Published online Nov 7, 2007. doi: 10.3748/wjg.v13.i41.5432
Revised: August 4, 2007
Accepted: September 10, 2007
Published online: November 7, 2007
AIM: To investigate the multicellular resistance of human hepatocellular carcinoma HepG2 cells in three-dimensional culture to delisheng, 5-fluorouracil and adriamycin, and the possible molecular mechanisms of delisheng.
METHODS: Human hepatocellular carcinoma HepG2 cells were cultured with a liquid overlay technique. After the formation of multicellular spheroids, morphology was analyzed by phase contrast microscopy, scanning electron microscopy and transmission electron microscopy. Sensitivity of HepG2 cells to delisheng, 5-fluorouracil and adriamycin was investigated by MTT assay in multicelluar spheroids and monolayers. Vascular endothelial growth factor (VEGF) and endostatin expression were analyzed in multicellular spheroids treated with delisheng, 5-fluorouracil, adriamycin and negative control PBS, with immunohistochemical staining.
RESULTS: Multicellular spheroids exhibited structural characteristics somewhat different to those in monolayers. The cells in three-dimensional cell culture turned out to be less sensitive to delisheng, 5-fluorouracil and adriamycin than the cells cultured in monolayer. This showed that delisheng had a satisfactory cells inhibition ratio compared to 5-fluorouracil and adriamycin. Immunohistochemical staining showed that VEGF and endostatin expression was positive during growth as multicellular spheroids, and endostatin expression in spheroids with treatment of delisheng was higher than that with 5-fluorouracil, adriamycin and PBS (139.35 ± 7.83, 159.23 ± 10.34, 162.83 ± 3.47 and 148.48 ± 11.06, P < 0.05).
CONCLUSION: Chinese medicine compound delisheng has satisfactory anti-tumor activity in HepG2 cells in three-dimensional culture, and the effects are associated with up-regulation of endostatin.
- Citation: Cui J, Nan KJ, Tian T, Guo YH, Zhao N, Wang L. Chinese medicinal compound delisheng has satisfactory anti-tumor activity, and is associated with up-regulation of endostatin in human hepatocellular carcinoma cell line HepG2 in three-dimensional culture. World J Gastroenterol 2007; 13(41): 5432-5439
- URL: https://www.wjgnet.com/1007-9327/full/v13/i41/5432.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i41.5432
Hepatocellular carcinoma (HCC) is a highly malignant tumor with a very high morbidity and mortality, and a poor prognosis. Its incidence is increasing both in Asian countries and in the USA. A majority of HCC patients presents with advanced or unresectable disease. Even for those with resected disease, the recurrence rate can be as high as 50% at 2 years. Despite extensive efforts by many investigators, systemic chemotherapy for HCC has been quite ineffective, as demonstrated by low response rates and no survival benefits[1-4]. With the continuous development of the traditional Chinese medicine industry in recent years, it has been proven that traditional Chinese medicines (TCMs) have a marked effect on treating HCC, with unique advantages, and have gained wide acceptance as a safe, palliative and effective treatment in China[5-9]. Delisheng is a Chinese medicinal compound and is usually used in combination with chemotherapy for HCC. Furthermore, it has been reported that it can improve the clinical symptoms and quality of life, without severe adverse reactions, in patients with late-stage HCC. It is composed of ginseng, milk vetch root, secretion bufonis and Cantharidium. As delisheng is attractive as a natural product for medicinal use, increasing attention is being paid to its scientific evaluation and its possible molecular mechanisms.
Three-dimensional cell culture has been widely used for studying the various molecular processes and the development of therapy in recent years, for subtle changes in phenotypic expression and biological activity not demonstrated in conventional monolayer culture. In contrast, multicellular spheroids of tumor cells provide an excellent three-dimensional in vitro model to facilitate detailed investigations, including the response to various antineoplastic agents and their possible molecular mechanisms, since spheroids mimic the solid tumors more closely than monolayers do[10]. Tumor resistance to anticancer drugs is a real phenomenon, partly because of the so-called multicellular resistance (MCR), and it may be the most important obstacle to cancer treatment[11]. The resistance encountered in cells cultured as spheroids seems to be analogous to the natural resistance observed in patients, so the usage of three-dimensional cell culture may provide a model for studies on the development of anti-cancer drugs.
In this study, cells were cultured with a liquid overlay technique[12,13]. After the formation of multicellular spheroids, morphology was analyzed by phase contrast microscopy, scanning electron microscopy and transmission electron microscopy. Sensitivity of human hepatocellular carcinoma HepG2 cells to delisheng, 5-fluorouracil and adriamycin was investigated by MTT assay in multicelluar spheroids and monolayers. Vascular endothelial growth factor (VEGF) and endostatin expression was analyzed in multicellular spheroids treated with delisheng, 5-fluorouracil, adriamycin and negative control PBS, with immunohistochemical staining.
The human hepatocellular carcinoma cell line used in the present study was HepG2 preserved in The Center of Molecular Biology of Xi'an Jiaotong University.
Each HepG2 cell line was maintained in DMEM (Gibco, USA) medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 U/mL streptomycin in 5% CO2/95% air at 37°C. Cell cultures were maintained in the exponentially growing state by passaging twice weekly. Exponentially growing cells were harvested in a monolayer cell culture, while for three-dimensional cell culture obtained by liquid overlay technique, a single cell suspension in complete medium was seeded in each culture flask coated with 2% agarose. The conditions for three-dimensional cell culture were exactly the same as for monolayer culture, except for the presence of an agarose layer. After 3 or 4 d incubation, multicellular spheroids were obtained from each culture flask.
After 3 or 4 d culture, monolayer cells and multicellular spheroids were observed by phase contrast microscopy. After which, samples were washed with PBS, pH 7.4, and fixed with 2.5% glutaraldehyde for 2 h at 4°C. After three washes in PBS, they were post-fixed with 1.0% osmium tetroxide in PBS for 2 h at 4°C, then washed once in PBS, followed by dehydration with an increasing ethanol series (30, 50, 70, 90 and 100%). The samples were then treated with isoamyl acetate for 10 min, dried to the critical point, and coated with gold. Finally, the samples were observed with a scanning electron microscope (JEOL, JSM-840, Japan).
Additional samples were fixed and dehydrated as described for scanning electron microscopy, and embedded in Epon812 epoxy resin. Thin sections were prepared and examined with a transmission electron microscope (HITACHI, H-600, Japan).
