Published online Jun 21, 2007. doi: 10.3748/wjg.v13.i23.3232
Revised: March 5, 2007
Accepted: March 15, 2007
Published online: June 21, 2007
AIM: To determine the efficacy of tacrolimus on clinical status, histopathological status and biochemical markers in patients with steroid refractory autoimmune hepatitis (AIH).
METHODS: Retrospectively, clinical parameters, biochemistry and histology were obtained from patient records.
RESULTS: Nine patients [8 females/1 male, median age 32 (range 16-64) years] were identified to have received tacrolimus for a median duration of 18 (12-37) mo. Before initiation of tacrolimus treatment the patients were maintained on a prednisolone dose of 20 mg daily (range 20-80 mg/d), which was tapered to 7.5 (5-12.5) mg/d (P = 0.004). Alanine aminotransferase and immunoglobulin-G concentrations decreased from 154 (100-475) to 47(22-61) U/L (P = 0.007), and from 16 (10-30.2) to 14.5 (8.4-20) g/L (P = 0.032), respectively. All patients showed improvement of the liver inflammatory activity, as determined by the Ishak score (P = 0.016), while the degree of fibrosis tended to decrease (P = 0.049).
CONCLUSION: The use of low dose tacrolimus can lead to biochemical and histologic improvement of inflammation with no progression of the stage of fibrosis in patients with steroid refractory AIH. Low dose tacrolimus therapy also allows substantial reduction of prednisone dose.
- Citation: Larsen FS, Vainer B, Eefsen M, Bjerring PN, Hansen BA. Low-dose tacrolimus ameliorates liver inflammation and fibrosis in steroid refractory autoimmune hepatitis. World J Gastroenterol 2007; 13(23): 3232-3236
- URL: https://www.wjgnet.com/1007-9327/full/v13/i23/3232.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i23.3232
Autoimmune hepatitis (AIH) is mediated by T suppressor cells that result in cytotoxic reactions against hepatic cells[1]. Most patients with AIH respond to standard medical therapy (SMT) consisting of a combination of corticosteroids and azathioprine (AZA). Patients with manifest cirrhosis receiving appropriate treatment experience decreases in the degree of liver fibrosis, and compared to placebo, the ten-year survival improves[1,2].
The prognosis of patients with AIH is determined not only by factors existing prior to initiation of medical treatment, but also to the amount of immunosuppressive drugs needed to control the disease[1,3]. Approximately 10% of patients with AIH are refractory to treatment and some patients have severe adverse effects of either corticosteroids or AZA[4]. Both of these groups are at high risk of developing progressive liver disease and decompensated cirrhosis. Currently, there is no consensus for a pharmacological second line management strategy, but several alternative immunosuppressive agents have been used in smaller non-randomised controlled trials with variable success. Among such drugs, the reported clinical experience with cyclosporine (CsA), a calcineurin inhibitor, and mycophenolate mofetil (MMF) in patients unresponsive to SMT indicates some beneficial effects[5,6]. However, it remains unknown if CsA and MMF or any other drug, like corticosteroid can prevent or decrease the degree of fibrosis, i.e. the disease stage.
Tacrolimus is a macrolide immunosuppressant produced by streptomyces tsukubaensis. Tacrolimus is a potent calcineurin inhibitor that has multiple effects on the immune system, mainly inhibition of CD4+ T helper cells that results in a net inhibitory effect on both B and T lymphocytes, in theory making it especially suitable for patients with autoimmune hepatitis.
The wide application of tacrolimus in the transplant setting indicates that it may also be of value in patients with steroid refractory AIH. However, the reported experience with this immunosuppressive drug in the non-transplant setting is even less than the experience with CsA and MMF.
In this study, we aimed at determining whether treatment with tacrolimus is able to decrease the inflammatory activity and the progression of fibrosis in AIH patients who are refractory to SMT.
The diagnosis of AIH was based on the aggregate score of the International Autoimmune Hepatitis Group including evaluation of a liver biopsy in all patients[7]. Steroid refractoriness was defined as persistent elevation of transaminases (twice above the upper limit, i.e. > 90 U/L) despite adequate treatment with prednisolone for at least 3 months together with AZA or MMF. Prednisone was initiated at a dose of 40 mg/d, and tapered by 10 mg every 2 wk if possible. The dose was adjusted according to the clinical and biochemical response. Slower prednisolone tapering (5 mg every 2 wk) was considered in patients with suboptimal biochemical response and in those with relapse. AZA (1-2 mg/kg per day) and MMF (1 g twice daily) were initiated 4 weeks after start of prednisolone therapy and continued for the entire follow-up period.
Liver biopsy and laboratory tests were obtained at baseline prior to treatment. Laboratory tests were obtained weekly during the first month of treatment, thereafter at 1-3 mo interval or when the patients reported signs or symptoms suggestive of relapse or tacrolimus toxicity. The laboratory tests included alanine aminotransferase (ALT), immunoglobulin G (IgG), total bilirubin, complete blood count, albumin, creatinine, urea, electrolytes, and trough tacrolimus levels.
One liver pathologist (BV) evaluated the liver biopsies obtained routinely by using a Menghini needle 1.6 under ultrasound guidance. The biopsies were processed for histopathological analysis before initiation of tacrolimus treatment and again after a median of 18 mo (range 12-37). The tissue specimens were fixed in 4% buffered formalin for at least 24 h and embedded in paraffin. Serial 4-µm thick sections were cut and mounted on glass slides and stained with a diagnostic panel of histochemical and immunohistochemical stains, including serial hematoxylin and eosin stains, PAS with diastase digestion, sirius red, reticulin, iron, orcein and cytokeratin 7 (CK7). A total of 15 slides were produced, each containing a minimum of 4 sections on each slide, except for the CK7 staining, which contained only 2 sections.
The tissue sections were evaluated by light microscopy. The histopathologic diagnosis of autoimmune hepatitis was confirmed, and each series was assessed for inflammatory activity according to Ishak et al[8] (Table 1) and the METAVIR criteria[9,10], including the degree of portal inflammation, presence of interface hepatitis and the degree of parenchymal inflammation, as well as for the degree of fibrosis and architectural changes (i.e. the stage) using the METAVIR criteria (Table 2).
| Score | |
| Portal inflammation | |
| None | 0 |
| Mild, some or all portal areas | 1 |
| Moderate, some or all portal areas | 2 |
| Moderate/severe, all portal areas | 3 |
| Severe, all portal areas | 4 |
| Periportal or periseptal interface hepatitis (“piecemeal necrosis”) | |
| Absent | 0 |
| Mild (focal, few portal areas) | 1 |
| Mild/moderate (focal, most portal areas) | 2 |
| Moderate (continuous around < 50% of tracts or septa) | 3 |
| Severe (continuous around > 50% of tracts or septa) | 4 |
| Focal parenchymal necrosis | |
| Absent | 0 |
| 1 focus or less per visual field of 100 × magnification | 1 |
| 2-4 foci per 100 × magnification | 2 |
| 5-10 foci per 100 × magnification | 3 |
| More than 10 foci per 100 × magnification | 4 |
| Confluent necrosis | |
| Absent | 0 |
| Focal confluent necrosis | 1 |
| Zone 3 necrosis in some areas | 2 |
| Zone 3 necrosis in most areas | 3 |
| Zone 3 necrosis and occasional portal-central bridging | 4 |
| Zone 3 necrosis and multiple portal-central bridging | 5 |
| Panacinar or multiacinar necrosis | 6 |
| Stage 0 | No fibrosis |
| Stage 1 | Mild fibrosis (portal/periportal fibrosis, no septa) |
| Stage 2 | Moderate fibrosis (few septa) |
| Stage 3 | Severe fibrosis (many septa, no cirrosis) |
| Stage 4 | Cirrhosis |
Patients were followed up every 1-3 mo in the outpatient clinic. Tacrolimus was initiated at 1 mg twice daily. Tacrolimus levels and dose were adjusted according to the clinical and biochemical response. No therapeutic range was targeted. But, the goal was to keep the blood tacrolimus concentration of below 6 ng/mL. Clinical outcome was assessed at the end of follow-up, including ALT, IgG, current steroid use, and histological evaluation.
Laboratory parameters and scores from liver biopsies at baseline and at follow-up were described as medians and ranges and compared using the Wilcoxon test for paired data. P < 0.05 was considered statistically significant.
Of the 123 consecutive patients with AIH treated in our tertiary university clinic of hepatology, 9 patients (8 women) with refractory AIH were treated with tacrolimus. The median patient age was 32 years (range 16-64 years). All patients fulfilled the IAHG aggregate score criteria for diagnosis of Definite AIH [median score 18 (range 16-19)] and were refractory to steroids and had significant weight gain and Cushingoid appearance due to corticosteroid treatment. Their renal function was normal (Table 3). Baseline liver biopsies demonstrated ongoing inflammation with stage 1 to 3 fibrosis.
| Patient | Gender | Age | Prednisolone | Additional | HLA type | ALT | IgG | INR | Bilirubin | Albumin | Creatinine | Follow-up |
| No. | (yr) | dose | immonusupression | (U/L) | (g/L) | (μmol/L) | (g/L) | (μmol/L) | (mo) | |||
| 1 | F | 64 | 20 | MMF | N.A. | 271 | 15 | 1.1 | 9 | 37.8 | 88 | 37 |
| 2 | F | 32 | 20 | AZA | DR-3 | 156 | 29 | 1.6 | 30 | 28.0 | 71 | 20 |
| 3 | F | 49 | 20 | MMF | DR-4,15 | 109 | 18 | 1.1 | 13 | 34.7 | 85 | 34 |
| 4 | F | 20 | 40 | AZA | N.A. | 102 | 12.3 | 0.9 | 13 | 43.0 | 55 | 18 |
| 5 | F | 17 | 20 | AZA | DR-17, DQ2 | 475 | 30.2 | 1.0 | 146 | 39.1 | 59 | 13 |
| 6 | M | 16 | 80 | AZA | DR-7,17 | 154 | 16 | 1.5 | 8 | 37.1 | 65 | 17 |
| 7 | F | 55 | 25 | AZA | DR-4,13 | 100 | 17 | 1.1 | 58 | 37.1 | 49 | 12 |
| 8 | F | 49 | 20 | AZA | DR-4,7 | 202 | 10 | 1.2 | 15 | 42.2 | 66 | 12 |
| 9 | F | 32 | 20 | MMF | N.A. | 126 | 16 | 1.1 | 6 | 33.3 | 68 | 36 |
Tacrolimus was initiated at a median starting dose of 2 (2-4) mg/d and adjusted to achieve a blood tacrolimus concentration of below 6.0 ng/mL (-3 ng/mL). The median treatment duration with tacrolimus was 18 mo (12-37 mo) and the median tacrolimus dose at the end of follow-up was 3.0 (2.0-4.0) mg/d. All patients remained on tacrolimus during the entire follow-up period.
Before initiation of tacrolimus treatment, the patients were maintained on a prednisolone dose of 20 mg daily (range 20-80 mg/d), this dose was tapped to 7.5 (5-12.5) mg/d (P = 0.004).
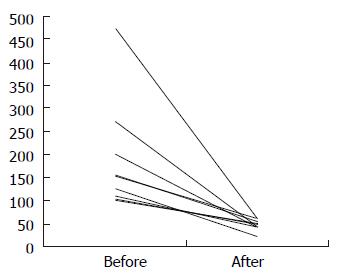
During the study period, a decrease of ALT from 154 U/L (range 100-475) to 47 U/L (22-61) (P = 0.0066) (Figure 1) and IgG from 16.0 g/L (10.0-30.2) to 14.5 g/L (8.4-20.0) (P = 0.032) was observed, whereas creatinine and urea remained within the normal ranges, i.e. 66 μmol/L (49-88) vs 56 μmol/L (47-89) and 5.4 mmol/L (3-8) vs 4.7 mmol/L (2.7-10.7), respectively.
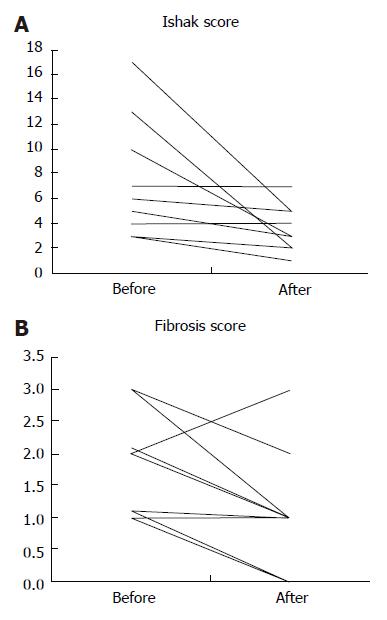
In the follow-up, liver biopsies obtained after a minimum of 12 months, improvement of the inflammatory activity was demonstrated, as determined by the Ishak score (P = 0.016), and the degree of fibrosis (stage) tended to decrease (P = 0.049) (Figure 2). Tacrolimus treatment was well tolerated in all patients without major side effects such as renal insufficiency, arterial hypertension, headache, or generalized edema in the study period going up to 37 mo. Nor did any of the patients develop opportunistic or serious infections. One patient developed mild tremor that did not require dose reduction.
In the majority of patients with AIH, SMT attenuates liver inflammation, inhibits the advancement of fibrosis to cirrhosis and prevents the development of hepatic decompensation[2]. SMT may even cause regression of fibrosis or cirrhosis through anti-inflammatory actions and the effect on fibrogenic compounds[1,4]. Such anti-fibrogenic action of corticosteroids and AZA is known to improve the 10 years survival[2]. However, it remains to be seen whether second line immunosuppressive drugs have the same efficacy on histology and survival.
The diagnostic criteria of AIH have been reviewed and a scoring system proposed by the International Autoimmune Hepatitis Group that was based on demographic, clinical, biochemical and histological data has been suggested[7]. This aggregate score reflects the certainty of the diagnosis of AIH and can be applied both before and after initiation of corticosteroid treatment or treatment with alternative immunosuppressive drugs as a useful tool to monitor treatment effect. The patients in this study all had definite AIH according to this system with a median score of 18 (range 16-19).
Generally, 10%-20% of AIH patients do not respond or are intolerant to current SMT with corticosteroids and AZA[4]. It remains uncertain if addition or replacement with other immunosuppressive agents can prevent or improve the fibrosis score in patients with SMT-resistant AIH. In the cohort of 123 AIH patients from our clinic, 9 patients with SMT-resistant AIH did not respond sufficiently to the treatment with additional steroid pulse, MMF and/or CsA. Based upon our experience with liver grafted recipients, we, therefore, decided to carry out a salvage treatment attempt using tacrolimus.
Our study demonstrated that steroid refractory patients with AIH could administer tacrolimus with its blood level and side effects carefully monitored. The use of tacrolimus in low therapeutic levels was successful as assessed by biochemical parameters and inflammatory activity and by preventing aggravation of the fibrosis. Furthermore, none of the patients experienced any severe side effects such as renal impairment, arterial hypertension, diabetes or headache. These results are largely in accordance with Aqel et al[11] who reported biochemical remission in 10/11 (91%) of patients. Aqel et al[11] also reported that 9 patients (83%) could be completely weaned off steroids. In contrast, we succeeded in decreasing the prednisolone doses from 20 (20-80) mg to 7.5 (5-12.5) mg, but none of our patients achieved complete freedom of steroid use. This apparent discrepancy may be related to the fact that our patients had more severe disease due to their younger age, higher aggregate score and insufficient response to treatment with other second line immunosuppressive drugs.
It could be argued that sampling error during the two liver biopsies may hamper our conclusion. Though this is controversial[1,12], we did not see any change or a decline in the inflammatory activity score in our patients. None of these cases had a rise in inflammatory activity. Also the fibrosis score remained unchanged or decreased in all patients except in one. These findings indicate that it is less likely that sampling error accounts for our conclusions.
In the early days of the tacrolimus era, Van Thiel et al[13] studied the application of tacrolimus in 21 newly diagnosed AIH patients, and remission defined by improvement in the biochemical biomarkers was obtained in 18 of the patients, which encouraged the authors to suggest a randomised, controlled trial. However, a rise in serum creatinine was noted during the study period and to our knowledge a randomised controlled trial study was never carried out. It is likely that the high level of blood tacrolimus may have accounted for the rise in creatinine in that study. Using a lower dose of tacrolimus to maintain the blood tacrolimus concentration below 6 ng/dL allowed us to avoid this complication in any of our treated patients (Table 3).
Heneghan et al[14] used lower doses of tacrolimus in combination with reduced dose of prednisolone (20 mg/d) and achieved significant improvement in ALT, bilirubin and albumin levels and in prothrombin time in 6 of 7 patients with severe new-onset AIH. However, the morphological changes in liver tissue were not evaluated.
In conclusion, tacrolimus therapy can be used in patients with severe AIH as a salvage therapy. The treatment seems to prevent progression of hepatic fibrosis by suppression of inflammation. However, this needs to be confirmed in a large multicenter randomised trial that includes patients with steroid refractory AIH. The long-term complications, i.e. beyond 37 mo, associated with tacrolimus use in this group of patients are unknown and need to be evaluated. Until such controlled data are provided, we recommend that the use of tacrolimus should take place in an institution with extensive experience in treating transplant recipients.
S- Editor Wang J L- Editor Wang XL E- Editor Liu Y
| 1. | Manns MP, Vogel A. Autoimmune hepatitis, from mechanisms to therapy. Hepatology. 2006;43:S132-S144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Kirk AP, Jain S, Pocock S, Thomas HC, Sherlock S. Late results of the Royal Free Hospital prospective controlled trial of prednisolone therapy in hepatitis B surface antigen negative chronic active hepatitis. Gut. 1980;21:78-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 175] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Czaja AJ, Manns MP, McFarlane IG, Hoofnagle JH. Autoimmune hepatitis: the investigational and clinical challenges. Hepatology. 2000;31:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 604] [Article Influence: 31.8] [Reference Citation Analysis (1)] |
| 5. | Alvarez F, Ciocca M, Cañero-Velasco C, Ramonet M, de Davila MT, Cuarterolo M, Gonzalez T, Jara-Vega P, Camarena C, Brochu P. Short-term cyclosporine induces a remission of autoimmune hepatitis in children. J Hepatol. 1999;30:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Richardson PD, James PD, Ryder SD. Mycophenolate mofetil for maintenance of remission in autoimmune hepatitis in patients resistant to or intolerant of azathioprine. J Hepatol. 2000;33:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 134] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 1985] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 8. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3782] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 9. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3081] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 10. | Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2159] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 11. | Aqel BA, Machicao V, Rosser B, Satyanarayana R, Harnois DM, Dickson RC. Efficacy of tacrolimus in the treatment of steroid refractory autoimmune hepatitis. J Clin Gastroenterol. 2004;38:805-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 169] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Van Thiel DH, Wright H, Carroll P, Abu-Elmagd K, Rodriguez-Rilo H, McMichael J, Irish W, Starzl TE. Tacrolimus: a potential new treatment for autoimmune chronic active hepatitis: results of an open-label preliminary trial. Am J Gastroenterol. 1995;90:771-776. [PubMed] |
| 14. | Heneghan MA, McFarlane IG. Current and novel immunosuppressive therapy for autoimmune hepatitis. Hepatology. 2002;35:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |