INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies related to a high mortality glo-bally[1,2]. Recent studies have noted a significant rise in the incidence of HCC in the United States in the past 2 decades[2]. Less than 1% of HCC patients underwent a radical surgical resection in the US between 1974 and 1996[3]. HCC's limited treatment remedies and the poor prognosis emphasize the importance in developing an effective chemoprevention for this disease.
Milk thistle (Silybum marianum) has been widely utilized as a folk remedy for liver diseases. It is a popular dietary supplement widely used in the United States and Europe[4]. Silibinin is a polyphenolic flavonoid and the major biologically active compound of milk thistle[4-6]. It is well known that milk thistle is safe and well-tolerated, and it protects the liver from drug or alcohol-related injury[7,8]. Studies demonstrated silibinin's inhibitory effects on multiple cancer cell lines, including prostate[9-12], colon[13,14], skin[15-17], bladder[18,19] and lung cancers[20]. Recently, we and Varghese et al[21,22] reported silibinin's anti-HCC effects, but further studies are needed to define silibinin's inhibitory effects and mechanisms on human HCC cell growth.
Searching for non-invasive biomarkers is another important filed of HCC chemoprevention. Plasma alpha-fetoprotein (AFP) has been used as a clinical marker for diagnosing and monitoring recurrent HCC[23-25]. However, AFP's value in monitoring effect of HCC chemoprevention has not been tested before.
Phosphatase and tensin homolog deleted on chromo-some ten (PTEN), phosphatidylinositol 3'-kinase (PI3K) and Akt (PTEN/PI3K/Akt) pathway has been associated with carcinogenesis[26]. Activated PI3K-Akt signaling promotes carcinogenesis[27,28]. PTEN is a negative regulator of PI3K-Akt signaling[29] and one of the most frequently inactivated genes in malignancies[30,31]. Akt is a downstream protein kinase of PI3K (PTEN) and is a signal transduction protein that has been identified as one of the key elements in protecting cells from apoptosis. If unregulated, Akt promotes uncontrolled cell replication[32,33]. It was reported that silibinin affects Akt expression in prostate cancer cells[16], but it remains unknown whether silibinin affects HCC growth through a PTEN/PI3K/Akt pathway in human liver cancer cells.
Histone acetylation modifies nucleosome structure that leads to DNA relaxation, reduces the affinity of histone complexes with DNA, and enhances the access of transcriptional factor to DNA[34]. Accumulating evidence has indicated that alteration of histone acetylation plays an important role in carcinogenesis[35,36], but it remains unknown whether it is associated with silibinin's anti-HCC effects.
In the present study, we demonstrated that silibinin significantly inhibited the growth of HuH7, HepG2, Hep3B, and PLC/PRF/5 human HCC cells that was associated with decreased Ki-67 expression, and cell cycle progression by arresting G1-S transition, and promoted apoptosis. These effects of silibinin were associated with increased PTEN activity and decreased p-Akt production, indicating the role of PTEN/PI3K/Akt pathway in silibinin-mediated anti-HCC effects. We also demonstrated that silibinin increased AC-H3 and AC-H4 expression, indicating that altered histone acetylation is involved in silibinin-reduced HCC cell proliferation.
MATERIALS AND METHODS
Reagents
The cell culture media were the same, as previously reported[37,38]. Anti-activated caspase-3 antibody was purchased from Sigma Chemical Co. (St. Louis, MO). The antibodies against human Ki-67, AFP, p-Rb, E2F1, DP1, CD1, CDK4, p21 and p27, activated caspase-9, bcl-2, survivin, CD34, metalloproteinase (MMP)-2, MMP-9, phosphorylated-AktThr308, PTEN, AC-histone3 and AC-histone4, and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). PTEN activity assay kit was from Biomol Research Laboratories, Inc (Plymouth Meeting, PA). An EIA kit for cell death detection was from Roche Applies Science (Indianapolis, IN).
Cell culture
Human HCC cell lines, HuH7, HepG2, PLC/PRF/5, and Hep3B cells[37,38], were used in the present study. All the cells were cultured, as previously reported[37,38]. The experiments were performed when cells reached about 80% confluence and cultured in FBS-free media for 24 h to synchronize the cell growth[37,38].
Cell proliferation assay
Cell proliferation was determined using MTT assay, as previously reported[37,38]. Briefly, the effects of silibinin on HCC cell growth were then determined after 24 h of incubation by optical density absorbance at 490 nm according to the manufacturer's instruction[37,38].
Apoptosis assays
Apoptosis was determined in duplicate using an EIA kit for cell death detection, as previously reported[37,38].
Immunoprecipitation (IP) and immunoblot (IB) assays
After 24 h of treatment with silibinin at 25% inhibitory concentration (IC25) or IC50 dose, the cell pellets were lysed and the supernatants were used to detect Ki-67, AFP, p-Rb, E2F1, CD1, CDK4, p21waf1/cip1, p27kip1, bcl-2, survivin, activated caspase-3 and caspase-9, CD34, MMP-2, MMP-9, phosphorylated-AktThr308, PTEN, and AC-H3 and AC-H4. The IP assays were same, as previously reported[37,38]. β-actin was used as an internal control. The relative amount of each protein was quantified by digitally scanning its hybridizing bands, as previously reported[37,38].
PTEN activity assay
PTEN protein was immunoprecipitated with 10 μL of rabbit anti-human antibody at 4°C overnight, followed by addition of 25 μL of anti-rabbit IgG-conjugated agarose beads for 2 h at 4°C, washing and centrifugation. The phosphatase reaction was performed in 50 μL of assay buffer containing 200 μmol/L water-soluble diC8-PIP3 and the immunoprecipitated PTEN protein. The release of phosphate from the substrate was measured in a colorimetric assay using the Biomol Green Reagent (Plymouth Meeting, PA)[39]. The OD absorbance at 650 nm was recorded in an ELISA plate reader[37,38].
Statistical analysis
The descriptive statistics was provided with mean ± SD. A repeated-measure ANOVA test was used to assess dose dependent effects of silibinin on HuH7, HepG2, Hep3B, and PLC/PRF/5 cells. An independent sample t-test was used to assess the effects (i.e., mean differences) of silibinin treatment on apoptosis, and IB results. A P value < 0.05 was considered statistically significant.
RESULTS
Potent dose-dependent anti-proliferative effects of silibinin on human HCC cells
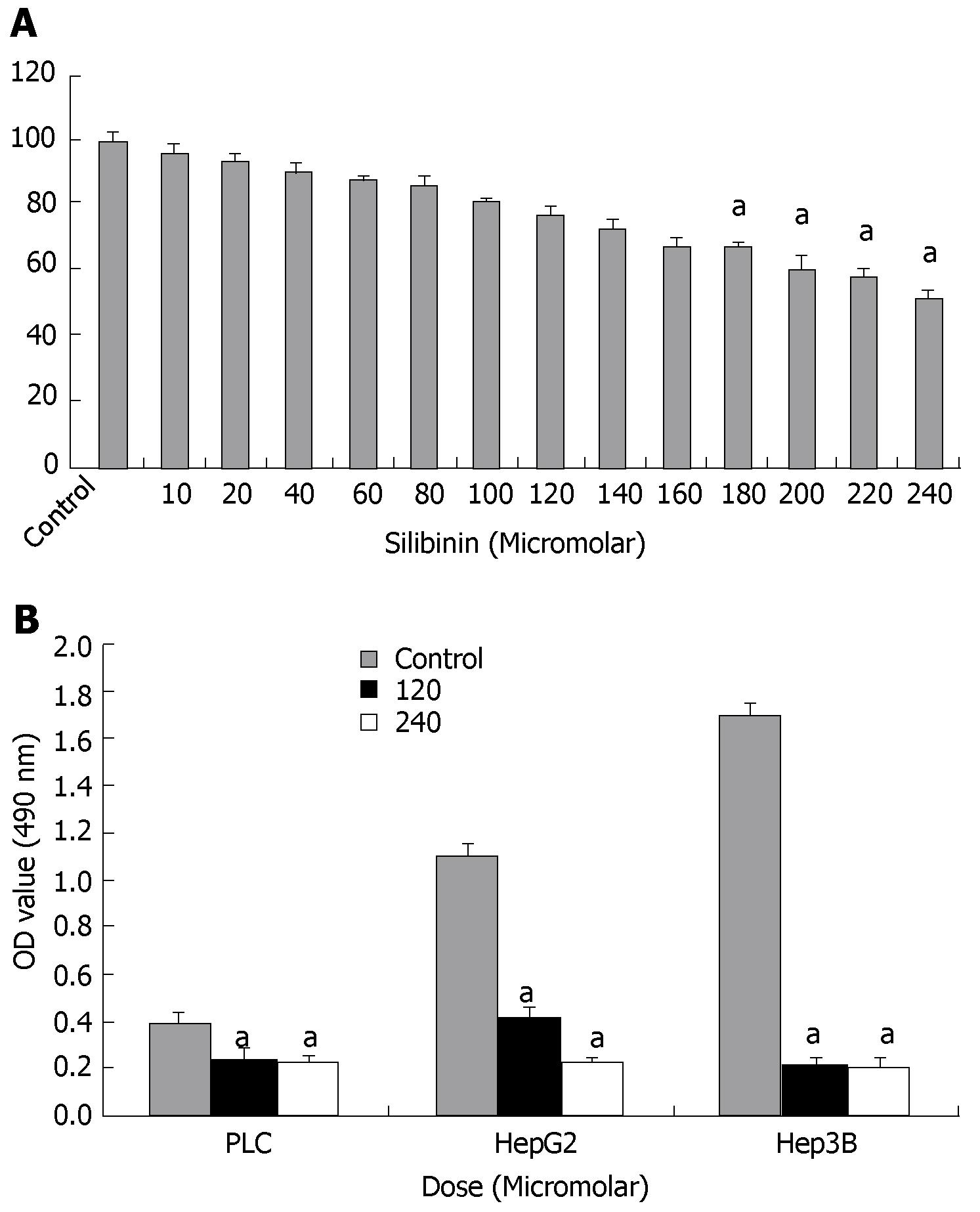
Effects of silibinin were initially assessed in HuH7 cells by MTT assay. As shown in Figure 1A, silibinin resulted in a dose-dependent inhibition of HuH7 cell growth. Compared to the control, there was a dose-dependent inhibitory which became significant at the dose greater than 180 μmol/L (P < 0.05). As shown in Figure 1B, silibinin also significantly inhibited the growth of HepG2, Hep3B, and PLC/PRF/5 human HCC cell lines, indicating a wide spectrum of silibinin's inhibitory effects on human HCC cell growth, as previously reported[21,22]. Because the HuH7 cell line is one of the most commonly used human HCC lines[37,38], it was then used to further determine silibinin's anti-HCC effects and mechanisms. For further characterization of dose-related mechanistic effect of silibinin, approximate IC25 (i.e., 120 μmol/L) and IC50 (i.e., 240 μmol/L) concentration were subsequently used for the remainder of the study.
Figure 1 Silibinin's effects on growth of human hepatoma cells.
A: MTT assay showed silibinin's dose dependent anti-proliferative effects on HuH7 cells. A significant decrease in proliferation compared to control was noted from silibinin ≥ 180 μmol/L. The IC25 is determined to be 120 μmol/L and the IC50 is determined to be 240 μmol/L; B: Silibinin's effects on other human HCC cell growth. PLC/PRF/5, HepG2, and Hep3B HCC cells were treated with silibinin at IC25 and IC50 doses for HuH7 cells. Silibinin significantly reduced growth of all three HCC cells in different rates. aP < 0.05 vs control.
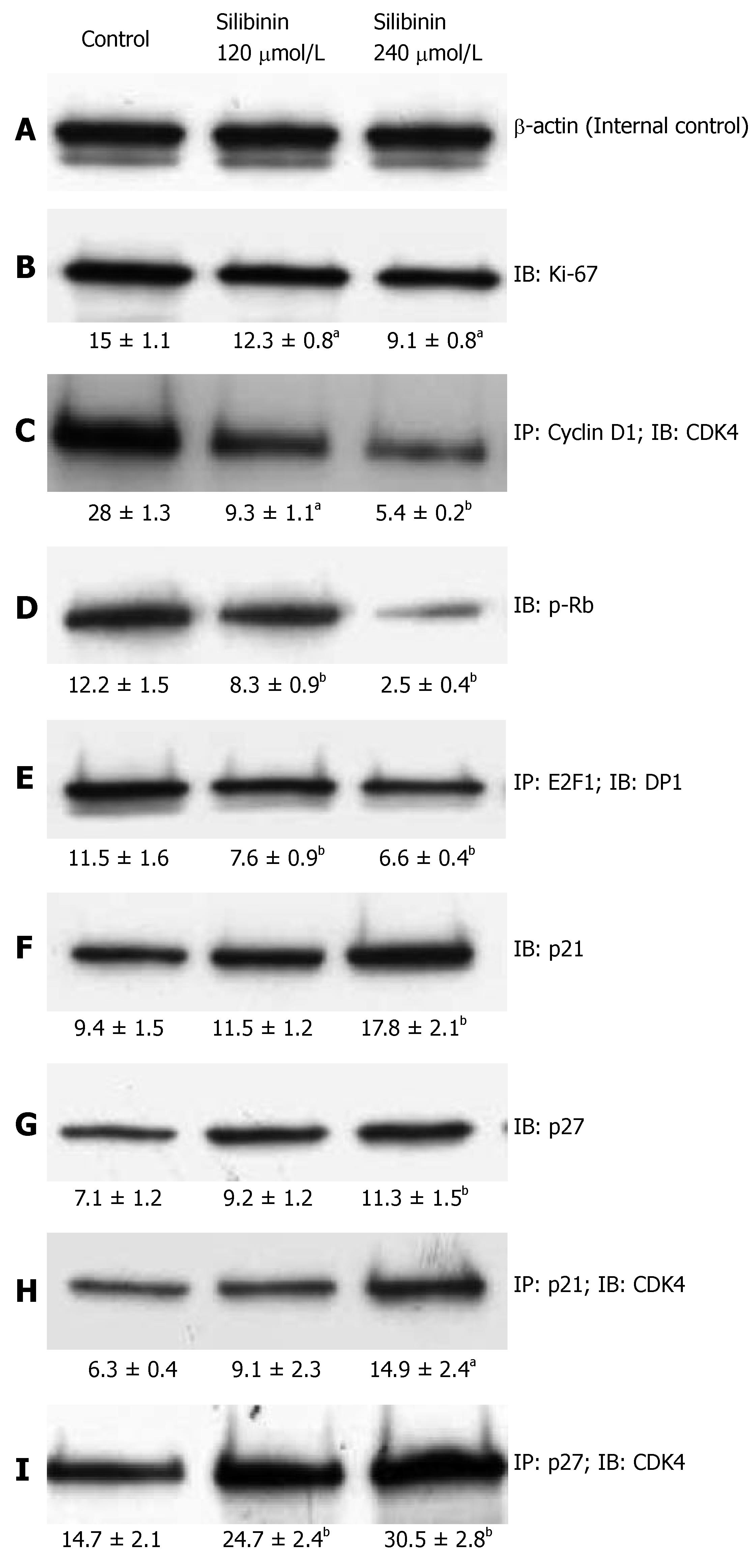
Ki-67 is a commonly used biomarker for cell prolifera-tion[40]. Consistent with the data derived from MTT as-say, silibinin treatment resulted in a significantly dose-dependent decrease in Ki-67 expression, as shown in Figure 2B (P < 0.05). These data further demonstrated silibinin's significant dose-dependent anti-proliferative effects on human HCC cells.
Figure 2 Silibinin's effects on proliferation of HCC cells.
After HuH7 cells were treated with silibinin at IC25 and IC50 doses, immunoprecipitation (IP) and immunoblots (IB) were performed in triplicate for each specimen. A mean densitometer reading was expressed in the respective box and used for statistical analysis. A: β-actin for internal control; B: Ki-67; C: CD1/CDK4 complex; D: p-Rb; E: E2F1-DP1 complex; F: p21Waf1/Cip1; G: p27Kip1; H: p21Waf1/Cip/CDK4 complex; I: p27Kip1/CDK4 complex. aP < 0.05, bP < 0.01, vs control.
Effects of silibinin on cell cycle progression
Uncontrolled progression of the cell cycle promotes growth of cancer cells[41]. A major activity of the CD1/CDK4 complex is to initiate phosphorylation of retinoblastoma (Rb) that then fails to maintain it's binding to E2F1, and thus releases the transcription factor to promote cell cycle progression[42]. Previous studies on other cancer cell lines showed a significant inhibitory effect of silibinin on the cell cycle progression[11-13]. In the present study, we found that silibinin resulted in a significant dose-dependent inhibition of CD1/CDK4 complex that was associated with reduced Rb phosphorylation and, E2F1/DP1 complex in HuH7 cells (P < 0.01), as shown in Figure 2C-E.
By binding to the cyclin/CDK complexes, cyclin dependent kinase inhibitors (CDKIs), such as p21 and p27, halt uncontrolled cell proliferation[43]. As noted in previous studies on other cancer cell lines[12-14], we demonstrated that silibinin not only significantly increased p21 and p27 expression (P < 0.01), but also increased formation of p21/CDK4 and p27/CDK4 complexes (Figure 2F-I) in a dose-dependent fashion. Thus, our results demonstrate silibinin inhibits the growth of human hepatoma cells through inhibiting CDK activity.
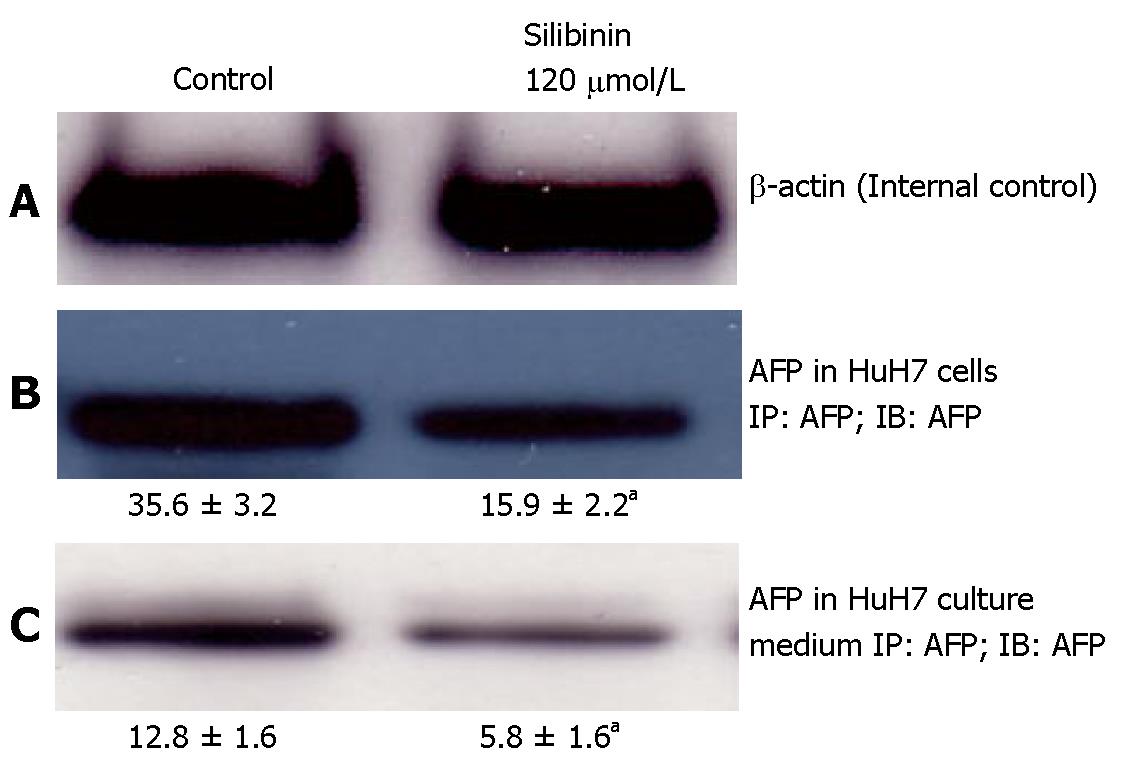
Effects of silibinin on AFP production and secretion from HuH7 cells
As shown in Figure 3B, compared to untreated HuH7 cells, silibinin at the dose of 120 μmol/L resulted in a significant decrease in AFP production in HuH7 cells (P < 0.05) that was associated with a reduced AFP level in the culture medium (Figure 3C, P < 0.05).
Figure 3 Silibinin's effects on AFP production and secretion from HuH7 cells.
A: β-actin for internal control; B: Silibinin at IC25 dose decreased AFP production in HuH7 cells; C: Silibinin at IC25 dose decreased AFP secretion from HuH7 cells. aP < 0.05 vs control.
Effects of Silibinin on Apoptosis
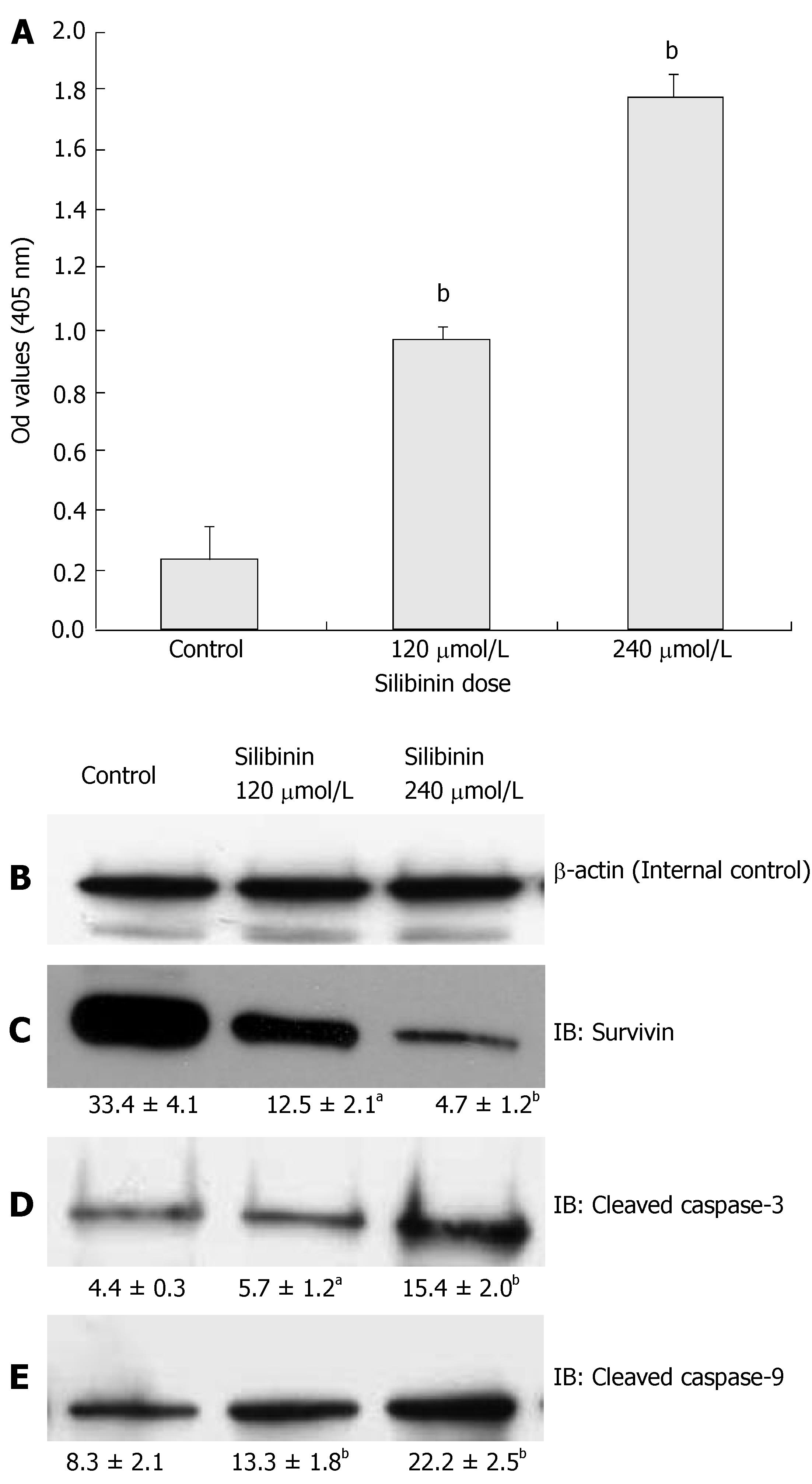
Apoptosis is another important regulatory step in contro-lling cancer cell proliferation[4]. Studies indicated that silibinin induces apoptosis in several malignant cell lines[13,14,18], but such effects have not been tested in human hepatoma cells. We demonstrated silibinin dose-dependently increases of apoptosis in HuH7 cells, as shown in Figure 4A (P < 0.01). To understand the mechanisms of silibinin-induced apoptosis, we examined the expression of Bcl-2, survivin, and activated caspase-3 and 9. Our results showed that silibinin-induced apoptosis did not alter bcl-2 expression (data not shown), but resulted in a dose-dependent inhibition of survivin expression (Figure 4C, P < 0.01) that was associated with increased levels of activated caspases-3 and -9 (Figure 4D and E, P < 0.01).
Figure 4 Silibinin's effects on apoptosis in HuH7 cells.
A: Silibinin at IC25 and IC50 doses significantly promoted apoptosis of HuH7 cells; B: β-actin for internal control. Silibinin at IC25 and IC50 doses decreased survivin expression (C), but increased activated caspase-3 (D), and activated caspase-9 (E). aP < 0.05, bP < 0.01, vs control.
Possible effects of silibinin on angiogenesis
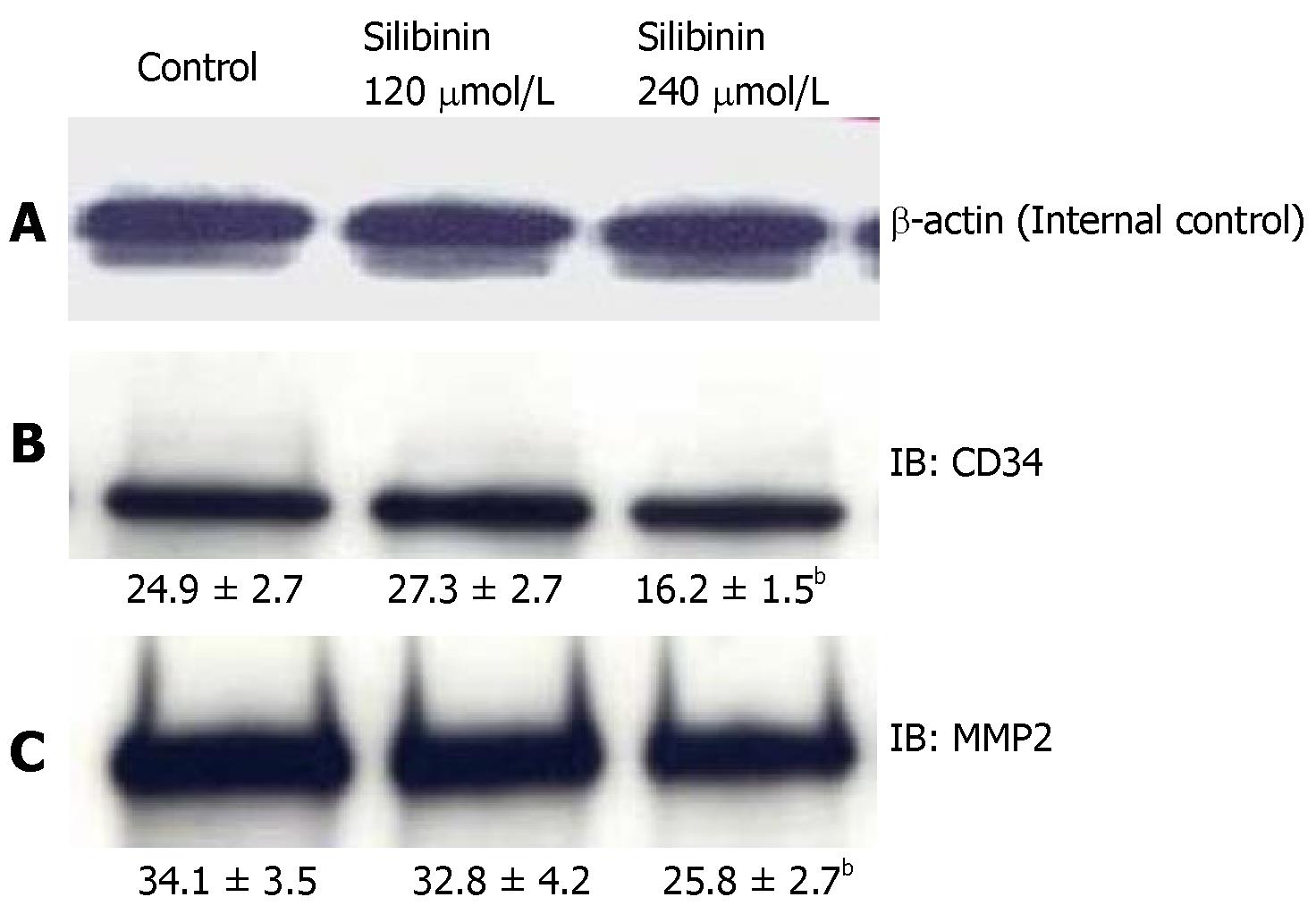
In previous studies, silibinin has been reported to inhibit angiogenesis in non-HCC cancer cell lines[13]. To evaluate whether silibinin affects angiogenesis in human HCC cells, we measured the expression of CD34, a transmembrane glycoprotein on vascular cells associated with angiogenesis[44], and MMP-2 and MMP-9, which are markers associated with angiogenesis as well as metastatic invasion[45]. As shown in Figure 5B, silibinin at IC50, but not IC25 dose decreased the expression of CD34 (P < 0.01). At the higher dose, Silibinin also resulted in decrease of MMP-2 (Figure 5C, P < 0.01), but not MMP-9 (data not shown) in HuH7 cells.
Figure 5 Silibinin's effects on angiogenesis in HuH7 cells.
A: β-actin for internal control. Silibinin decreased CD34 (B), and MMP-2 (C). bP < 0.01 vs control.
Effects of silibinin on PTEN/PI3K/Akt pathway
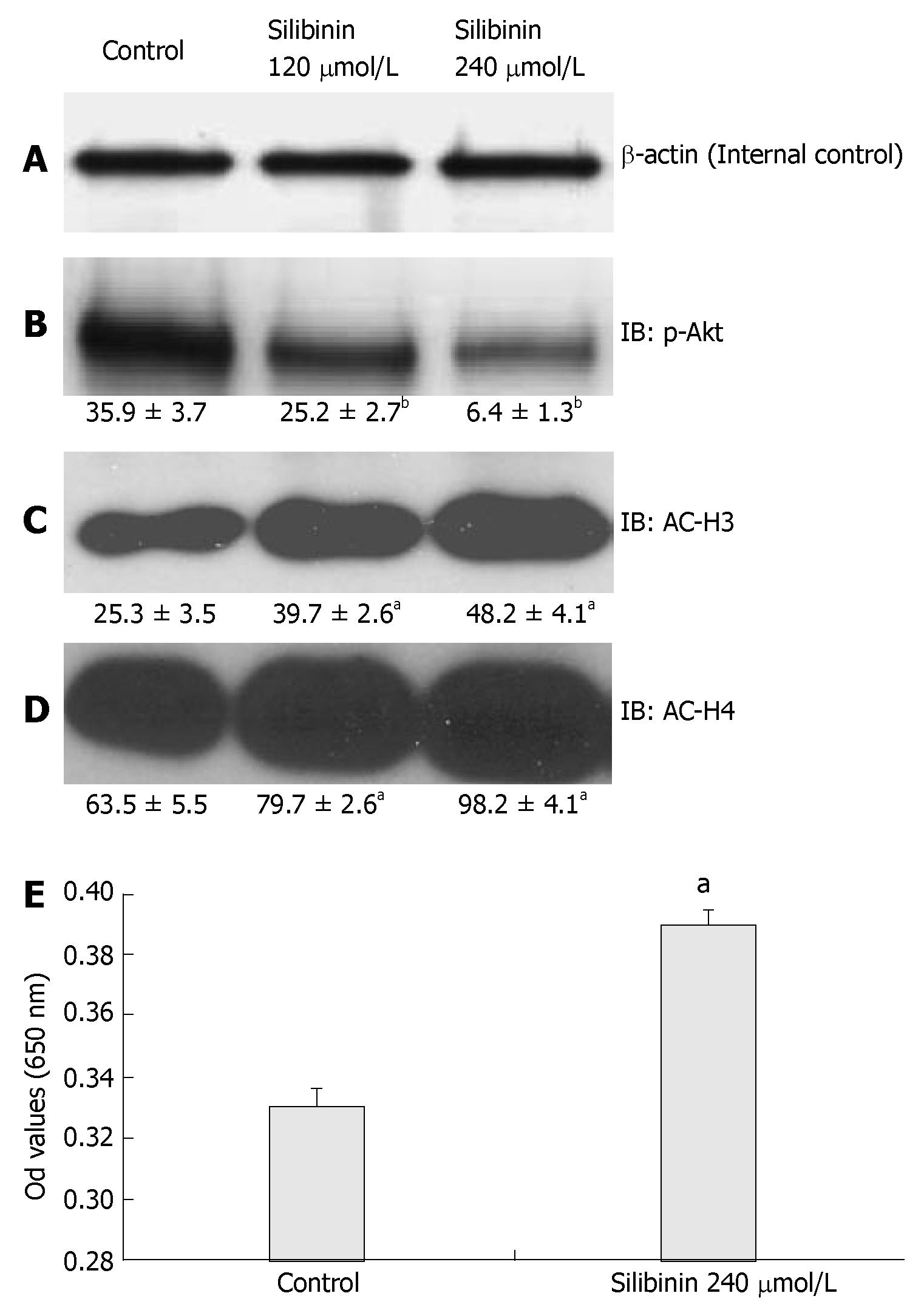
It has been reported that the PTEN/PI3K/Akt pathway is involved in cancer growth[16,46], As shown in Figure 6B, silibinin-reduced HuH7 cell proliferation was associated with a dose-dependent decrease in p-Akt (P < 0.01) in these cells. PTEN is an upstream negative regulator of Akt. It was reported that altered PTEN expression or activity is associated with the pathogenesis of HCC[47-49]. In the present study, we found that silibinin at IC50 dose did not significantly change PTEN expression (data not shown), but significantly increased PTEN activity (Figure 6E). These results suggested that the PTEN/PI3K/Akt pathway is involved in silibinin-reduced growth of human HCC cells.
Figure 6 Silibinin's Effects on PTEN, p-Akt, AC-H3 and AC-H4 in HuH7 Cells (A).
β-actin for internal control. Silibinin significantly decreased p-Akt (B); increased AC-H3 (C); AC-H4 (D). Silibinin at 240 µmol/L significantly increased PTEN activity in HuH7 cells (E). aP < 0.05, bP < 0.01, vs control.
Effects of silibinin on AC-H3 and AC-H4 expression
We then examined the association of AC-H3 and AC-H4 expression with silibinin-reduced HCC cell growth. Our results demonstrated that silibinin-reduced HuH7 cell growth was associated with increased AC-H3 and AC-H4 expression (Figure 6C and D, P < 0.05). These results suggest that increased AC-H3 and AC-H4 expression may play an important role in silibinin-reduced HCC growth.
DISCUSSION
Searching for an effective chemoprevention of HCC has been an active field of research. Silibinin is a polyphenolic flavonoid and the major biologically active compound of milk thistle. It is well known that milk thistle is safe and well tolerated, and it protects the liver from drug or alcohol-related injury[7,8]. Recent demonstration of silibinin's anti-HCC effects[21,22] provided us with a rationale to further define the related effects and mechanisms of HCC chemoprevention. In the present study, we examined the effects and mechanisms of silibinin on growth of human HCC cells.
Using MTT assay[37,38], we demonstrated that silibinin treatment resulted in a potent inhibition of four different human HCC cell lines, indicating its broad spectrum of anti-HCC effects. We also revealed silibinin's linear dose-dependent inhibition of HuH7 cell growth. Silibinin at IC25 and IC50 doses for HuH7 cells also resulted in reduced growth of HepG2, Hep3B, and PLC/PRF/5 cells, confirming the previous reports[21,22], These results promote us to further test silibinin for HCC chemoprevention.
Both PCNA and Ki-67 are biomarkers for cell proliferation[40]. Singh et al[11] reported silibinin significantly decreases PCNA and Ki-67 expression in nude mice bear-ing xenografts of human prostate cancer. Consistent with this, we have demonstrated that silibinin significantly reduced Ki-67 expression in HuH7 cells in a dose-dependent fashion. These suggest that silibinin reduces growth of human HCC cells by down regulating their proliferation.
AFP is associated with HCC differentiation and has been widely used for diagnosing HCC and assessing treatment effects or recurrence of HCC in humans[24,25]. Our results showed that silibinin treatment resulted in significant decrease in AFP production and secretion that was well correlated with growth inhibition of HuH7 cells. These findings suggest silibinin may promote HCC cell differentiation, and AFP may serve as a non-invasive biomarker to determine silibinin's in vivo anti-HCC effects.
An uncontrolled G1-S progression results in continued proliferation with potential malignant transformation and carcinogenesis. Increased CDK4/CD1 complex enhances Rb phosphorylation that results in release of E2F1 from p-Rb/E2F1 complex and promotes E2F1/DP1 complex formation and stimulates cell cycle progression[42]. Tyagi et al[10] reported that silibinin causes a significant decrease in p-Rb in human prostate cancer cells. Our results indicate that silibinin-inhibited CDK4/CD1 complex formation is one of the important steps that inhibit Rb phosphorylation, followed by reduction of E2F1/DP1 complex formation.
CDKIs are important regulators of the activity of the CD1/CDK4 complex. By binding to the cyclin/CDK complexes, two very important CDKIs, p21 and p27, inhibit their activities. Varghese, et al[22] reported that silibinin increases levels of p27 in human hepatoma cells. Silibinin was also reported increasing expression of p21 in several non-HCC cancer cells[14]. In the present study, we demonstrated that silibinin resulted in a significantly dose-dependent increase in both p21 and p27, which was well correlated with their respective binding to CDK4, the bioactive forms of these CDKIs. Taken together, our data demonstrated that silibinin reduces cell cycle progression in human hepatoma cells by arresting G1-S transition that involves a comprehensive signaling of cell cycle modulators.
Previous data on other cancer cell lines have demon-strated that silibinin has effects on the apoptotic con-trol[10,14,21]. We demonstrated that silibinin causes a signifi-cant increase in apoptosis of HuH7 cells which was asso-ciated with decreased survivin expression and increased activated caspase-3 and -9. Because survivin can bind with caspases[19,50,51], our results suggest that silibinin-induced apoptosis of HuH7 cells is mediated by decreased survivin that results in increased caspase-3 and 9 activation.
Angiogenesis is an important aspect of cancer invasion and survival. CD34 is a valuable marker to demonstrate this issue[44]. A previous study demonstrated that silibinin decreases angiogenesis in colon cancer cells[15]. In the present study, we revealed that silibinin at IC50 decreased CD34 protein expression. MMP-2 and MMP-9 have been used as markers for angiogenesis and malignant invasion[45]. It was reported that silibinin resulted in a significant decrease in MMP-2, but not MMP-9 levels in human lung cancer cells[20]. In the present study, we found that silibinin also resulted in a dose-dependent and significant decrease in MMP-2, but not MMP-9 expression. Although our data suggest that silibinin may reduce angiogenesis in human HCC cells, further in vivo studies with quantification of microvessel density[52] will be needed to validate these findings.
There is growing evidence of PTEN/PI3K/Akt path-way in hepatocarcinogenesis[27,32,47-49]. PTEN is a tumor suppressor gene and the deletion or inactivation of this gene has been described in a variety of cancer cell lines[30,33,53]. As a result, the tumor suppressive properties of PTEN relates in part to its ability to down-regulate the Akt pathway and thus inhibit cell proliferation[32,33]. Paramio et al[54] showed that PTEN decreases p-Rb and resulted in down-regulation of CD1. Furthermore, Weng et al[55] demonstrated through the use of breast cancer cells that PTEN also up-regulates p27 and down-regulates CD1. However, it remains to be determined whether PTEN/PI3K/Akt pathway is involved in silibinin-reduced growth of cancers. In the present study, we found that silibinin significantly increased PTEN activity in association with decreased p-Akt in HuH7 cells. Since silibinin treatment also resulted in significant decrease of p-Rb and proliferation in these cells, it is evident that silibinin alters PTEN activity to assist in cellular growth control through downstream regulation of Akt and also possibly in promoting the up-regulation of p27 and the down-regulation of both p-Rb and CD1 as suggested by previous studies[54]. It was also reported that overexpression of PTEN reduces survivin expression[56]. We found that silibinin-mediated increase in PTEN activity and decrease in p-Akt was associated with decreased survivin expression and enhanced apoptosis in HuH7 cells. These data support the notion that PTEN/PI3K/Akt pathway may mediate cancer cell apoptosis by modulating surviving expression, and silibinin may play an important role in this interaction. Additional studies will be needed to further detail the role of PTEN/PI3K/Akt signaling in silibinin-reduced growth of human HCC cells.
Histone acetylation alters chromatin conformation by making promoter regions more accessible to transcription factors and permissive to transcriptional activation[34]. Studies have reported that histone acetylation is involved in cell proliferation, differentiation, and cell cycle regulation[34]. Decrease in acetylation status in the cell is associated with carcinogenesis[35,36]. Our results demonstrated that silibinin-reduced HuH7 cell growth was significantly associated with increased AC-H3 and AC-H4 expression, suggesting that increased histone acetylation may mediate silibinin-reduced HCC growth. Our findings not only indicate silibinin's novel anti-cancer mechanisms, but also provide additional targets for searching new agents for HCC chemoprevention.