The Chinese medicinal compound delisheng was dispensed into a physic liquor to give a clinical dosage of 80 mL (recommended dose is 50-100 mL/d). Delisheng was attenuated with Hanks balanced salts solution, and the final concentration was 12.5, 25, 50, 100 and 200 μL/mL. MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] (Sigma, St. Louis, MO, USA) was dissolved in PBS at 5 mg/mL and sterilized by filtration. After treatment with 12.5, 25, 50, 100 or 200 μL/mL delisheng; 6.25 × 10-3, 12.5 × 10-3, 25 × 10-3, 50 × 10-3 and 100 × 10-3 g/L 5-fluorouracil; 0.15625 × 10-3, 0.3125 × 10-3, 0.625 × 10-3, 1.25 × 10-3 and 2.5 × 10-3 g/L adriamycin in monolayer and three-dimensional cell culture for 48 h, the cells were freshly disaggregated by enzymatic dissociation, and the cell number was determined with a hemocytometer. A cell suspension (150 μL) of each sample was added to a 96-well plate. The cell number per well was approximately 2 × 105, Each well was added to stock MTT solution (20 μL, 5 mg/mL). After incubation in the presence of 5% CO2 and 95% air at 37°C for 4 h, the supernatant was discarded. DMSO (150 μL) (Sigma) was added to each well and mixed thoroughly to dissolve the dark blue crystals. After 30 min at room temperature to ensure that all crystals were dissolved, the plates were read with a Micro Elisa plate reader at a wavelength of 492 nm. All samples were read five times. The cell inhibition rate was calculated by the following formula: cell inhibition rate (%) = (1-OD of treated cells)/(OD of control cells) × 100%.
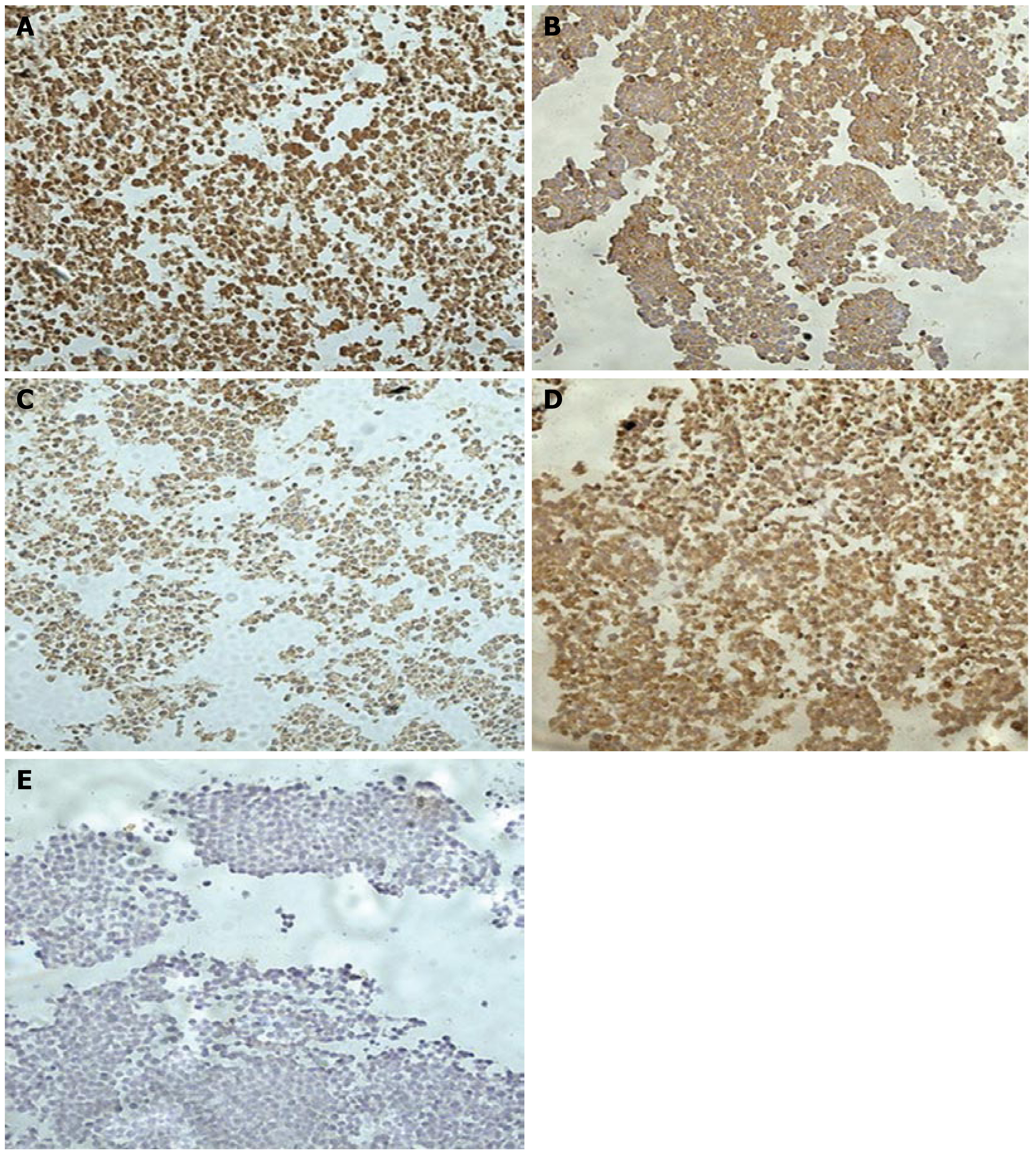
Antibody staining was performed with multicellular spheroids. The monoclonal anti-VEGF antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at a dilution of 1:25. The monoclonal anti-endostatin (Santa Cruz Biotechnology) was used at a dilution of 1:25. Spheroids treated with delisheng (25 μL/mL), 5-fluorouracil (40 × 10-3 g/L), adriamycin (1 × 10-3 g/L) and negative control PBS for 48 h were washed in PBS, and fixed in 4% paraformaldehyde in PBS at 4°C for 1 h, placed in a small tissue processing cassette full of 3% agarose for 1 h, then dehydrated and embedded in paraffin. Four-micrometer sections from the representative blocks were immunohistochemically stained with mouse anti-human monoclonal antibodies against VEGF, and rabbit anti-human monoclonal antibodies against endostatin. Four-micrometer thick sections were deparaffinized, rehydrated and washed in PBS for 15 min, before endogenous peroxidase activity was blocked. Primary antibody was substituted by normal mouse and rabbit serum as a negative control .Then endogenous peroxidase activity was blocked by 30 min incubation in 3% hydrogen peroxide solution. The specimens were washed with PBS, pH 7.5. Non-specific binding was blocked by incubating the slides with normal goat serum in PBS for 15 min at 37°C, then incubated overnight at 4°C with the primary antibodies. After washing three times with PBS, the sections were incubated with secondary antibody, biotinylated antibodies for 40 min at 37°C. After washing three times with PBS, the sections were immunostained with avidin-biotin complex for 40 min at 37°C. Visualization of the immunoreactions was conducted with 3, 3'-diaminobenzidine (DAB, Sigma, UK) for 5 min. Finally, sections were counterstained with hematoxylin. The degree of the expression of immunohistochemical products was classified into negative (< 10% of cells had a positive reaction) and positive (> 10% of cells had a positive reaction). Simultaneously, VEGF- and endostatin-positive staining particles were quantitatively analyzed by a LeicaQ550cw imaging analysis system (Germany) to determine the mean grey values, the lower mean grey values, and the stronger substrate coloration. The mean grey values are an inverse ratio with the protein expression quantity.
