestine and mesentery[1,5,6]. The propensity of MFH for invasion of contiguous organs and local recurrence result in its poor prognosis.
The radiographic features of MFH are non-specific. On CT, FMH usually presents as a poorly demarcated or a well circumscribed mass, and exhibits peripheral solid enhanced component with intralesional hypodense areas of myxoid change, hemorrhage or necrosis. Although the vascularity of MFH is variable, the majority of lesions are moderately hypervascular with tumor supply derived from multiple surrounding vessels[1,7]. Because of the rich vascularity of the lesion, preoperative transarterial embolization may be considered to shrink the tumor and minimize intraoperative hemorrhage.
MFH can develop variable degrees of tumor hemo-rrhage either spontaneously or as a result of response to chemotherapy[1,8]. Approximately 5% MFHs will develop apparent intra-tumoral hemorrhage spontaneously, especially when MFH arises from the peritoneum or omentum[1]. The intratumoral hemorrhage can be so extensive as to obscure the underlying neoplasms and create a large cyst-like space that might be mistaken as a hematoma, abscess or cystic tumor. Previous reports described that intra-peritoneal MFHs situated at the mesovarium and gastrocolic ligament with marked intra-tumoral hemorrhage manifested as large cystic tumors, causing diagnostic confusion[2-3]. Nevertheless, these intra-peritoneal MFH lesions with extensive tumor hemorrhage were still confined to the tumor mass itself, rather than complicated with extra-tumoral bleeding into the peritoneal cavity. However, in the present case, the subhepatic peritoneal MFH not only experienced intra-tumoral hemorrhage but also developed tumor rupture with extra-tumoral bleeding into the peritoneal cavity, mimicking a ruptured hepatocellular carcinoma. Three previous cases of MFH causing extra-tumoral massive bleeding had been reported, all involving metastatic MFH lesions at the alimentary tract causing gastrointestinal bleeding[9-11]. A case of post-irradiation induced dermal MFH with spontaneous tumor hemorrhage, causing massive skin bleeding had also been reported[12]. Another case of primary aortic MFH presenting as aortic dissection was described as well[13]. However, to the best of our knowledge, intra-peritoneal MFH bleeding complicated with massive hemoperitoneum causing acute abdominal emergency has not been previously reported.
Tumor-associated hemoperitoneum is frequently related to rupture of underlying intra-abdominal hypervascular tumors. Hepatocellular carcinoma (HCC) rupture is the most common cause of tumor-associated hemoperitoneum in male patients of all ages in Asia and Africa, especially patients with large and peripherally located tumors. Compared to the incidence of intraperitoneal visceral organ tumor rupture causing hemoperitoneum, the occurrence of primary peritoneum-origin tumor bleeding presenting with hemoperitoneum is rare. A review of the literature found only one similar case report which described a hypervascular peritoneal hemangiopericytoma with spontaneous tumor bleeding causing a small amount of hemoperitoneum[14]. By contrast, in our present case, large amounts of bloody ascites were found, indicating that the MFH had tumor rupture with active tumor bleeding rather than minimal tumor bleeding only.
In the present case, CT showed that the vast majority of subhepatic tumor was situated in the extra-hepatic peritoneal cavity and the center of the mass was outside of the liver parenchyma. These findings suggest that the subhepatic mass may be a tumor of extra-hepatic origin rather than arising from the liver parenchyma. Furthermore, the subhepatic tumor was large enough not only to compress but also to directly invade the liver and gallbladder. However, the subhepatic tumor and the compressed liver tip parenchyma still exhibited negative “beak sign” on CT study, seen as an obtuse angle between the subhepatic mass and the compressed liver tip surface with partially preserved dull edges of the liver. These findings suggest that the subhepatic mass did not arise from the liver. Moreover, a cleavage plane between the subhepatic mass and the compressed lower-most liver tip parenchyma was also identified as negative “embedded organ sign” on CT scan, indicating the subhepatic mass was compressing the liver, but did not arise from the liver. In addition, CT also revealed enlarged omental arteries supplying the subhepatic mass, presenting as “prominent feeding artery sign”. This suggests the subhepatic mass may be a tumor of peritoneal omentum origin. Angiography also confirmed the subhepatic tumor was mainly supplied by multiple enlarged omental arteries, whereas the hepatic artery only accounted for a minority of tumor feeding. Advanced HCC with omentum invasion can develop extra-hepatic collateral vessels from the omental branches of the gastroepiploic artery, but HCC usually still preserves its dominant feeding arteries from the hepatic artery rather than from the extra-hepatic omental artery. The more prominent blood supply from the omental arteries than the hepatic artery in the present case is an unusual phenomenon in HCC. Careful analysis of the CT imaging features of this case indicate that a subhepatically located bleeding peritoneal tumor with liver invasion should be taken into account as a possible cause of peri-hepatic tumor complicated with hemopertitoneum. Since MFH is the most common sarcoma in the elderly, primary peritoneal MFH, although rare, should be included among the causes of tumor-related hemoperitoneum. However, the radiographic features of MFH are indistinguishable from those of other peritoneal malignant neoplasms, such as leiomyosarcoma, gastrointestinal stromal tumor, fibrosarcoma or angiosarcoma.
In conclusion, MFH arising from the peritoneal subhepatic region is rare, but the potential exists for a hemorrhagic peritoneal sarcoma to directly invade adjacent organs, mimicking an exophytic hepatic tumor rupture. The possibility of MFH should be carefully considered when a patient presents with a hemorrhagic subhepatic tumor and no prior documented hepatitis episode and liver cirrhosis.
S- Editor Ma N L- Editor Ma JY E- Editor Yin DH
| 1. | Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41:2250-2266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Ko SF, Ng SH, Lin JW, Lee TY. Cystic malignant fibrous histiocytoma of the mesovarium. AJR Am J Roentgenol. 2001;176:549-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Huang CC, Ko SF, Ng SH, Lee TY, Wan YL, Lin JW, Chen WJ. Cystic malignant fibrous histiocytoma of the gastrocolic ligament. Br J Radiol. 2001;74:651-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | O'Brien JE, Stout AP. Malignant fibrous xanthomas. Cancer. 1964;17:1445-1455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Ros PR, Viamonte M, Rywlin AM. Malignant fibrous histiocytoma: mesenchymal tumor of ubiquitous origin. AJR Am J Roentgenol. 1984;142:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Bruneton JN, Drouillard J, Rogopoulos A, Laurent F, Normand F, Balu-Maestro C, Monticelli J. Extraretroperitoneal abdominal malignant fibrous histiocytoma. Gastrointest Radiol. 1988;13:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Goldman SM, Hartman DS, Weiss SW. The varied radiographic manifestations of retroperitoneal malignant fibrous histiocytoma revealed through 27 cases. J Urol. 1986;135:33-38. [PubMed] |
| 8. | Panicek DM, Casper ES, Brennan MF, Hajdu SI, Heelan RT. Hemorrhage simulating tumor growth in malignant fibrous histiocytoma at MR imaging. Radiology. 1991;181:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Blaauwwiekel EE, Koopal S, Kreeftenberg HG. Metastasized malignant fibrous histiocytoma as a cause of gastrointestinal tract haemorrhage. Scand J Gastroenterol. 1997;32:1272-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Adams HW, Adkins JR, Rehak EM. Malignant fibrous histiocytoma presenting as a bleeding gastric ulcer. Am J Gastroenterol. 1983;78:212-213. [PubMed] |
| 11. | Santoro MJ, Abdulian JD, Chase RL, Griffin RA, Solinger MR, Collen MJ. Malignant fibrous histiocytoma metastatic to the colon presenting as a lower gastrointestinal bleed. Am J Gastroenterol. 1992;87:1051-1053. [PubMed] |
| 12. | Yamamoto Y, Arata J, Yonezawa S. Angiomatoid malignant fibrous histiocytoma associated with marked bleeding arising in chronic radiodermatitis. Arch Dermatol. 1985;121:275-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Chen WJ, Chen CL, Liau CS, Chu SH, Lee YT. Primary malignant fibrous histiocytoma of the aorta associated with aortic dissection. Chest. 1991;99:1049-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Crusco F, Chiodi M, Pugliese F, Mosca S, Fischer MJ, Lupattelli L. Benign omental hemangiopericytoma presenting with hemoperitoneum: radiologic findings. AJR Am J Roentgenol. 2005;184:S67-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |