INTRODUCTION
Ulcerative colitis is a worldwide, chronic, idiopathic, inflammatory bowel disease (IBD) of the rectal and colonic mucosa. In the past, this disease was thought to occur infrequently in the Asia Pacific region. However, new evidence is showing that IBD is on the rise in the region, including in Hong Kong and mainland China[1-3]. Although glucocorticoid and salicylazosulfapyridine have been mainly used for the treatment of this disease, their side effects remain a major clinical problem. Therefore, there is an increasing interest in using traditional Chinese medicines (TCM) as alternative therapy in addition to the conventional therapies that are used to treat UC[4].
The dried root of Scutellaria baicalensis Georgi (Scutellaria Radix, common name Huangqin) is widely used in TCM. It is officially listed in the Chinese Pharmacopoeia and is one of the most widely used Chinese herbal medicines for the treatment of bacterial infection of the respiratory and gastrointestinal tract. In Japan and China, Scutellariae Radix has been employed for centuries as an important medicine to treat chronic inflammatory and ulcerative disease. The main components of Scutellariae Radix (and of all Scutellaria species) are baicalein, baicalin and wogonin. Scutellariae Radix and its major flavonoids possess multiple biological and pharmacological effects, including anti-inflammation[5], anti-viral[6], anti-tumor[7], anti-proliferative[8] and anti-bacterial[9], etc. Recent studies also suggest that Scutellariae Radix-containing TCM formula, such as Oren-gedoku-to (Huang Lian Jie Du Tang) may have therapeutic potential against murine colitis[10-12].
In this study, an experimental model of UC was established in SD rats using DSS. The effect of the total extract of Scutellariae Radix on DSS-rats was evaluated using macroscopic, histological, biochemical and electrophysiological assessments.
MATERIALS AND METHODS
Materials
Male SD rats, initially weighing 170-180 g, were housed five per cage and maintained in an animal holding room controlled at a constant temperature of 24°C ± 2°C with a relative humidity of 70% ± 5% and a 12 h light-dark cycle. Animals were fed on a standard pellet chow with free access to fresh tap water. The study was approved by the Animal Research Ethics Committee of our university. DSS was obtained from MP Biochemicals Inc. Hexadecyltrimethylammonium bromide, forskolin were obtained from Sigma. SRE was purified from the ground roots of S. baicalensis Georgi with hexane, acetone, and finally, with methanol as described previously[13,14].
Experimental design
The induction of colitis was modified from a previously described work[15]. The experiment lasted for 8 d. The rats were randomly divided into four groups. In the DSS Group, 4% DSS in drinking water ad libitum was given from d 0 to d 7. In DSS + SRE group, SRE (100 mg/kg per day) was orally administrated with exposure to 4% DSS drinking water. In the normal control group (Ctr), the rats had free access to a water bottle containing tap water. In Ctr + SRE group, SRE alone (100 mg/kg per day) was administered orally to the rats. In this model, the colonic damage was evaluated using macroscopic, histological, electrophysiological and biochemical assessments (see below). From d 3 to d 7, rats were sacrificed by CO2 asphyxiation on each day. Postmortem, the entire colon was removed from the cecum to the anus and placed on an ice cold plate and cleaned of fat and mesentery. The length of each specimen was measured, which is an indirect marker of inflammation. The colon was divided into three parts (proximal, middle and distal) based on total length: 10% regions from three parts were fixed for histological examination; the distal portion was used for electrophysiological studies; and the adjacent distal 10% was snap-frozen in liquid nitrogen for later quantification of MPO activity.
Macroscopic assessment
Animals were checked daily for the three main clinical symptoms-body weight changes, stool consistency and fecal occult blood. Weight loss is usually observed in animals with colitis, thus body weight changes recorded could be an indicator for the severity of colitis. The colonic damage was quantified by a clinical scoring system assessing stool consistency and rectal bleeding[16]. For stool consistency, 0 points were given for well formed pellets, 2 points for pasty and semiformed stools that did not stick to the anus, and 4 points for liquid stools that did stick to the anus. Bleeding was scored 0 for no blood in hemoccult, 2 points for positive hemoccult, and 4 points for gross bleeding. These scores were added, forming a total clinical score that ranged from 0.0 (healthy) to 8.0 (maximal activity of colitis).
Histological assessment
For light microscopy, we used tissue samples from three parts (distal, middle and proximal) of colon of each animal fixed in 4% (40 g/L) buffered paraformaldehyde, dehydrated in increasing concentrations of ethanol, and embedded in paraffin. Thereafter, sections of tissue were cut at 5 μm on a rotary microtome, mounted on clean glass slides and dried overnight at 37°C. The sections were cleaned, hydrated, and stained with hematoxylin and eosin (HE) for histological evaluation of inflammatory infiltrate and tissue damage, according to standard protocols, and the slides were coded to prevent observer bias during evaluation. All tissue sections were examined in a blinded fashion by two investigators. Histological damage was scored using the criteria of Siegmund B et al[17] which considers the inflammatory infiltrate (maximum score = 3) and tissue damage (maximum score = 3). Photographs of colon samples were digitized using a ZEISS Axioskop 2 plus camera. Analysis of the figures was carried out with Axio Vision 3.1 image analysis program.
Electrophysiological assessment
Mucosal ion transport was examined in colonic segments (approximately distal colon) mounted in Ussing chambers according to a well established protocol in our laboratory[18]. In brief, tissues (surface area = 0.45 cm2) were bathed in 20 mL of warm (37°C), oxygenated Krebs buffer. The spontaneous potential differences were maintained at 0 mV by a voltage clamp amplifier (Physiologic Instruments), and the short-circuit current (ISC in μAcm-2) was continuously measured as an index of electrogenic ion transport. A transepithelial potential difference of 1 mV was applied periodically, and the resultant change in current was used to calculate the transepithelial resistance (Rt) using Ohm's law. Stimulated ion transport was evoked by the addition of an adenylate cyclase-activating agent, forskolin (1 μmol/L), to the mucosal bathing solution. In all instances, the effect of the treatment was recorded as the maximum change in ISC (∆ ISC) to occur within 5 min.
Biochemical assessment
The MPO activity was determined following a published protocol[19]. Briefly, frozen tissue samples were weighed and suspended in potassium phosphate buffer (20 mmol/L, pH 7.4) at a ratio of 50 mg tissue to 1 mL of buffer. Tissue was homogenized by a polytron tissue homogenizer three times for 20 s, and homogenate was decanted into sterile Eppendorf tubes and centrifuged at 10000 ×g for 10 min at 4°C. The pellet was then resuspended in 1 mL potassium phosphate buffer (50 mmol/L, pH 6.0) containing 5 mg/mL hexadecyltrimethylammonium bromide (TMB) at a tissue concentration of 50 mg/mL. Samples were sonicated three times for 10 s, freeze-thawed three times, and centrifuged at 10000 ×g for 5 min at 4°C. The reaction was started by mixing 20 μL of the supernatant at 25°C with 30 μL TMB. After 110 s, the reaction was terminated by addition of 50 μL of 0.18 mol/L H2SO4. The change in absorbance was read at 450 nm. MPO activity (1 unit) was expressed as the amount of enzyme necessary for the degradation of 1 μmol H2O2/min per 100 mg tissue at 25°C.
Statistical analysis
All data are presented as means ± SE. One way ANOVA followed by post hoc statistics with Tukey test was used for statistical evaluation of the parametric data. Non-parametric data was analyzed by Kruskal-Wallis One Way Analysis using Student-Newman-Keuls method. P < 0.05 was considered as statistically significant. The values of n refer to the number of experiments undertaken using different rats. Non-parametric data are expressed as the means ± SE.
RESULTS
Macroscopic assessment
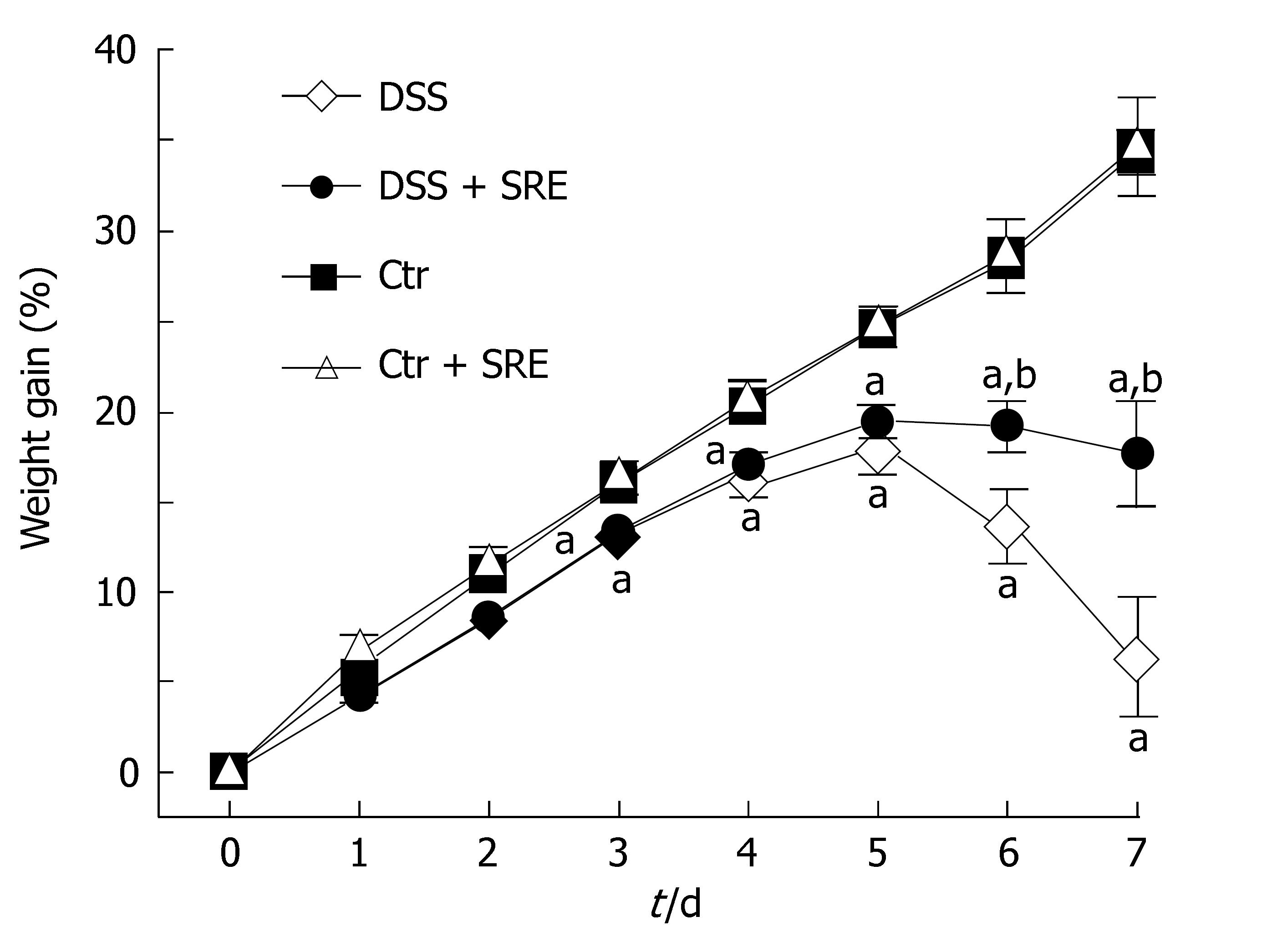
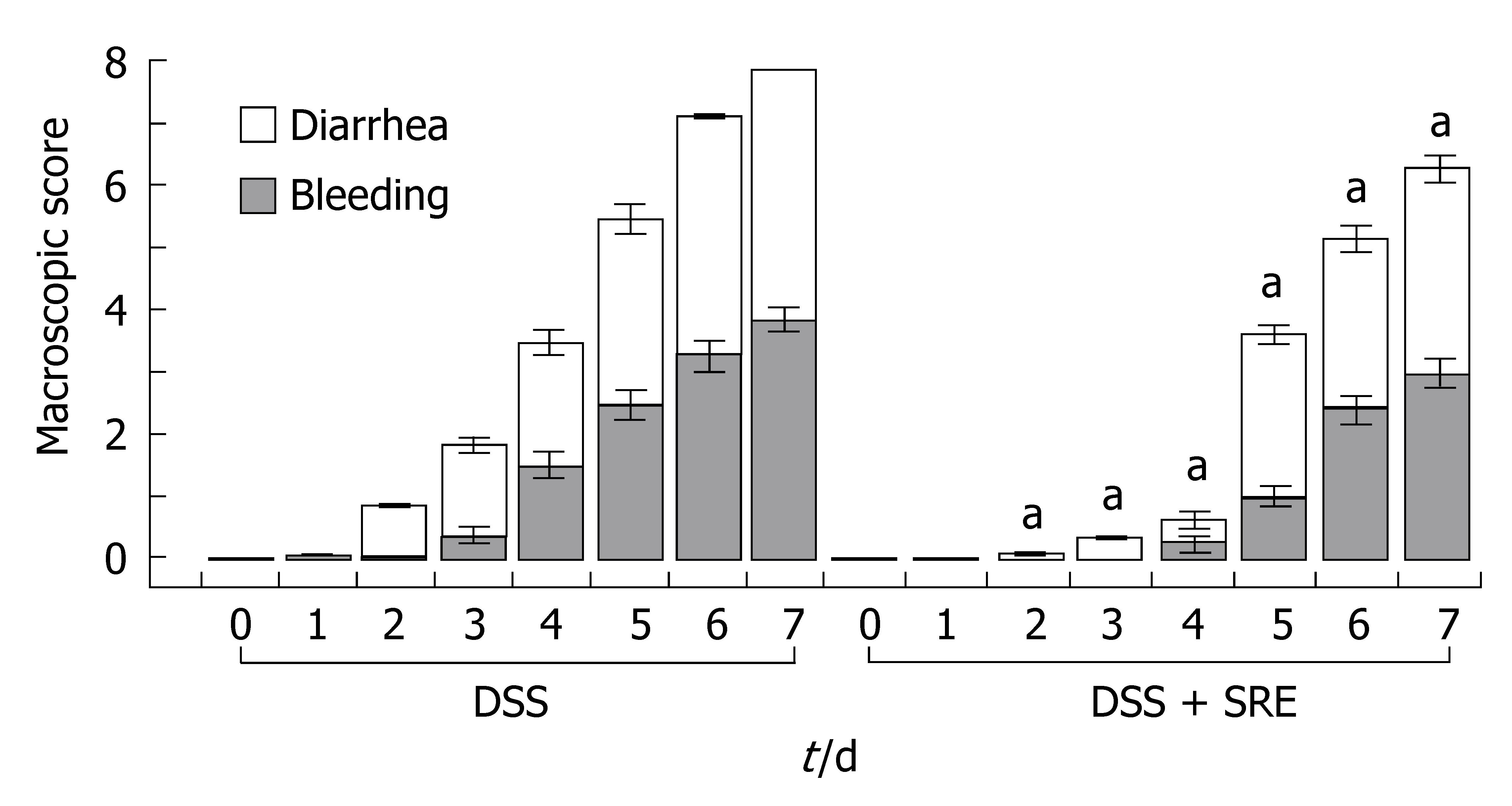
The weight gain % over the entire study period is shown in Figure 1. The weight gain % of rats in DSS group and DSS + SRE group were significantly lower than the Ctr group and Ctr + SRE group from d 3 until the end of experiment. The body weight of rats in DSS group then dramatically decreased from d 5 onwards. However, in the case of DSS + SRE group, the weight gain % became stabilized and the weight loss was found to be less severe than DSS group from d 6 to d 7. Figure 2 shows the macroscopic score recorded throughout the experimental period in DSS and DSS + SRE groups. Oral administration of SRE resulted in a significant reduction in the clinical activity of colitis compared with DSS-treated rats. Moreover, the occurrences of those clinical signs were found to be delayed in DSS + SRE group.
Figure 1 Figure showing the weight gain percentage (%) in different groups of rat from d 0 to d 7.
Body weight was assessed daily and expressed as percentage increase of basal body weight. Values are expressed as the means ± SE, (n = 8), aP < 0.05 vs Ctr group, bP < 0.05 vs DSS group, one-way ANOVA followed by Tukey multiple comparison test.
Figure 2 Ameliorative effect of SRE treatment on the time-course changes in the macroscopic score over the 8-d experimental period.
The macroscopic score of Ctr and Ctr + SRE groups is 0 (data not shown). aP < 0.05 vs DSS group. Non-parametric data are expressed as the means ± SE, Kruskal-Wallis One Way Analysis using Student-Newman-Keuls method (n = 6-8).
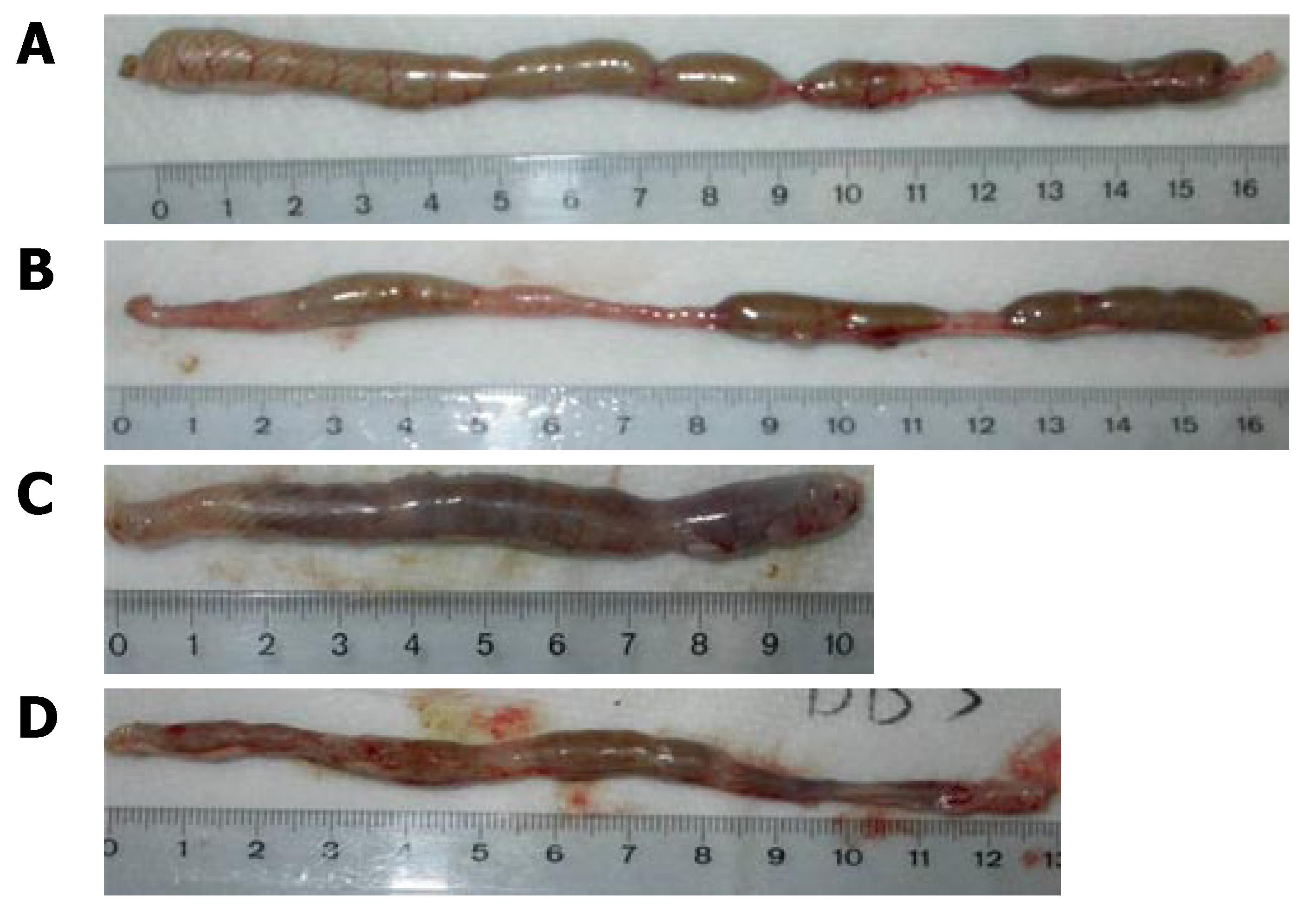
The colon length is a useful indication of colitis and is, therefore, measured as a marker of inflammation (Figure 3). After 8 d treatment with DSS in drinking water, there was a significant shortening of the colon length (Figure 3C) compared with the Ctr group (Figure 3A) and the Ctr + SRE group (Figure 3B). The oral administration of SRE significantly improved this inflammatory marker (Figure 3D). On d 7, the colon length of DSS group (11.2 cm ± 0.4 cm, n = 10) was significantly shorter than the control group (16.8 cm ± 0.6 cm, n = 8). The colon length of DSS-rats treated with SRE (13.5 cm ± 0.4 cm, n = 9), however, was significantly longer than that of untreated rats.
Figure 3 Macroscopic view of the colon showing the changes in colon length in different groups of rat on d 7.
Not much difference was observed between the colon of the Ctr (A) and Ctr + SRE group (B), but significant difference could be found in the DSS group (C) which displayed extensive hyperemia and edema. In the DSS + SRE group, the shortening of colon was less severe (D) when compared with the control (A).
Histological assessment
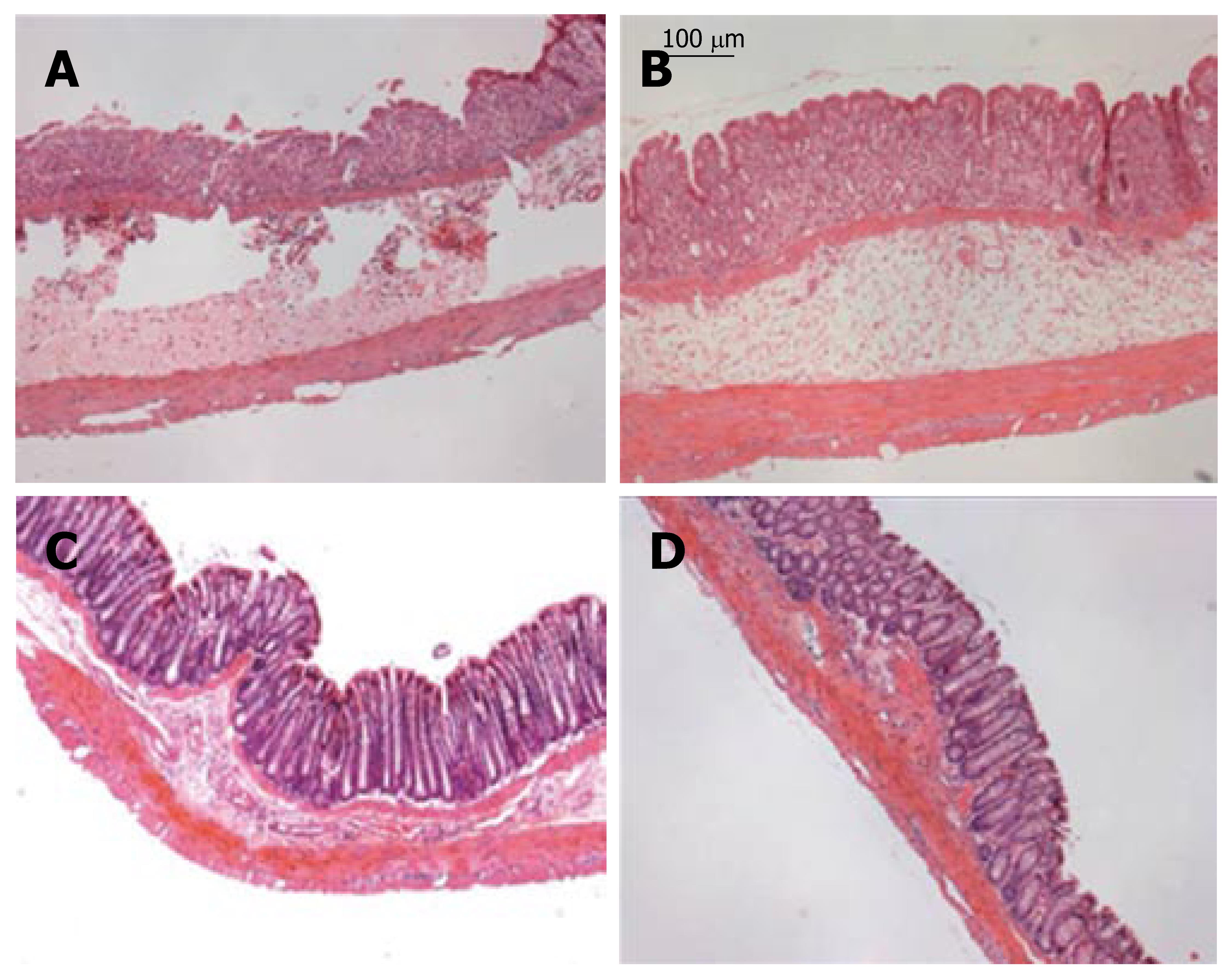
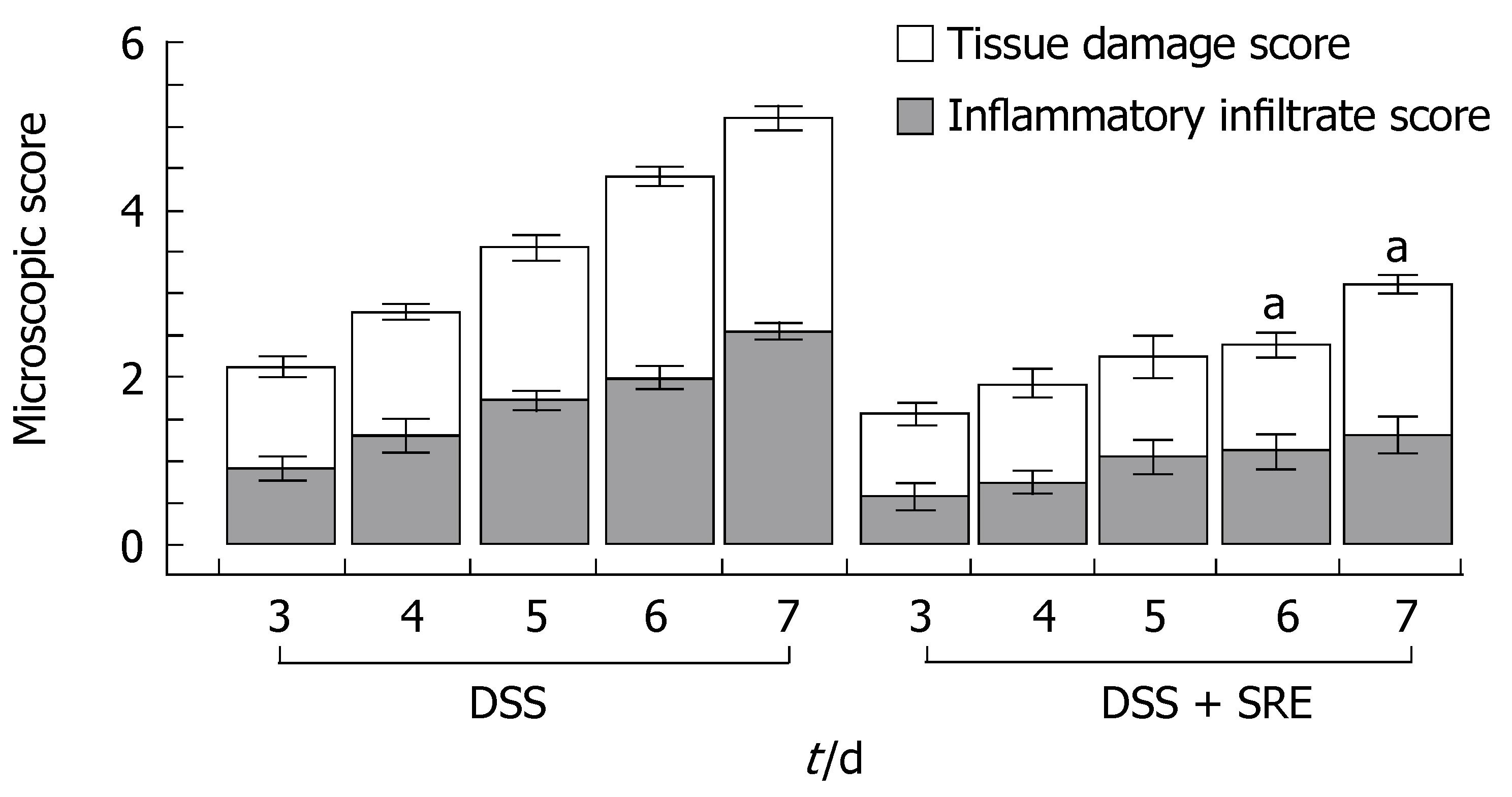
Histological damage was evaluated by the grading method described above in Materials and Methods. The occurrence of UC was confirmed on the basis of histological damage and inflammatory infiltrate as shown in Figure 4. Figure 5 summarized the damage scores from DSS rats and DSS rats treated with SRE. The microscopic score of samples from different regions (distal, middle and proximal) of colon were not significantly different from each other (data not shown). The histological index began to increase on d 3 in both groups. However, the rats with SRE administration showed a significantly lower value than the untreated animals on d 6 and d 7.
Figure 4 Histological sections from different groups of rats on d 7.
A: DSS group showing extensive ulceration with a severe inflammatory cell infiltrate; B: DSS + SRE group showing recovery in the inflammatory cell infiltration with less severe ulceration; C: Non-colitic Ctr group showing the normal histology of the colon; D: Ctr + SRE group showing the normal morphology of the colon. (HE staining; original magnifications, x 100).
Figure 5 Ameliorative effect of SRE treatment on the time-course changes in the microscopic score from d 3 to d 7.
The microscopic score of Ctr and Ctr + SRE groups is 0 (data not shown). aP < 0.05 vs DSS group. Non-parametric data are expressed as the means ± SE, Kruskal-Wallis One Way Analysis using Student-Newman-Keuls method (n = 4-5).
Electrophysiological assessment
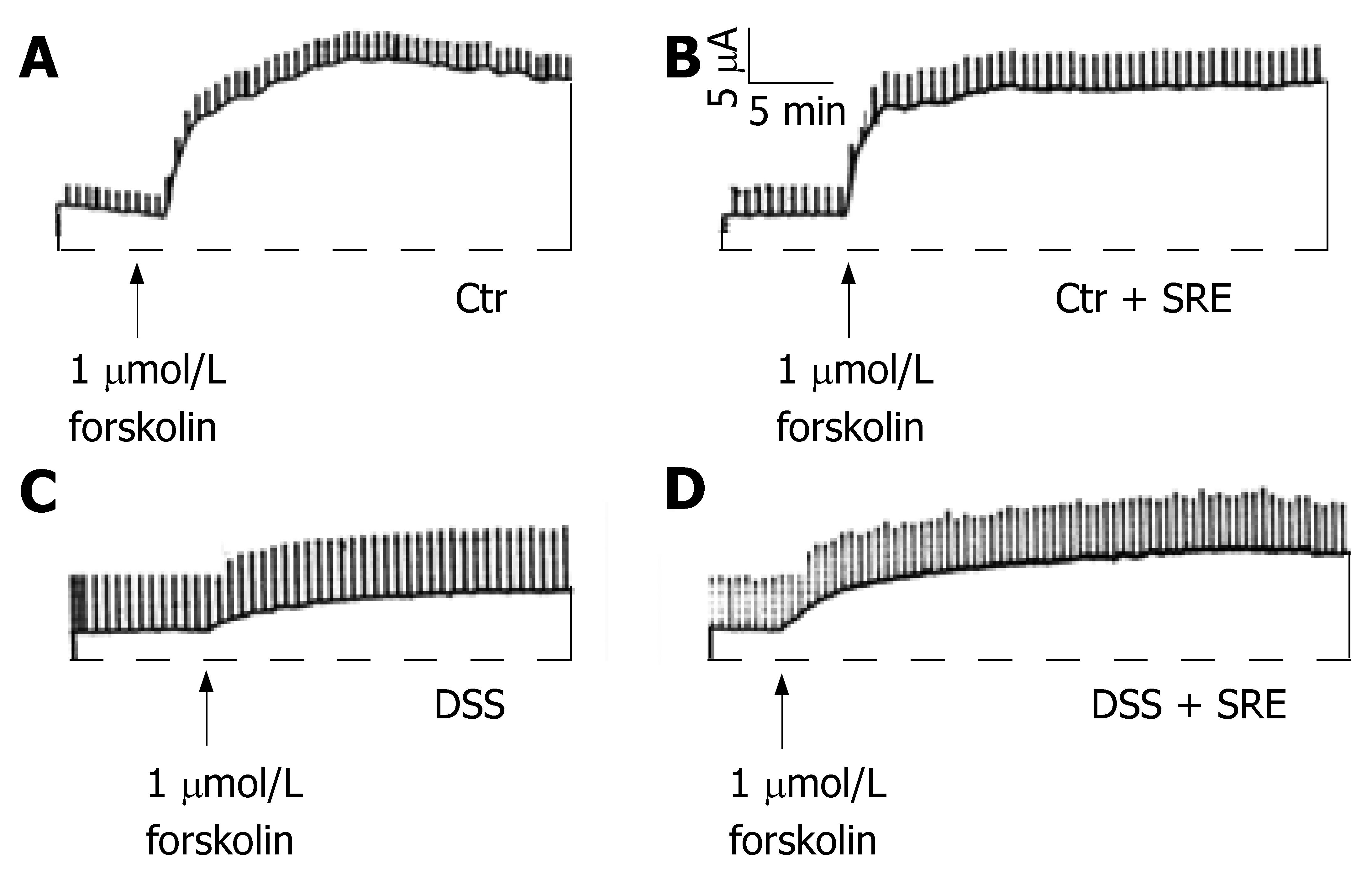
Electrogenic ion transport function was assessed by stimulating the colon with an adenylate cyclase activator, forskolin (1 μmol/L), using short-circuit current measurement technique (Figure 6). Under the experimental conditions, the increase in ISC is mainly due to the cAMP-mediated Cl- secretion via the cystic fibrosis transmembrane conductance regulator (CFTR)[18]. The basal ISC and Rt in the tissues were recorded and these parameters were not significantly different (data not shown) when tissues from different groups were compared. On d 7, the secretory response to the cAMP-elevating agent was significantly diminished in tissues from rats treated with DSS (∆ISC = 4.94 ± 1.65 μAcm-2, n = 6) when compared with Ctr (∆ISC = 60.97 ± 7.62 μAcm-2, n = 8) and Ctr + SRE (∆ISC = 61.48 ± 6.78 μAcm-2, n = 7). On the other hand, the reduced responsiveness was ameliorated in the colitic rats with SRE administration (∆ISC = 20.67 ± 3.30 μAcm-2, n = 9).
Figure 6 Representative tracings showing the change in ISC evoked by forskolin in different groups of rat on d 7.
All four groups showed an increase in ISC but with different magnitudes (A-D). The ISC response was greatly reduced in the DSS group (C) when compared with the control (A). On the other hand, the reduction in secretory response appears to be ameliorated in the DSS + SRE group (D). The transient current pulses were the results of intermittently clamping the potential at 1 mV. The horizontal lines represent zero ISC. The record is representative of at least six experiments.
Biochemical assessment
The MPO activity in the groups without DSS treatment remained at a low value throughout the entire experiment and there was no effect of SRE on the control (d 7: Ctr 0.09 ± 0.01 mU/mg, n = 6; Ctr + SRE 0.06 ± 0.0003 mU/mg, n = 5). In comparison, rats with colitis were accompanied by a significant increase in MPO activity (d 7: 0.52 ± 0.07 mU/mg, n = 8). On d 7, the increase in MPO activity (0.34 ± 0.05 mU/mg, n = 8) was significantly reduced in rats treated with SRE.
DISCUSSION
Ulcerative colitis is an inflammatory disease that causes ulcerations of the mucosa in the colon. The incidence of UC is around 1 in 1000 people with a higher prevalence among Caucasians[20]. In the past, this disease has been considered to occur rarely in the Asia Pacific region, but recent evidence indicates that both UC and Crohn's disease (CD) are becoming increasingly prevalent in local populations[3]. For example, from 1991 to 2000, there has been a three-fold increase in the number of cases of UC in China[1]. In Hong Kong, from 1986-1989 to 1999-2001, there was also a three-fold increase in the incidence of CD in the Chinese population[2]. Although the etiology of IBD remains essentially unknown[21], results from many studies in human patients and animal models suggest that it may be related to an abnormal immune response in the gastrointestinal tract, possibly associated with genetic and environmental factors[22,23]. Although progress has been made in the overall management of UC, the pharmacological treatments that are available are still unsatisfactory. Glucocorticoids, sulfasalazine and immunosuppressive drugs have been mainly used for the treatment and maintenance of UC, but the side effects or toxicity of these drugs remain a major clinical problem[24]. As a result, there is an increasing interest in using TCM as alternative therapy in addition to the conventional therapies that are used to treat UC[25]. In Japan and China, the most commonly used alternative remedies are herbal and these have been widely used in patients with mild to moderate disease, as well as an adjunct to therapy in patients with moderate to severe disease[1]. Interestingly, a recent survey showed that one-third of both Chinese and Caucasian IBD patients had used complementary and alternative medicines and therapies[4]. Several TCM formulae have been shown to possess an anti-colitic effect in rats[26-28]. Recent studies suggest that Scutellariae Radix may have a pharmacological effect against murine colitis[10-12] and therefore in this study we aim to further evaluate the therapeutic effect of SRE on DSS-induced rat colitis.
In the present study, 4% DSS in drinking water was administrated for 8 d to induce acute colitis in rats. All DSS-treated rats showed numerous clinical symptoms such as body weight loss, diarrhea, bloody stools and shortening of colon length. Body weight loss is a common symptom of UC because of the loss of body fluid and damage to the digestive system. In Ctr and Ctr + SRE groups, rats showed a steady increase in body weight throughout the experiment. In comparison, rats exposed to DSS had a lower rate of body weight increase followed by a dramatic decrease from d 5 onwards. Treatment with SRE to the colitic rats showed a less severe weight loss from d 6 to d 7. In DSS group, diarrhea and rectal bleeding occurred in d 1 and d 2, respectively. The SRE administration delayed the occurrence of both symptoms and with less severity. Colon shortening is always found in UC patients, which can act as an indirect marker of colonic inflammation. Although the colitic rats with SRE treatment also showed a decrease in colon length on d 7 when compared with the control, the shortening was much less severe than that of DSS rats. Taken together, the data suggested that SRE treatment could induce a decrease in the extent of colitis accompanied by reducing the severity and delaying the occurrence of the associated clinical symptoms.
Colitis induced by DSS was histologically characterized by severe disruption of tissue architecture, edema, a massive mixed immune cell infiltrate, ulceration and muscle thickening. To quantify the histological damage, we used a scoring system modified from a previous study[17]. We found that the overall histological damage of colitis was significantly reduced by the SRE treatment on d 6 and d 7 of the experiment. Together with the reduced MPO activity, our results showed that SRE treatment ameliorated the DSS-induced colitis possibly via its anti-inflammatory and protective effect against the colonic tissue damage.
The colonic epithelium, in addition to its absorptive and secretory properties, presents an efficient barrier to commensal flora and pathogens[29,30]. In fact, the secretion of water and electrolytes is one of the most important responses of the mucosa, purging the gut of offending agents and delivering anti-microbial mediators (e.g., antibodies) to the luminal surface[31]. In addition, mucus forms a gel layer covering the mucosal surface, and it has been hypothesized that changes in mucin structure and/or quantity may influence the protective functions of the mucosal surface, and affect the pathogenesis of IBD[32]. However, published data on the electrolyte transport mechanisms in an inflamed colon are inconsistent[33,34]. Some studies have documented the acute effects of immune and inflammatory agents in directly or indirectly stimulating intestinal anion secretion, while others do not support such a notion[35,36]. In most cases, however, disease models of colonic mucosa exhibit reduced ion transport responses to secretory agonists, especially in the setting of chronic inflammation[37-45].
The mechanism underlying the typical hyporesponsiveness of tissues from animal models of colitis has yet to be satisfactorily explained. For example, it has been reported that responsiveness to both Ca2+- and cAMP-dependent secretagogues is reduced in mouse and rat colitis models when compared to normal tissue[37-45]. Similar reduction in secretory responsiveness, as measured by changes in ISC has also been observed in tissue resections from patients with IBD[46,47]. It has been proposed that prolonged hyporesponsiveness to secretagogues is due to the upregulation of inducible nitric oxide synthase (iNOS), resulting in an ongoing synthesis of NO and chronic suppression of epithelial secretory function[41,42]. However, another recent study suggests that it may be related to a disturbance of the enteric nervous system resulting in defective mucosal cAMP production and inhibition of ionic secretion, although the epithelial secretory machinery (e.g., CFTR) appears to be normal[43]. Others suggested that prolonged hyporesponsiveness to secretagogues is due to the disruption of normal cholinergic control of ion secretion[40,48]. Nonetheless, intestinal secretion is an important component of mucosal defense. Reduced secretory responses will compromise the ability of the mucosal defense mechanism to clear bacteria, bacterial products, or antigens away from the epithelium, which may then predispose the colon to inflammation[49]. In this study, the ISC responses of the colonic mucosa to forskolin were suppressed after the induction of colitis. Although the stimulated ion transport activity of DSS-rats treated with SRE was still reduced, they displayed improvement in the secretory responsiveness which may at least partly contribute to the therapeutic effect of SRE.
In summary, our findings indicated that SRE was effective in treating acute DSS-induced ulcerative colitis, as gauged by reduced clinical disease, improved macroscopic and histological damage scores, and enhanced recovery of normal colonic secretory function. The results support further evaluation of the therapeutic potential of SRE for the treatment of IBD.
COMMENTS
Background
Scutellariae Radix, also known as Huangqin, is the dried root of Scutellaria baicalensis Georgi (Lamiaceae). It is officially listed in the Chinese Pharmacopoeia and is one of the most widely used Chinese herbal medicines for the treatment of bacterial infection of the respiratory and gastrointestinal tract. In Japan and China, Scutellariae Radix has been employed for centuries as an important medicine. Although scientific evidence confirming the traditional use of Scutellariae Radix as an inflammatory modulator in experimental colitis is now accumulating, the physiological basis and the precise mechanism of action of Scutellariae Radix or its individual constituents remain largely unknown.
Research frontiers
Cytokine dysregulation is currently an important focus of both basic and clinical research in IBD, with recent immunologically based therapeutic interventions using highly specific agents demonstrating a promising clinical efficacy. The treatment of steroid-refractory Crohn's Disease with anti-TNF-α (infliximab) is an example of this kind of therapeutic approach. In addition, there is an increasing interest in using TCM as alternative therapy in addition to the conventional therapies that are used to treat IBD. However, there is still a paucity of scientific and clinical data so far to support the use of TCM in colitis patients. Therefore, there are unmet needs for further mechanistic, pharmacological and pharmacokinetic studies to delineate the biological basis underlying the therapeutic effects of TCM. More controlled clinical trials of the potential efficacy and safety of herbal TCM therapies are also required.
Innovations and breakthroughs
The authors showed for the first time that the therapeutic potential of Scutellariae Radix extract on experimental colitis may be related to the restoration of ion transport function of the colonic mucosa.
Applications
The results support further evaluation of the therapeutic potential of this herbal extract and its active component(s) for the treatment of IBD.
Terminology
Colonic ion transport: Intestinal fluid secretion is a passive process driven by osmotic forces generated by ion transport. In the colon, the main determinant of a luminally-directed osmotic gradient is the mucosal transport of chloride ions into the lumen. Intestinal secretion is an important component of mucosal defense. Reduced secretory responses will compromise the ability of the mucosal defense mechanism to clear bacteria, bacterial products, or antigens away from the epithelium, which may then predispose the colon to inflammation.
Peer review
This is an interesting paper which shows the anti-inflammatory effect of Scutellariae Radix on experimental colitis. It also demonstrates that TCM has a scientific and biological basis for its effectiveness.