Data were reported as means ± SE. The t test was used for statistical analysis, P < 0.05 was considered statistically significant.
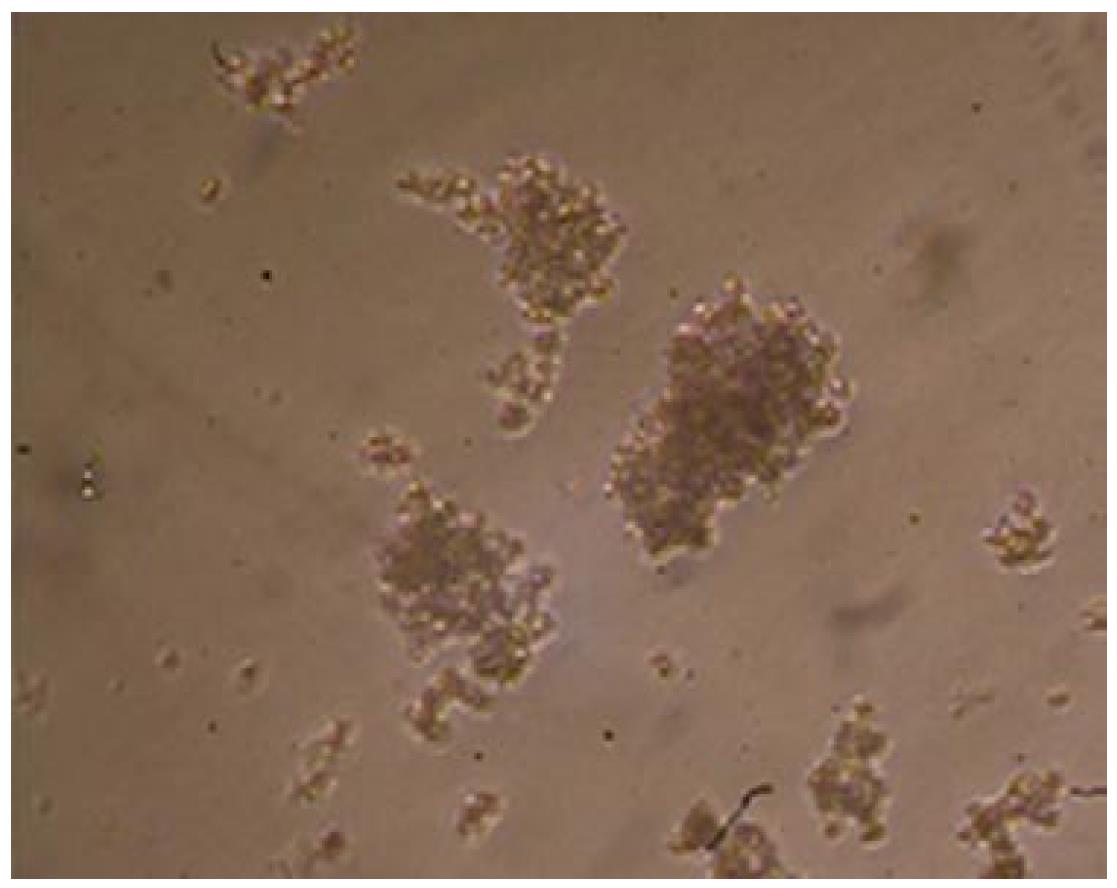
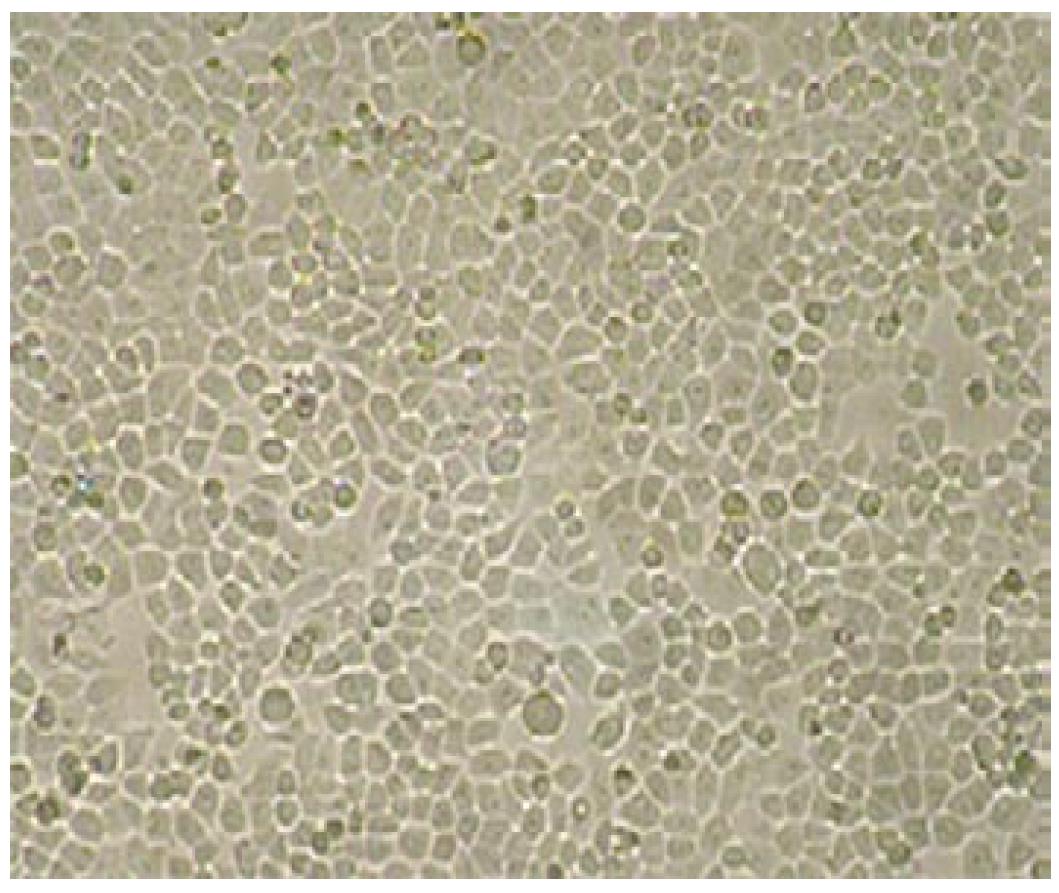
Multicellular spheroids and monolayer cells were observed with phase contrast microscopy. The cells were oval spheroids in three-dimensional cell culture (Figures 1 and 2).
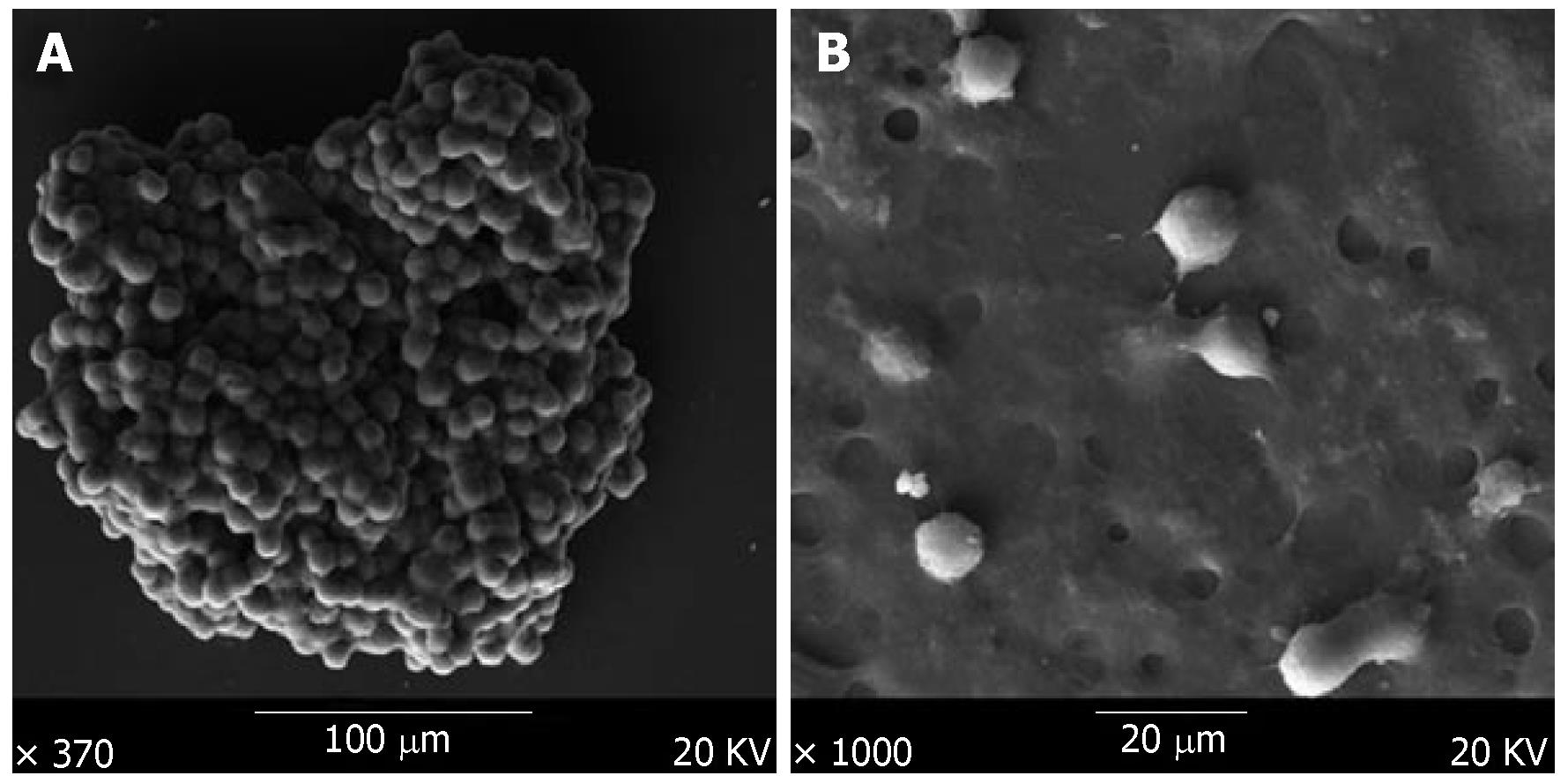
Scanning electron microscopy showed that the multicellular spheroids were irregular, with a diameter of up to 200 μm at 3-4 d. The cells were oval spheroids or polyhedrons, with tight cell junctions, compared to monolayer cells that were spread in a dispersed manner and had few cell junctions (Figure 3).
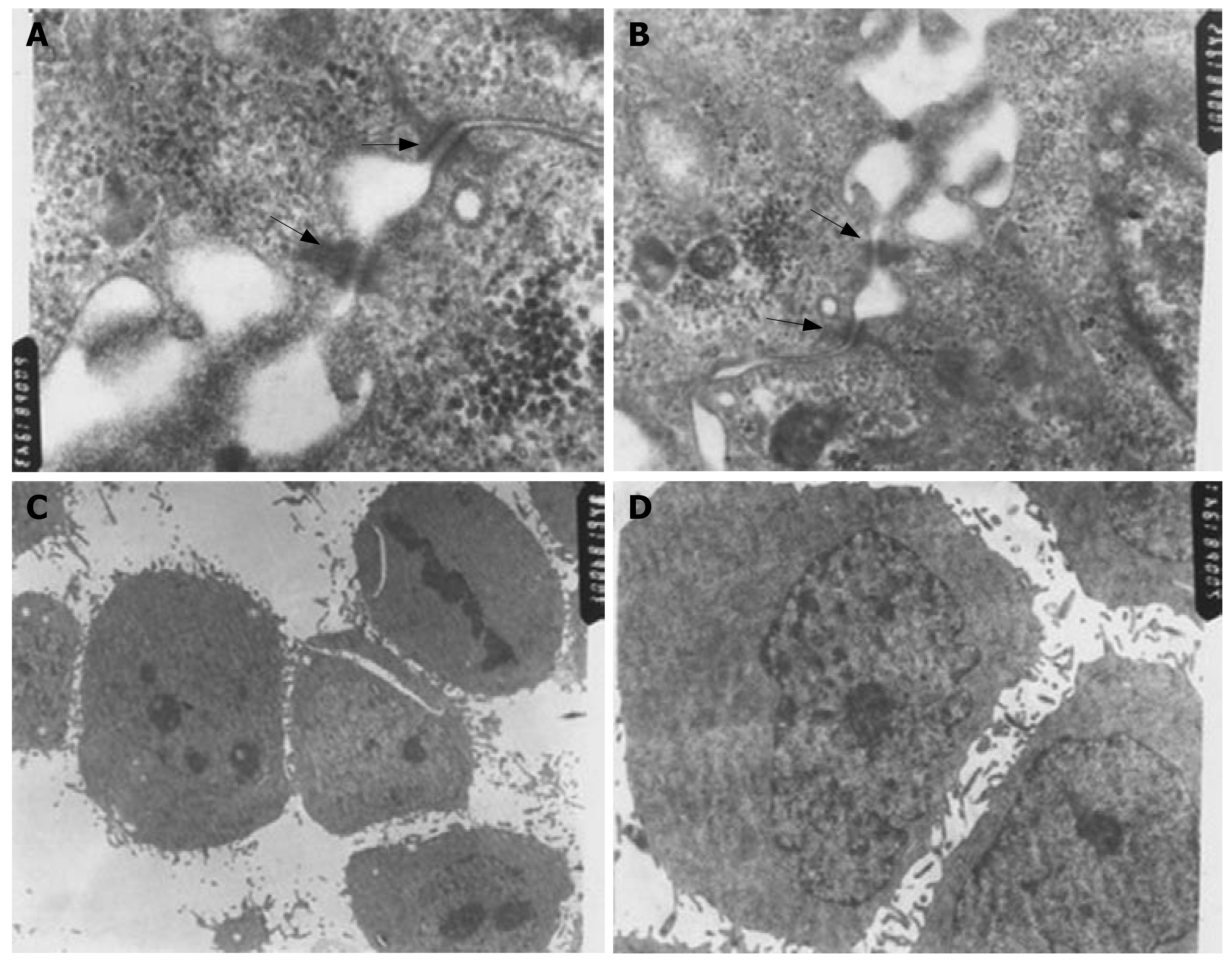
Transmission electron microscopy showed that desmosome and intermediate junctions were observed in three-dimensional cell culture, but such structures were rarely observed in monolayer cell culture. Multicellular spheroids of approximately 200 μm diameter showed no obvious signs of extensive central apoptosis or necrosis, although there was some swelling and there were fewer microvilli on the surface of multicellular spheroids compared to monolayer cells (Figure 4).
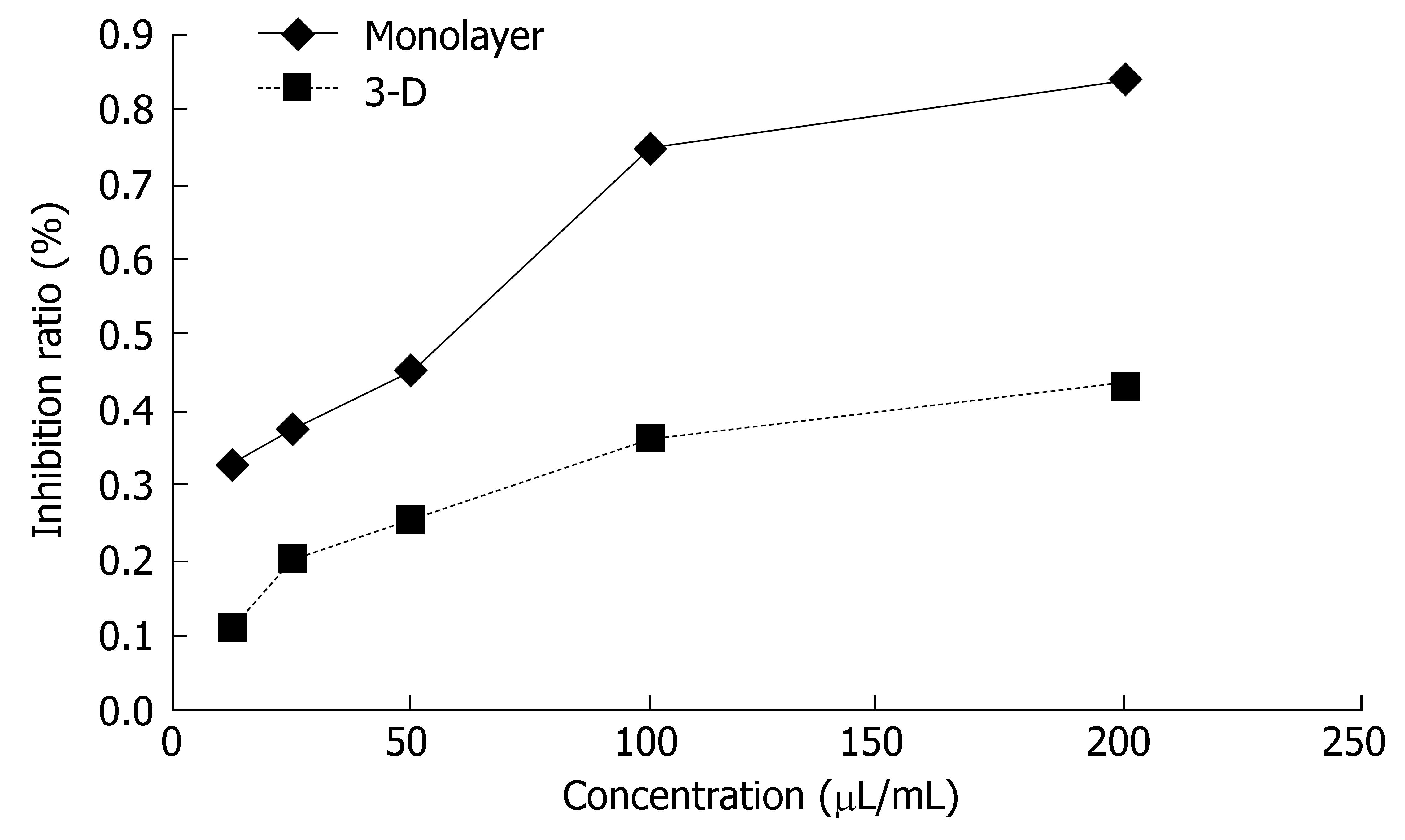
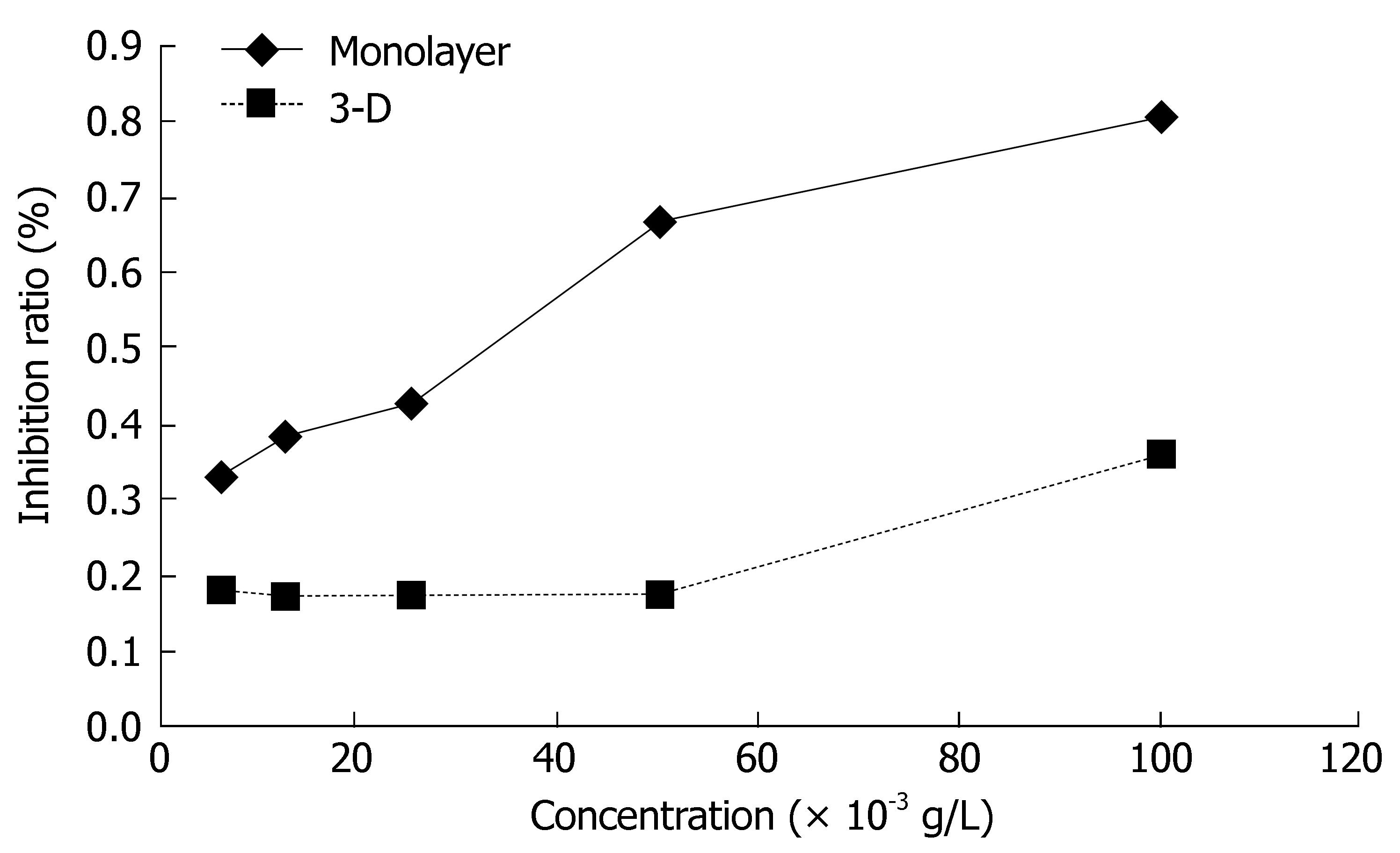
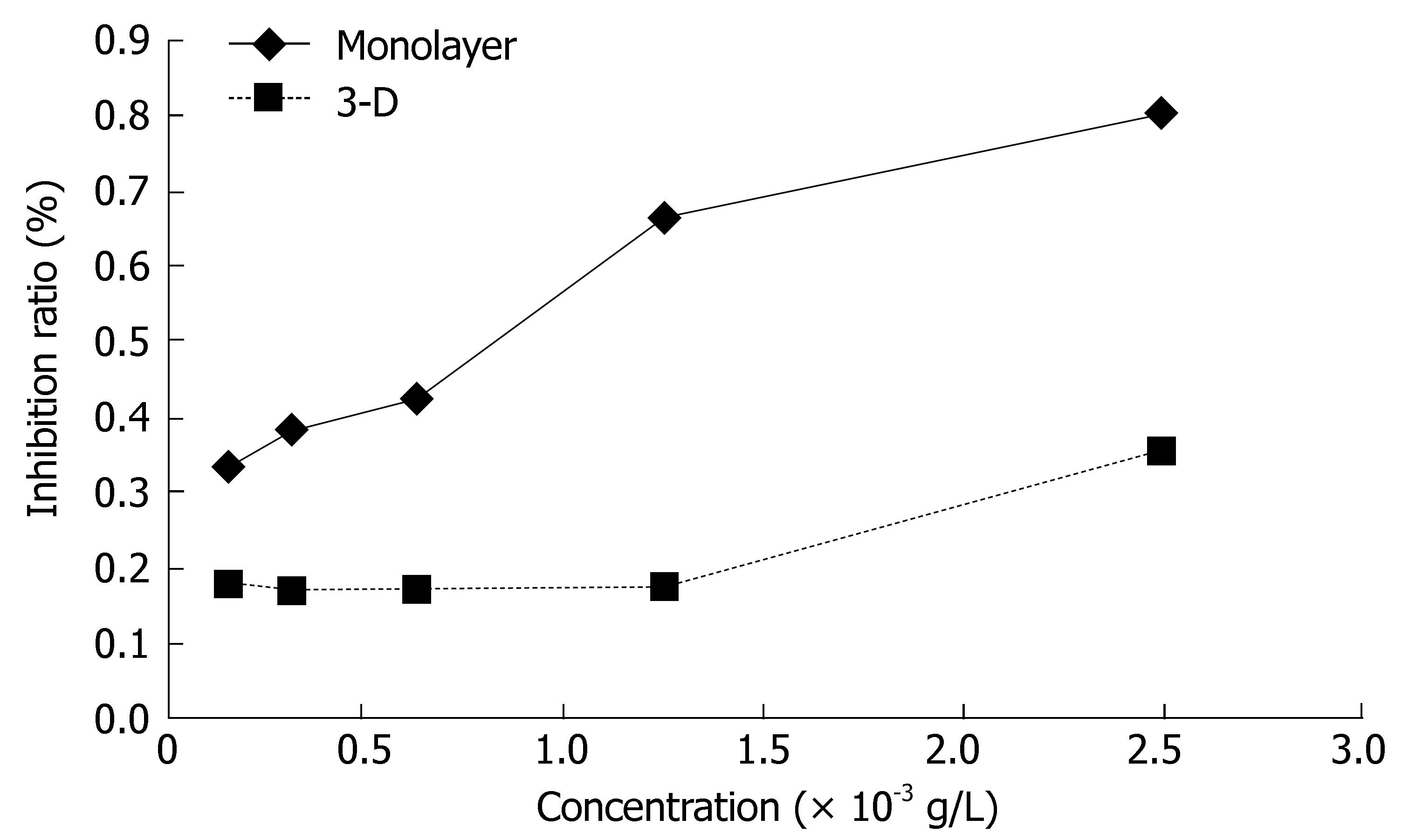
The sensitivity of monolayer and three-dimensional cell cultures to delisheng, 5-fluorouracil and adriamycin was investigated by MTT assay. The cell inhibition ratio in three-dimensional cell culture treated with delisheng, 5-fluorouracil and adriamycin was lower than that in monolayer culture (P < 0.01) (Tables 1, 2 and 3, Figures 5, 6 and 7), which indicated the HepG2 cells in three-dimensional culture became resistant to delisheng, 5-fluorouracil and adriamycin. Cell inhibition ratio increased with concentration of delisheng, 5-fluorouracil and adriamycin. The results showed that delisheng had a satisfactory cell inhibition ratio compared to 5-fluorouracil and adriamycin.
| Concentration (μL/mL) | Inhibition ratio (%) | ||
| 3-d | Monolayer | P value | |
| 12.5 | 11.73 ± 11.58 | 33.81 ± 10.54 | 0.014 |
| 25 | 20.94 ± 8.29 | 37.79 ± 9.55 | 0.018 |
| 50 | 25.45 ± 5.62 | 46.01 ± 6.32 | 0.001 |
| 100 | 36.69 ± 5.37 | 75.65 ± 10.47 | < 0.001 |
| 200 | 43.93 ± 7.81 | 84.62 ± 3.24 | < 0.001 |
| Concentration (g/L) | Inhibition ratio (%) | P value | |
| 3-d | Monolayer | ||
| 6.25 × 10-3 | 18.25 ± 7.85 | 33.89 ± 9.41 | 0.021 |
| 12.50 × 10-3 | 17.51 ± 7.28 | 39.08 ± 7.74 | 0.002 |
| 25.00 × 10-3 | 18.18 ± 11.34 | 43.18 ± 5.92 | 0.002 |
| 50.00 × 10-3 | 17.87 ± 10.93 | 67.72 ± 2.18 | < 0.001 |
| 100.00 × 10-3 | 36.42 ± 10.54 | 81.17 ± 2.81 | < 0.001 |
| Concentration (g/L) | Inhibition ratio (%) | P value | |
| 3-d | Monolayer | ||
| 0.15625 × 10-3 | 11.40 ± 2.71 | 10.39 ± 5.07 | 0.705 |
| 0.31250 × 10-3 | 12.17 ± 6.49 | 21.31 ± 2.85 | 0.02 |
| 0.62500 ×10-3 | 18.61 ± 9.41 | 25.69 ± 8.19 | 0.24 |
| 1.25000 × 10-3 | 29.45 ± 5.26 | 41.66 ± 6.52 | 0.012 |
| 2.50000 × 10-3 | 38.09 ± 20.71 | 84.56 ± 7.97 | 0.002 |
VEGF and endostatin protein expression were confirmed in multicellular spheroids in three-dimensional culture. Immunohistochemical analysis demonstrated that VEGF and endostatin were expressed in the cytoplasm. Endostatin expression in spheroids treated with delisheng was higher than that with 5-fluorouracil, adriamycin and negative control PBS (P < 0.05). However, VEGF expression in spheroids treated with delisheng was similar with 5-fluorouracil, adriamycin and PBS (P > 0.05) (Tables 4 and 5; Figure 8).
HCC is one of the most common types of human malignancy worldwide. The prognosis in patients with untreated HCC is very poor, with a median survival of 6 mo in patients who receive no specific treatment[14]. Curative therapy such as surgery, liver transplantation[15], or percutaneous treatments benefit only 25% of patients. Systemic chemotherapy has been widely used in an attempt to prolong this short survival time or to provide symptomatic relief[16], but it appears to be less efficient, possibly because it is used when metastases are already present, and the tumor is large and spreading; moreover, anticancer drug resistance is frequent. Tumor resistance to anticancer drugs is a real phenomenon, but the most prevalent mechanism may not correspond to multidrug resistance[17]. Instead, the so-called MCR, first described in 1972 by Durand and Sutherland may be the most important obstacle to cancer treatment. The resistance encountered in cells cultured as spheroids seems to be analogous to the natural resistance observed in patient tumors. Tumor cells often form compact multicellular spheroids when maintained in a three-dimensional culture system. Various changes in molecular expression and even in biological activity have been reported to exist between the three-dimensional and conventional monolayer cultures[18,19].
There are abundant resources in TCMs that have been used clinically for > 5000 years in China and Asia, and increasing attention is being paid to their scientific evaluation. With continuing development of TCM, it has a marked effect on the treatment of several, including tumors, with unique advantages. Delisheng is a common Chinese medicinal compound, whose composition includes ginseng, milk vetch root, secretion bufonis and Cantharidium. Satisfactory effects of delisheng have been reported in patients with late-stage HCC that may improve clinical symptoms and quality of life, without severe adverse reactions. However, the mechanisms responsible for this treatment are unknown. Many kinds of solid tumors in vivo and tumor cells in three-dimensional cell culture in vitro exhibit intrinsic or acquired resistance to cytotoxic drugs, which is one of the major obstacles to clinical treatment.
In this study, human HepG2 cells were cultured with a liquid overlay technique to form multicellular spheroids. The results indicated that the cells were oval spheroid or polyhedral, with fewer microvilli on the surface and more desmosome and intermediate junctions, compared to monolayer cells. The cells in three-dimensional culture turned out to be less sensitive to delisheng, 5-fluorouracil and adriamycin than those cultured in monolayer. Some studies have indicated that more cells in multicellular spheroids shift into a quiescent state. However, the majority of conventional cytotoxic anticancer drugs preferentially kill cycling cells. The increase in quiescent cells might result in decreased sensitivity. VEGF and endostatin expression were confirmed in three-dimensional culture. The data indicated that endostatin expression in spheroids treated with delisheng was higher than that with 5-fluorouracil and adriamycin and negative control PBS, and a previous study has shown that delisheng has a satisfactory cell inhibition ratio compared to 5-fluorouracil and adriamycin. This suggests that the satisfactory effects of delisheng on HCC were associated with the up-regulation of endostatin. As we know, one of the components of delisheng, ginseng, has some antiangiogenic activity. Recent studies have reported that ginseng extract exerts anti-tumor activity through its effect on the vascular system; furthermore, some investigators have suggested that ginsenoside Rg3, a saponin extracted from ginseng, alone or combined with cyclophosphamide (CTX), inhibits growth and angiogenesis of ovarian cancer by decreasing the microvessel density (MVD value) and VEGF expression[20-23]. Endostatin as an angiogenesis inhibitor has been shown to inhibit VEGF-induced endothelial cell migration in vitro, and to have anti-tumor activity in vivo[24,25]. Some investigators have studied tumor growth in transgenic mice overproducing endostatin specifically in the endothelial cells (a 1.6-fold increase in the circulating levels), and found that tumor growth was 3-fold slower than in wild-type mice[26-30]. Therefore, we think that the endostatin up-regulation induced by delisheng in our experiment was possibly due to the antiagiogenic activity of its ginseng component, and this may explain why delisheng has satisfactory anti-tumor activity too. Although major emphasis has been placed on the down-regulation of VEGF, the potential role of endostatin increase induced by ginseng as an endogenous inhibitor of angiogenesis in tumor growth inhibition can not be ignored.
Further understanding of the mechanisms involved in the activity of delisheng will help in the development of new approaches to therapy of HCC. The satisfactory activity of delisheng on HCC was associated with ginseng up-regulation of endostatin expression.
We would like to offer special thanks to the Department of Medical Oncology, The First Affiliated Hospital of The School of Medicine of Xi'an Jiaotong University and The Center of Molecular Biology of Xi'an Jiaotong University for their help with these experiments.
