Published online Oct 7, 2007. doi: 10.3748/wjg.v13.i37.4986
Revised: July 2, 2007
Accepted: July 26, 2007
Published online: October 7, 2007
AIM: To define NGF (nerve growth factor) and its high-affinity receptor trkANGF presence and distribution in fibrotic liver and in HCC, and to verify if NGF might have a role in fibrosis and HCC.
METHODS: Intracellular distribution of NGF and trkANGF were assessed by immunohistochemistry and immuno-electron microscopy in liver specimens from HCC, cirrhosis or both. ELISA was used to measure circulating NGF levels.
RESULTS: NGF and trkANGF were highly expressed in HCC tissue, mainly localized in hepatocytes, endothelial and some Kupffer cells. In the cirrhotic part of the liver they were also markedly expressed in bile ducts epithelial and spindle-shaped cells. Surprisingly, in cirrhotic tissue from patients without HCC, both NGF and trkANGF were negative. NGF serum levels in cirrhotic and/or HCC patient were up to 25-fold higher than in controls.
CONCLUSION: NGF was only detected in liver tissue with HCC present. Intracellular distribution suggests paracrine and autocrine mechanisms of action. Better definition of mechanisms may allow for therapeutic and diagnostic/prognostic use of NGF.
- Citation: Rasi G, Serafino A, Bellis L, Lonardo MT, Andreola F, Zonfrillo M, Vennarecci G, Pierimarchi P, Vallebona PS, Ettorre GM, Santoro E, Puoti C. Nerve growth factor involvement in liver cirrhosis and hepatocellular carcinoma. World J Gastroenterol 2007; 13(37): 4986-4995
- URL: https://www.wjgnet.com/1007-9327/full/v13/i37/4986.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i37.4986
NGF (nerve growth factor) is a prototypical member of the neurotrophin family essential for survival, differentiation, and maintenance of neuronal cells in the central and peripheral nervous system[1]. In recent years, many findings have indicated that NGF could also have a role outside the central and peripheral nervous system. In particular, it may be involved in lung and skin tissue repair[2] as well as in allergic inflammation and fibrosis[3]. Increased levels of circulating NGF were observed in several autoimmune, chronic inflammatory and fibrotic disorders[4,5]. Numerous data also indicate that NGF is involved in tumour growth, invasion and metastasis[6-14].
Two types of receptors mediate NGF effects: trkANGF, the high-affinity receptor with protein kinase activity, specific for NGF, and p75NTR, the low-affinity glycoprotein receptor, also binding other neurotrophins[15,16]. Most of the biological activities elicited by NGF are mediated by binding to trkANGF receptor[17,18]. TrkANGF, p75NTR receptors or both, together with NGF, are expressed in various cancers, including lung[7], breast[12] and prostate cancer[10,11,13], suggesting that the NGF autocrine or paracrine pathway may have a role in tumorigenesis. NGF is also shown to be over expressed in lung and skin fibrotic process[2] as well as in liver during experimental fibrotic injury[19]. However, the role of NGF in tissue remodelling, fibrotic process and cancer progression is still controversial.
Hepatocellular carcinoma (HCC) is one of the most common cancers in the world, showing a rapid progressive clinical course, poor response to pharmacological treatment and a severe prognosis[20,21]. A major risk factor for HCC is hepatitis C virus (HCV)-related cirrhosis[22-25]; many chronically infected patients remain asymptomatic for a long period, with liver cirrhosis developing after approximately 30 years[26,27]. The lack of predictive markers that can detect the beginning of liver cirrhosis in chronic HCV patients may also contribute to the late diagnosis of HCC, its progression and the poor prognosis.
The expression of NGF and its receptors in liver tissue during fibrotic injury and HCC has been previously investigated mostly in animal models with contradictory results. Some authors demonstrated that hepatocytes expressed NGF but not trkANGF during profibrotic liver injury, or in early preneoplastic lesions and HCCs. TrkANGF was only expressed in the walls of arteries associated with tumors[28,29]. These data suggest that in hepatocytes NGF may not be an autocrine factor, but may rather act in a paracrine fashion. On the contrary, other authors[19] reported the presence of trkANGF mRNA, but not of NGF mRNA in hepatocytes of fibrotic rat liver. It is not clear from the literature which cell types (apart the hepatic stellate cells) constituting the whole liver tissue are actually involved in the cross-talk mediated by NGF during liver tissue remodelling processes and HCC progression.
In this study we investigated the expression of NGF and its high-affinity receptor trkANGF in patients suffering from HCC, cirrhosis or both. This was performed to verify if the expression of this neurotrophin may be correlated with tissue remodelling processes and HCC progression. We also studied the expression of NGF and trkANGF in the different cell types inside the fibrotic and HCC tissues, analyzing the intracellular distribution in each cell type at an ultrastructural level, attempting to clarify their involvement in the hepatic damage. Circulating NGF levels were also measured.
20 human liver specimens, taken for diagnostic purposes or resected before transplantation, were used in this study: 5 near normal liver biopsy specimens, 4 with cirrhosis without HCC from transplanted patients (Child-Pugh A), 11 with cirrhosis and HCC post-hepatitis C [Child-Pugh A (n = 4); Child-Pugh C (n = 7)]. All the patients had voluntarily signed a written consent to participate the study. The diagnosis was based on histopathologic examination of routinely processed tissue together with clinical and laboratory data. Each specimen was received fresh, and fixed in part in 10% buffered formalin solution for immunohistochemistry, and the other part in 1% or 2.5% glutharaldehyde for immunogold labelling or morphological analysis by transmission electron microscopy observation, as described below.
Cell types in liver tissues from cirrhotic and HCC patients have been identified on the basis of microscopic and ultramicroscopic morphological feature. In particular: (1) Endothelial cells: elongated narrow cells composing the wall of sinusoids; (2) Kupffer cells: macrophage cells, exhibiting considerable phagocytic ability, bulging into sinusoidal lumen; (3) Biliary epithelial cells: tightly arranged cuboid or columnar cells, constituting the wall of bile ducts, lying on a basement membrane and bearing microvilli on the luminal surface; (4) Spindle-shaped cells: elongated fibroblast-like cells embedded in fibrous tissue, possessing characteristics of myofibroblast, as shown by positive staining for smooth muscle actin (SMA).
Expression of NGF and trkANGF in normal liver, cirrhotic and HCC tissues, obtained as described above, was investigated by Confocal Laser Scanning Microscopy (CLSM) using an indirect immunofluorescence technique. Immuno labelling was performed on sections obtained from paraffin embedded tissues using rabbit polyclonal antibodies against NGF (Chemicon Int., Temecula, CA, USA) or trkANGF (Santa Cruz Biothech., Santa Cruz, USA). Primary antibodies detection was obtained by a reaction with TRITC-conjugated goat anti-rabbit IgG. To test the specificity of the immunoreaction, for each liver specimen examined and on a section close to that used for NGF or trkANGF staining, negative controls were performed by substitution of primary antisera with non-immune rabbit serum. Fluorescently labelled samples were imaged by the confocal microscope LEICA TCS 4D (Leica, Heidelberg, Germany) supplemented with an Argon/Krypton laser and equipped with 40 × 1.00-0.5 and 100 × 1.3-0.6 oil immersion lenses. The excitation/emission wavelengths employed were 488 nm/510 nm, for green auto-fluorescence (used to visualize liver tissue morphology), and 568 nm/590 nm for TRITC-labelling. During the observation, both fluorescent signals were obtained simultaneously. To better visualize the intracellular distribution of NGF or trkANGF along the thickness of tissue sections, confocal sections were taken at intervals of 0.5 μm, a 3D reconstruction image for each fluorescent signal was recorded, and merged images of the two signals were obtained using the confocal microscope software.
To determine the nature of spindle-shaped cells, double staining using rabbit polyclonal antibodies against NGF (Chemicon Int.) and mouse monoclonal antibody against smooth muscle actin (SMA, Sigma-Aldrich Co., St. Louis, Mo, USA) was performed. Anti-SMA detection was obtained by a reaction with Alexa Fluor 488-conjugated rabbit anti-mouse IgG (Molecular Probes Inc., Eugene, OR).
For the assessment of staining intensity, a minimum of three section for each sample were examined and the different level of positive immunoreaction was evaluated on the basis of number of positive cells and fluorescence intensity. The following criteria were used for evaluation of staining: (-), same as the background obtained in control performed by substitution of specific anti-NGF or trkANGF antibodies with non-immune rabbit serum; (±), moderately higher than the background; (+), higher than the background; (++), much higher than the background.
For histological observation, sections obtained from paraffin embedded tissues and close to that used for immunohistochemistry were stained with haematoxylin-eosin (HE) and observed by an optical microscope.
For ultrastructural observation, samples of normal liver, cirrhotic and HCC tissues were fixed with 2.5% glutharaldehyde in 0.1 mol/L Millonig’s phosphate buffer (MPB), and then post-fixed with 1% OsO4 in the same buffer. Samples were dehydrated in increasing concentrations of ethanol and embedded in Spurr epoxy resin (Agar Scientific LTD, Stansted, Essex, UK). For immuno-electron microscopy, tissue samples were fixed with 1% glutharaldehyde in 0.1 mol/L MPB, dehydrated in increasing ethanol concentrations and embedded in LR White acrylic resin (Agar Scientific LTD). Immunogold labelling was performed on ultrathin sections using the same rabbit polyclonal antibodies against NGF or trkANGF used for immunohistochemistry. Negative controls were performed by substitution of primary antisera with non-immune rabbit serum. Detection of primary antibodies was obtained by the reaction with 10 nm gold particles-conjugated goat anti-rabbit IgG. For morphological and immunogold labelling observation, ultrathin sections were stained with uranyl acetate and lead citrate and observed under a Philips CM12 transmission electron microscope operating at 80 kV.
Circulating NGF levels were measured in sera from 27 patients with a documented cirrhosis, HCC or both, by a modified highly sensitive two-site immunoenzymatic assay (ELISA), using anti-NGF antibodies (clone 27/21, Chemicon Int.)[30,31]. This assay specifically identifies human NGF but not brain-derived neurotrophic factor, with a detection limit of 0.5 pg/mL.
The results of NGF and trkANGF immunostaining in all the human liver specimens examined are summarized in Table 1.
| N°11 Patients | Cirrhotic liver | HCC | ||||
| ID# | Etiology | Child-Pugh | NGF | trkANGF | NGF | trkANGF |
| 2 | HCV | A | ++ | ++ | + | + |
| 4 | HCV | A | + | + | ± | ± |
| 5 | HCV | A | + | + | + | ± |
| 10 | HBV | A | ± | + | ± | ± |
| 3 | HCV | C | ++ | ++ | + | + |
| 11 | HCV | C | ± | + | + | + |
| 14 | HCV/HBV/HDV | C | + | ++ | ± | + |
| 16 | HCV | C | + | ± | + | + |
| 15 | HCV | C | na | na | + | ++ |
| 17 | HCV | C | na | na | ± | ++ |
| 18 | HCV | C | na | na | ± | + |
| N° 4 Patients | Cirrhotic liver | |||||
| ID# | Etiology | Child-Pugh | NGF | trkANGF | ||
| 20 | HCV | C (transplantation) | - | - | ||
| 21 | HCV | C (transplantation) | - | - | ||
| 22 | HCV | C (transplantation) | - | - | ||
| 23 | HCV | C (transplantation) | ± | - | ||
| N° 5 patients | Normal liver | |||||
| ID# | NGF | trkANGF | ||||
| 1 | - | - | ||||
| 7 | - | - | ||||
| 8 | - | - | ||||
| 9 | - | - | ||||
| 13 | - | - | ||||
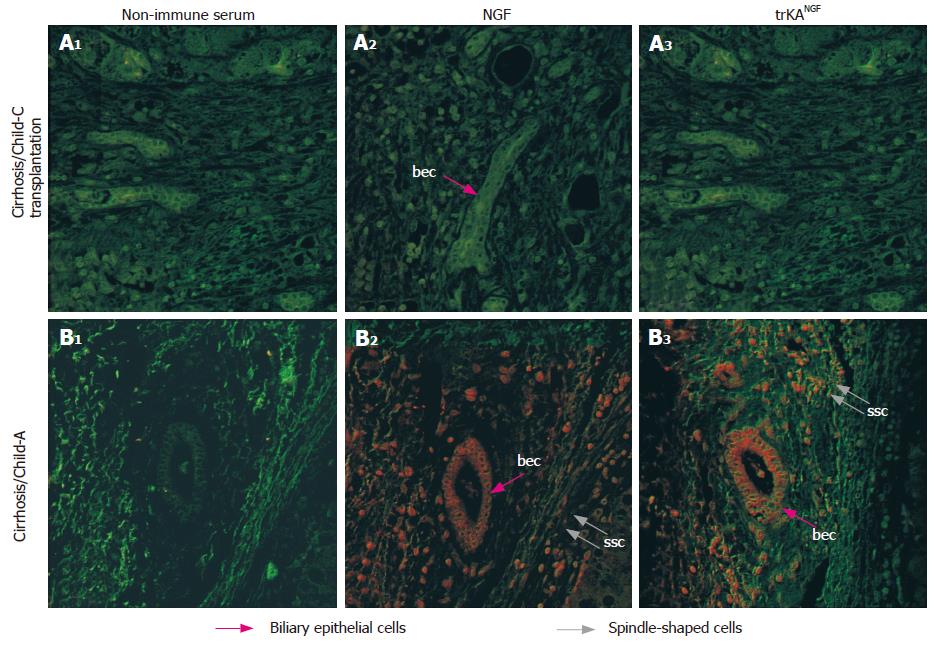
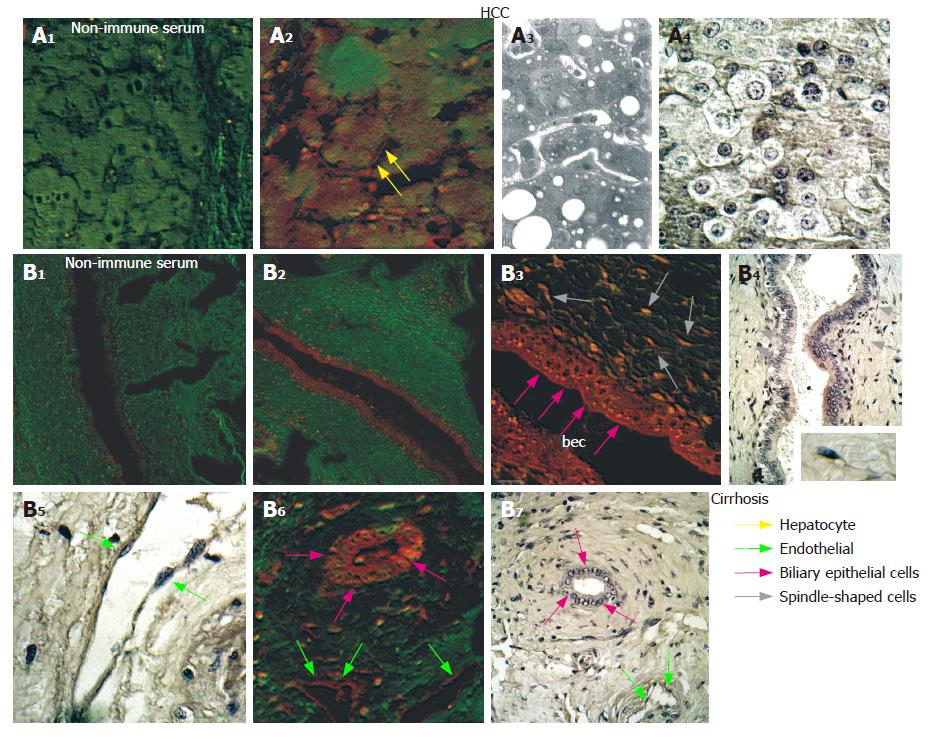
Analysis of NGF distribution by immunohisto-chemistry and CLSM observation indicated that all 11 patients with cirrhosis and HCC were positive, while the 5 normal liver biopsy specimens as well as the 4 samples from transplanted patients with cirrhosis without HCC, were negative. In HCC tissues NGF was detectable in a high number of cells at different levels of intensities, depending on the patient (Table 1, Figure 2) but never in normal liver tissue (Table 1, Figure 1). Interestingly, in liver from patients with cirrhosis (Child-Pugh C, undergoing transplantation) but without HCC, NGF and trkANGF were negative (Table 1 and Figure 3A2 and 3A3). Conversely NGF and trkANGF were markedly positive in patients with cirrhosis that had evolved into HCC, already at early staging (Child- Pugh A; Table 1 and Figure 3B2 and 3B3).
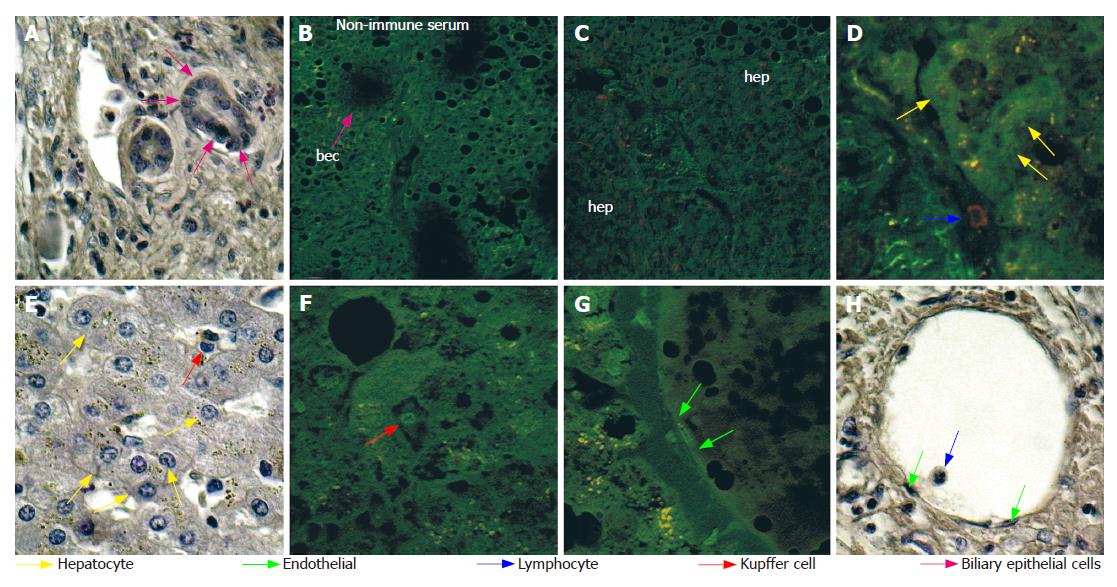
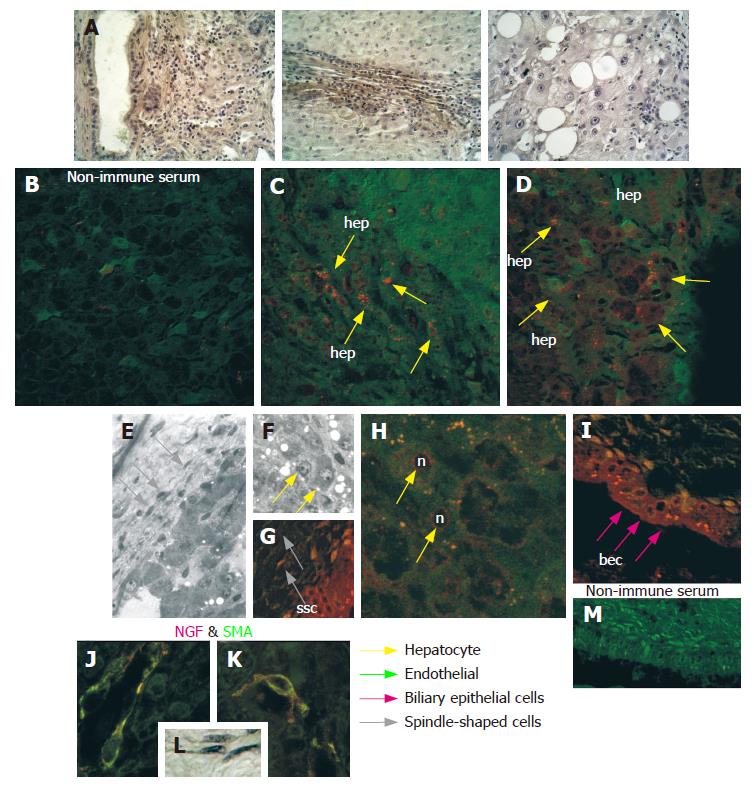
Furthermore, both the number of positive cells and the intensity of immunoreaction were significantly elevated in cirrhotic tissues with respect to the HCC tissues obtained from the same liver (Table 1 and Figure 4). Moreover in HCC tissues, NGF was mainly localized in the cytoplasm and on the nuclei of hepatocytes, and to a lesser extent on endothelial and Kupffer cells and some lymphocytes (Figure 2). However, in cirrhotic tissue, it was also markedly expressed on biliary epithelial cells constituting the wall of bile ducts, and on spindle-shaped cells embedded in fibrous tissue (Figure 4). Double staining using anti-NGF and antibody against SMA (Figure 4J and K) indicated that these spindle-shaped cells presumably correspond to the hepatic stellate cells described by other authors[19].
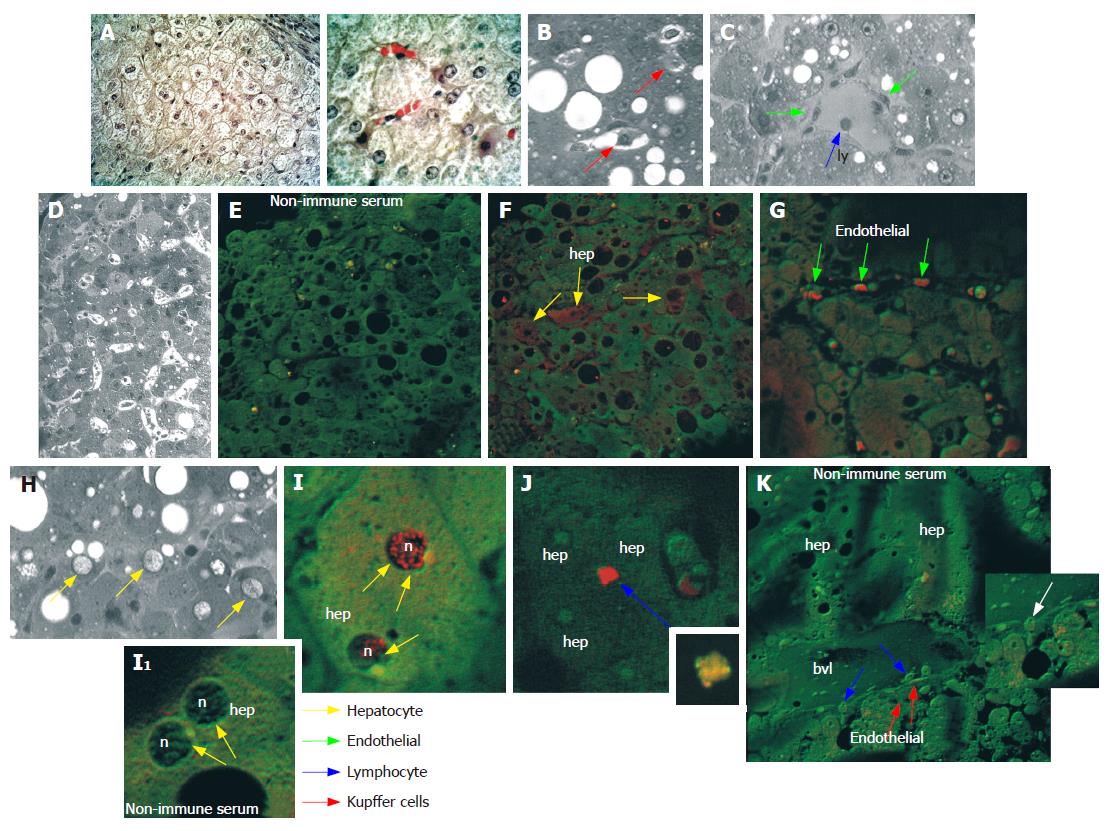
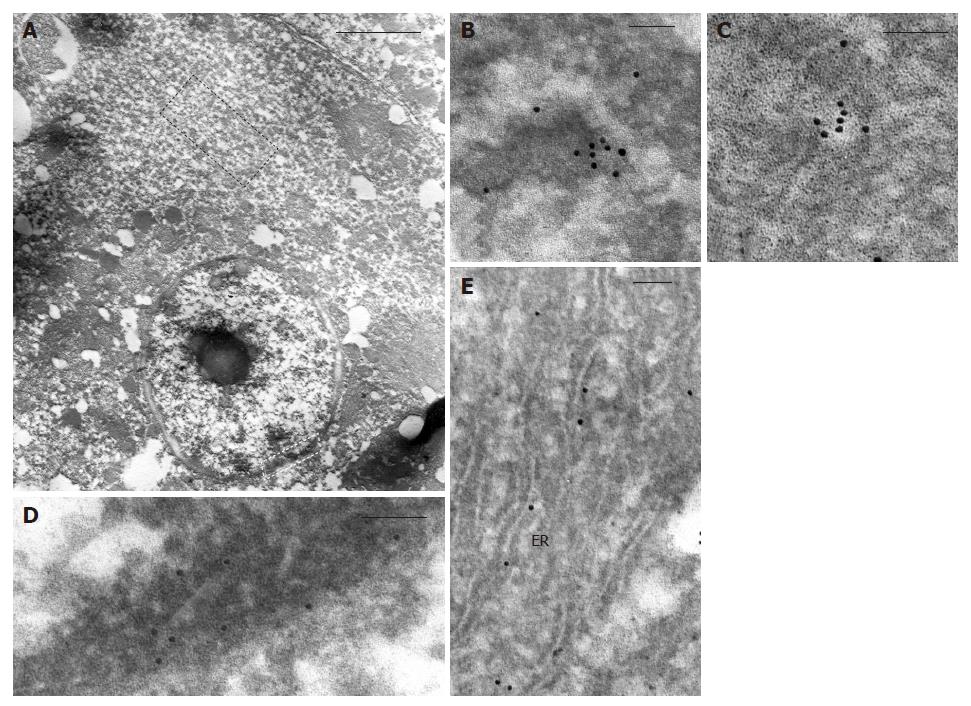
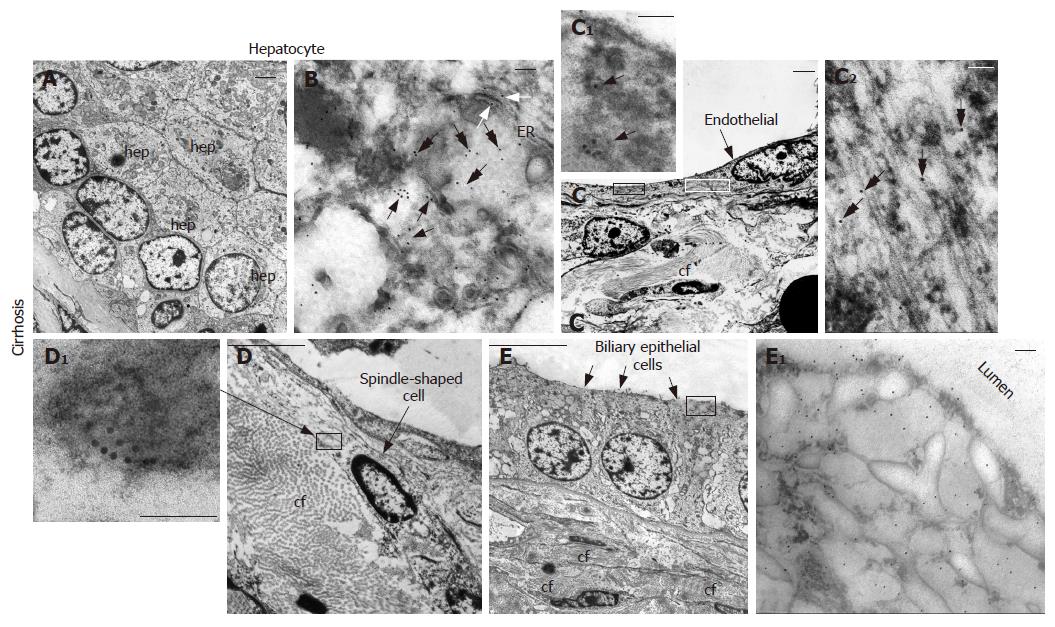
Ultrastructural observation by transmission electron microscopy after immunogold labelling showed that in hepatocytes of HCC tissue (Figure 5), positive reaction for NGF mainly localized on cytoplasmic vesicles (Figure 5B and C) and on endoplasmic reticulum (Figure 5E), suggesting that synthesis and accumulation of this molecule may occur. In some cases, immunogold labelling was also present on nuclei, beneath the nuclear membrane (Figure 5D). Furthermore, in cirrhotic tissue (Figure 6) a more intense immunoreaction, with respect to HCC tissue, was observed on cytoplasmic vesicles (Figure 6B), free in the cytoplasm (Figure 6B) and along endoplasmic reticulum (Figure 6B) of hepatocytes, indicating that in cirrhotic tissue NGF may be actively produced by hepatocytes. Positive immunogold reaction was also observed on cytoplasmic vesicles of endothelial cells (Figure 6C) and of spindle-shaped cells (Figure 6D). In biliary epithelial cells NGF localized in large cytoplasmic vacuoles that were present in the portion of cells near the ductal lumen (Figure 6E).
Immunohistochemical staining and CLSM observation showed that in HCC tissue trkANGF localized on the cell membrane of few hepatocytes (Figure 7A2), while in cirrhotic tissue an intense immunoreactivity was also observed on biliary epithelial cells (Figure 7B3 and 7B6). This was particularly evident beneath the cell membrane near the ductal lumen (Figure 7B3), and on spindle-shaped cells scattered throughout fibrotic tissue surrounding reactive bile ducts (Figure 7B6). Positive immunoreaction was also observed on endothelial cells constituting the wall of blood vessels (Figure 7B6).
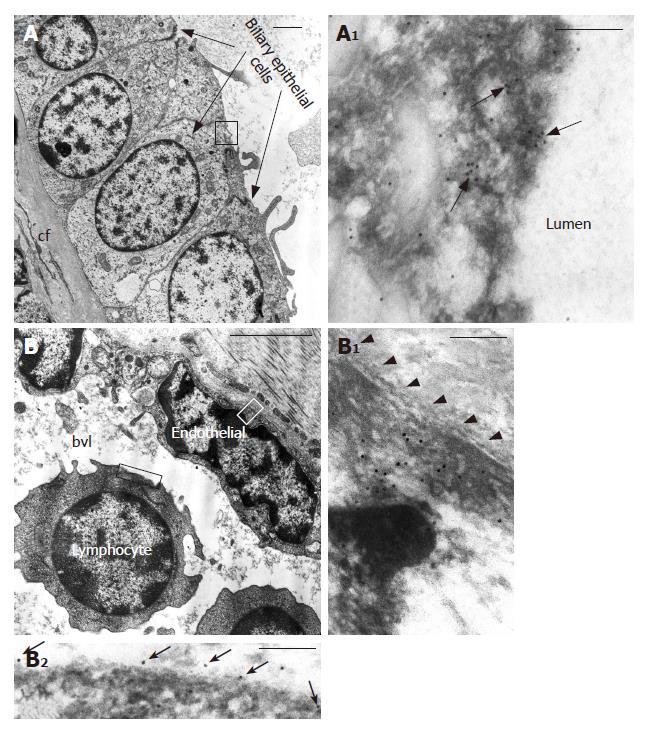
Furthermore, in cirrhotic tissue, immunogold labelling showed that in biliary epithelial cells (Figure 8A) trkANGF localized on cell membrane and on cytoplasmic vesicles beneath it (Figure 8A1), suggesting a possible receptor recycling after receptor-ligand reaction. Immunoreactivity was also observed in the cytoplasm of some endothelial cells (Figure 8B1) and on cell membrane of some lymphocytes (Figure 8B2).
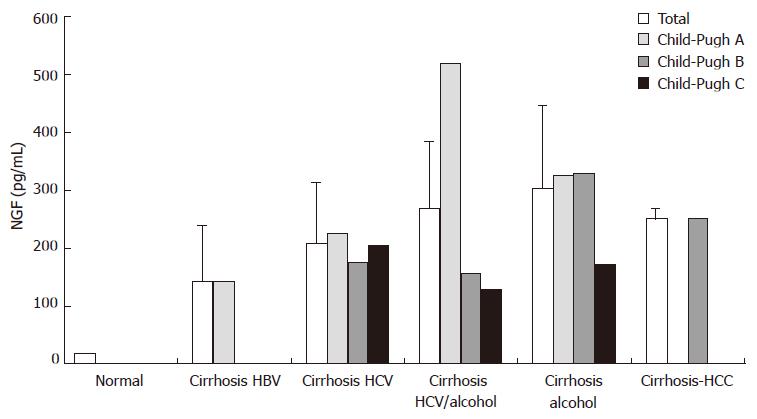
NGF levels were measured in sera obtained from 27 patients with documented cirrhosis, HCC, or both by immunoenzymatic assay. All patients disclosed with circulating NGF levels elevated 25-fold over the normal with (range 73-520 pg/mL, compared to a mean of 20 pg/mL in healthy donors, Figure 9).
The expression of NGF and its receptors in human and rodent liver cells has been investigated for some time in previous studies with contradictory results. Oakley et al[29] demonstrated for example, that hepatocytes expressed NGF, but not trkANGF during profibrotic liver injury, in early preneoplastic lesions and HCCs, suggesting that in the hepatocytes NGF may not be an autocrine factor but may rather act in a paracrine manner. Other studies[28] reported that in HCC tissue from male B6C3F1 mice, trkANGF is expressed exclusively in the walls of arteries associated with tumors, presumably in smooth muscle cells, whereas it is negative in other cell types including tumor cells. The same authors also demonstrated that NGF was expressed not only in HCCs but also in early preneoplastic lesions, possibly representing a very early change during mouse hepatic carcinogenesis. On the contrary, Cassiman et al[19] provided evidence that in human and rat cirrhotic tissues, NGF and other neurotrophins, as well as their receptors, were expressed not only in hepatic stellate cells, but also in regenerating bile ducts and in some hepatocytes with a cytoplasmic or nuclear staining patterns. Recently it has been reported that NGF is over expressed in approximately 60% of human HCC tissues compared to the surrounding liver tissue with cirrhosis and chronic hepatitis, suggesting a role for NGF in the progression of HCC[32]. Also, until now, to our knowledge, it has not yet been clarified what role NGF plays and which cell types in the liver tissue are actually involved in the NGF mediated cross-talk during liver fibrosis and HCC progression. In addition, even if increased levels of circulating NGF have been reported in several autoimmune, chronic inflammatory and fibrotic disorders[4,5], meanwhile data concerning circulating NGF levels in HCC or cirrhotic patients are not known.
In this study we provide immunohistochemical evidence that NGF and its high-affinity receptor trkANGF are over expressed in patients suffering from HCC and to a greater extent from HCC with cirrhosis. Surprisingly, NGF and trkANGF were negative in liver specimens from patients with cirrhosis undergoing transplantation but without HCC, supporting the hypothesis of an active role for NGF in HCC insurgence and progression. The elevated circulating NGF levels recorded in sera from patients with documented cirrhosis, HCC or both, as well as the tissue distribution of NGF and its receptor further support the correlation between NGF activity and the progression of liver fibrosis towards HCC. We also have been able to identify the NGF and trkANGF immuno positive cell types in liver tissues from cirrhotic and HCC patients, comparing the results obtained by immunohistochemistry and immuno-electron microscopy, as summarized in Table 2.
| Hepatocytes | Biliary epithelial cells | Endothelial cells | Spindle-shaped cells | Lymphocytes | Kupffer cells | Determination methods | |||||||
| Health | NGF | - | NGF | - | NGF | - | NGF | nd | NGF | + | NGF | - | CLSM (IF) |
| trkANGF | - | trkANGF | - | trkANGF | - | trkANGF | nd | trkANGF | nd | trkANGF | - | ||
| HCC | NGF | +1 | NGF | ± | NGF | + | NGF | nd | NGF | + | NGF | + | CLSM (IF) |
| trkANGF | ± | trkANGF | ± | trkANGF | ± | trkANGF | nd | trkANGF | nd | trkANGF | ± | TEM (IG) | |
| CIRR | NGF | ++1 | NGF | ++2 | NGF | ++ | NGF | + | NGF | + | NGF | ± | CLSM (IF) |
| trkANGF | ++ | trkANGF | ++2 | trkANGF | + | trkANGF | + | trkANGF | + | trkANGF | + | TEM (IG) | |
The evidence that hepatocytes in HCC and cirrhotic tissues from the same liver produce NGF (as suggested by the localization on cytoplasmic vesicles and on endoplasmic reticulum) and express its receptor strongly support the hypothesis that NGF may act by both autocrine and paracrine mechanisms, rather than a prevalent paracrine one, as previously suggested[29].
NGF and trkANGF have been reported over expressed in vivo in proliferating cholangiocytes after bile duct ligation. NGF also stimulates rat cholangiocyte proliferation in vitro[33]. We observed that in cirrhotic tissue both NGF and its receptor are over expressed on proliferating biliary epithelial cells, mainly localized on large cytoplasmic vacuoles in the ductal lumen portion of cells suggesting an intraductal lumen secretion during the progression from cirrhosis to HCC. Other studies[34,35] have shown that binding of NGF to its cognate receptor trkANGF induces formation of signalling endosomes containing both the NGF and activated trkANGF receptor, with the latter exhibiting very high dynamic trafficking between the cell surface and internal cell compartments. Therefore, the predominant staining on cytoplasmic vacuoles observed for trkANGF on biliary epithelial cells in cirrhotic tissue may represent the receptor endocellular trafficking. Taken together, these findings indicate that NGF and its related receptor play an important role also in modulating the physiopathology of the intrahepatic biliary epithelium in the course of liver tissue remodelling processes and HCC progression.
The mechanism of NGF involvement in liver tissue remodelling processes and HCC progression remain unclear. However, our observation, defining NGF distribution both inside the liver and in the intracellular compartments, indicate that it may function, in a paracrine and an autocrine manner, as a messenger molecule in the cross-talk between different cell types. This, in addition to the reported lack of NGF expression in cirrhotic tissue without concomitant HCC even if present at high level in serum, strongly support the need to further clarify the mechanism and to define the role of this neurotrophin.
This opens up an interesting perspective for the possible use of NGF, not only as a marker of progression and transformation, but also as an attractive target for a new therapeutic approach.
S- Editor Liu Y L- Editor Kremer M E- Editor Lu W
| 1. | Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2261] [Cited by in RCA: 2249] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 2. | Micera A, Vigneti E, Pickholtz D, Reich R, Pappo O, Bonini S, Maquart FX, Aloe L, Levi-Schaffer F. Nerve growth factor displays stimulatory effects on human skin and lung fibroblasts, demonstrating a direct role for this factor in tissue repair. Proc Natl Acad Sci USA. 2001;98:6162-6167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Micera A, Puxeddu I, Aloe L, Levi-Schaffer F. New insights on the involvement of Nerve Growth Factor in allergic inflammation and fibrosis. Cytokine Growth Factor Rev. 2003;14:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Aloe L, Tuveri MA. Nerve growth factor and autoimmune rheumatic diseases. Clin Exp Rheumatol. 1997;15:433-438. [PubMed] |
| 5. | Bonini S, Lambiase A, Bonini S, Levi-Schaffer F, Aloe L. Nerve growth factor: an important molecule in allergic inflammation and tissue remodelling. Int Arch Allergy Immunol. 1999;118:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Revoltella RP, Butler RH. Nerve growth factor may stimulate either division or differentiation of cloned C1300 neuroblastoma cells in serum-free cultures. J Cell Physiol. 1980;104:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Oelmann E, Sreter L, Schuller I, Serve H, Koenigsmann M, Wiedenmann B, Oberberg D, Reufi B, Thiel E, Berdel WE. Nerve growth factor stimulates clonal growth of human lung cancer cell lines and a human glioblastoma cell line expressing high-affinity nerve growth factor binding sites involving tyrosine kinase signaling. Cancer Res. 1995;55:2212-2219. [PubMed] |
| 8. | Bold RJ, Ishizuka J, Rajaraman S, Perez-Polo JR, Townsend CM, Thompson JC. Nerve growth factor as a mitogen for a pancreatic carcinoid cell line. J Neurochem. 1995;64:2622-2628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | McGregor LM, McCune BK, Graff JR, McDowell PR, Romans KE, Yancopoulos GD, Ball DW, Baylin SB, Nelkin BD. Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci USA. 1999;96:4540-4545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Djakiew D, Delsite R, Pflug B, Wrathall J, Lynch JH, Onoda M. Regulation of growth by a nerve growth factor-like protein which modulates paracrine interactions between a neoplastic epithelial cell line and stromal cells of the human prostate. Cancer Res. 1991;51:3304-3310. [PubMed] |
| 11. | Pflug BR, Onoda M, Lynch JH, Djakiew D. Reduced expression of the low affinity nerve growth factor receptor in benign and malignant human prostate tissue and loss of expression in four human metastatic prostate tumor cell lines. Cancer Res. 1992;52:5403-5406. [PubMed] |
| 12. | Descamps S, Lebourhis X, Delehedde M, Boilly B, Hondermarck H. Nerve growth factor is mitogenic for cancerous but not normal human breast epithelial cells. J Biol Chem. 1998;273:16659-16662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Sortino MA, Condorelli F, Vancheri C, Chiarenza A, Bernardini R, Consoli U, Canonico PL. Mitogenic effect of nerve growth factor (NGF) in LNCaP prostate adenocarcinoma cells: role of the high- and low-affinity NGF receptors. Mol Endocrinol. 2000;14:124-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Koizumi H, Morita M, Mikami S, Shibayama E, Uchikoshi T. Immunohistochemical analysis of TrkA neurotrophin receptor expression in human non-neuronal carcinomas. Pathol Int. 1998;48:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Meakin SO, Shooter EM. The nerve growth factor family of receptors. Trends Neurosci. 1992;15:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 467] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Raffioni S, Bradshaw RA, Buxser SE. The receptors for nerve growth factor and other neurotrophins. Annu Rev Biochem. 1993;62:823-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 84] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 962] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 18. | Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1379] [Cited by in RCA: 1464] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 19. | Cassiman D, Denef C, Desmet VJ, Roskams T. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology. 2001;33:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Colombo M. Malignant neoplasm of the liver. Schiff's Disease of the Liver. Philadelphia: Lippincott William & Wilkins 2003; 1377-1404. |
| 21. | Sherlock S, Dooley J. Malignant liver tumors. In: Oxford B. Diseases of the Liver and Biliary System 2002; 537-562. |
| 22. | Kato Y, Nakata K, Omagari K, Furukawa R, Kusumoto Y, Mori I, Tajima H, Tanioka H, Yano M, Nagataki S. Risk of hepatocellular carcinoma in patients with cirrhosis in Japan. Analysis of infectious hepatitis viruses. Cancer. 1994;74:2234-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 23. | Takano S, Yokosuka O, Imazeki F, Tagawa M, Omata M. Incidence of hepatocellular carcinoma in chronic hepatitis B and C: a prospective study of 251 patients. Hepatology. 1995;21:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 244] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Yamada G, Tanaka E, Miura T, Kiyosawa K, Yano M, Matsushima T, Tsubouchi H, Ishikawa K, Kohara M, Hino K. Epidemiology of genotypes of hepatitis C virus in Japanese patients with type C chronic liver diseases: a multi-institution analysis. J Gastroenterol Hepatol. 1995;10:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Semin Liver Dis. 1995;15:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Yano M, Kumada H, Kage M, Ikeda K, Shimamatsu K, Inoue O, Hashimoto E, Lefkowitch JH, Ludwig J, Okuda K. The long-term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 337] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 27. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2160] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 28. | Kishibe K, Yamada Y, Ogawa K. Production of nerve growth factor by mouse hepatocellular carcinoma cells and expression of TrkA in tumor-associated arteries in mice. Gastroenterology. 2002;122:1978-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Oakley F, Trim N, Constandinou CM, Ye W, Gray AM, Frantz G, Hillan K, Kendall T, Benyon RC, Mann DA. Hepatocytes express nerve growth factor during liver injury: evidence for paracrine regulation of hepatic stellate cell apoptosis. Am J Pathol. 2003;163:1849-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Weskamp G, Otten U. An enzyme-linked immunoassay for nerve growth factor (NGF): a tool for studying regulatory mechanisms involved in NGF production in brain and in peripheral tissues. J Neurochem. 1987;48:1779-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 284] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 31. | Laudiero LB, Aloe L, Levi-Montalcini R, Buttinelli C, Schilter D, Gillessen S, Otten U. Multiple sclerosis patients express increased levels of beta-nerve growth factor in cerebrospinal fluid. Neurosci Lett. 1992;147:9-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 176] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Tokusashi Y, Asai K, Tamakawa S, Yamamoto M, Yoshie M, Yaginuma Y, Miyokawa N, Aoki T, Kino S, Kasai S. Expression of NGF in hepatocellular carcinoma cells with its receptors in non-tumor cell components. Int J Cancer. 2005;114:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Gigliozzi A, Alpini G, Baroni GS, Marucci L, Metalli VD, Glaser SS, Francis H, Mancino MG, Ueno Y, Barbaro B. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Jullien J, Guili V, Derrington EA, Darlix JL, Reichardt LF, Rudkin BB. Trafficking of TrkA-green fluorescent protein chimerae during nerve growth factor-induced differentiation. J Biol Chem. 2003;278:8706-8716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Howe CL, Valletta JS, Rusnak AS, Mobley WC. NGF signaling from clathrin-coated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron. 2001;32:801-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 269] [Article Influence: 11.2] [Reference Citation Analysis (0)] |