HCC is a highly malignant tumor with a very high morbidity and mortality. Despite extensive efforts by many investigators, systemic chemotherapy for HCC has been quite ineffective. Delisheng is a Chinese medicinal compound and is often used in conjunction with chemotherapy for HCC, with satisfactory results. Our work tried to establish the mechanisms for these effects of delisheng on HCC. Three-dimensional cell culture has been widely used for studying the various molecular processes, since spheroids mimic solid tumors more closely than monolayers do, so the use of three-dimensional culture provides a model for the development of anti-cancer drugs. In this study, cells were cultured with a liquid overlay technique. After the formation of multicellular spheroids, we used the model to perform our experiments.
With the continuous development of TCM in recent years, it is been demonstrated that it can have a marked effect on treating HCC, with unique advantages, and it has gained wide acceptance as a safe, palliative and effective treatment in China. Delisheng is a Chinese medicinal compound and is often used in conjunction with chemotherapy for HCC. Furthermore, it has been reported in patients with late-stage HCC that delisheng may improve the clinical symptoms and quality of life, without severe adverse reactions. As delisheng is attractive as a natural product for medicinal use, increasing attention is being paid to its scientific evaluation and its possible molecular mechanisms. Three-dimensional cell culture has been widely used for studying the various molecular processes and development of therapy in recent years, as it detects subtle changes in phenotypic expression and biological activity not seen in conventional monolayer culture. In contrast, multicellular spheroids of tumor cells provide an excellent three-dimensional in vitro model to facilitate detailed investigations, including the response to various antineoplastic agents and their possible molecular mechanisms, since spheroids mimic solid tumors more closely than monolayers do. The resistance encountered in cells cultured as spheroids seems to be analogous to the natural resistance observed in patient tumors, so the usage of three-dimensional cell culture may provide a model for developing anti-cancer drugs.
We used three-dimensional cell culture to study a Chinese medicine and its anti-cancer effects. We showed that delisheng had satisfactory anti-cancer effects on HCC, and these were associated with the up-regulation of endostatin. This was possible because of the presence of ginseng in delisheng.
We confirmed that three-dimensional cell culture was suitable for the study of a traditional Chinese medicine, and this may help other researchers to find a better model for drug development. We also found that delisheng had satisfactory anti-cancer effects on HCC, and these were associated with the up-regulation of endostatin. This was made possible by one of delisheng's components, ginseng, and this may provide a new method of therapy for HCC.
Three-dimensional cell culture: this has been widely used for studying the various molecular processes and development of therapy in recent years, because it can detect subtle changes in phenotypic expression and biological activity not seen in conventional monolayer culture. This is because spheroids mimic solid tumors more closely than monolayers do. Delisheng: a Chinese medicinal compound that is often used in conjunction with chemotherapy for HCC. Furthermore, it has been reported in patients with late-stage HCC that it can improve clinical symptoms and quality of life, without severe adverse reactions. Its composition includes ginseng, milk vetch root, secretion bufonis and Cantharidium.
The article provides a new model to study TCM, and explains the test outcome rationally; furthermore, it introduces the Chinese medicinal compound delisheng and indicates its further applications.
S- Editor Liu Y L- Editor Kerr C E- Editor Lu W
| 1. | Okuda K. Hepatocellular carcinoma. J Hepatol. 2000;32:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 396] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 2. | Rougier P, Mitry E, Barbare JC, Taieb J. Hepatocellular carcinoma (HCC): an update. Semin Oncol. 2007;34:S12-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10:S115-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 514] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 5. | Chen RC, Su JH, Ouyang GL, Cai KX, Li JQ, Xie XG. Induction of differentiation in human hepatocarcinoma cells by isoverbascoside. Planta Med. 2002;68:370-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Efferth T, Davey M, Olbrich A, Rücker G, Gebhart E, Davey R. Activity of drugs from traditional Chinese medicine toward sensitive and MDR1- or MRP1-overexpressing multidrug-resistant human CCRF-CEM leukemia cells. Blood Cells Mol Dis. 2002;28:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Mabed M, El-Helw L, Shamaa S. Phase II study of viscum fraxini-2 in patients with advanced hepatocellular carcinoma. Br J Cancer. 2004;90:65-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3633] [Cited by in RCA: 3390] [Article Influence: 121.1] [Reference Citation Analysis (0)] |
| 9. | Cheng JT. Review: drug therapy in Chinese traditional medicine. J Clin Pharmacol. 2000;40:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 123] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol. 2000;36:193-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 292] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 11. | Hoffman RM. The three-dimensional question: can clinically relevant tumor drug resistance be measured in vitro? Cancer Metastasis Rev. 1994;13:169-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | O'Connor KC, Venczel MZ. Predicting aggregation kinetics of DU 145 prostate cancer cells in liquid-overlay culture. Biotechnol Lett. 2005;27:1663-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Carlsson J, Yuhas JM. Liquid-overlay culture of cellular spheroids. Recent Results Cancer Res. 1984;95:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 15. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5110] [Cited by in RCA: 5306] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 16. | Leung TW, Johnson PJ. Systemic therapy for hepatocellular carcinoma. Semin Oncol. 2001;28:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Pérez-Tomás R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem. 2006;13:1859-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 386] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 18. | O'Brien LE, Yu W, Tang K, Jou TS, Zegers MM, Mostov KE. Morphological and biochemical analysis of Rac1 in three-dimensional epithelial cell cultures. Methods Enzymol. 2006;406:676-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Hamilton G. Multicellular spheroids as an in vitro tumor model. Cancer Lett. 1998;131:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 178] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol. 1992;36:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 291] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Xu TM, Xin Y, Cui MH, Jiang X, Gu LP. Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide on growth and angiogenesis of ovarian cancer. Chin Med J (Engl). 2007;120:584-588. [PubMed] |
| 22. | Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1003] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 23. | Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RN, Sasisekharan R, Fan TP. Modulating angiogenesis: the yin and the yang in ginseng. Circulation. 2004;110:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 24. | O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3110] [Article Influence: 111.1] [Reference Citation Analysis (0)] |
| 25. | Shi W, Teschendorf C, Muzyczka N, Siemann DW. Adeno-associated virus-mediated gene transfer of endostatin inhibits angiogenesis and tumor growth in vivo. Cancer Gene Ther. 2002;9:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Sasaki T, Fukai N, Mann K, Göhring W, Olsen BR, Timpl R. Structure, function and tissue forms of the C-terminal globular domain of collagen XVIII containing the angiogenesis inhibitor endostatin. EMBO J. 1998;17:4249-4256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 247] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 27. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6485] [Article Influence: 259.4] [Reference Citation Analysis (0)] |
| 28. | Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 580] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 29. | Yamaguchi N, Anand-Apte B, Lee M, Sasaki T, Fukai N, Shapiro R, Que I, Lowik C, Timpl R, Olsen BR. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J. 1999;18:4414-4423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 340] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 30. | Sund M, Hamano Y, Sugimoto H, Sudhakar A, Soubasakos M, Yerramalla U, Benjamin LE, Lawler J, Kieran M, Shah A. Function of endogenous inhibitors of angiogenesis as endothelium-specific tumor suppressors. Proc Natl Acad Sci USA. 2005;102:2934-2939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |