Published online Jun 14, 2007. doi: 10.3748/wjg.v13.i22.3080
Revised: February 20, 2007
Accepted: March 1, 2007
Published online: June 14, 2007
AIM: To improve the preoperative diagnosis of liver metastasis from pancreatic cancer, we estimated computed tomography during arterial angiography (CTA) with/without administration of angiotensin-II (AT-II).
METHODS: Thirty-five patients with pancreatic cancer were examined in this study. After conventional CTA was performed, pharmacoangiographic CTA was performed with a 1-3 microgram/5 mL solution of angiotensin IIinjected through a catheter into the celiac artery during spiral computed tomography. We prospectively analyzed the relative region of interest (ROI) ratio of tumor to liver with/without AT-II.
RESULTS: In all patients, the relative ratio of each computed tomography (CT) number in the ROI was larger at pharmacoangiographic CT than at conventional angiographic CT. Administration of angiotensin-II enhanced the metastatic liver tumor as compared with normal tissue. Intratumoral blood flow increased in all patients with malignant tumors due to the pressure effect of AT-II. Furthermore, the metastatic lesions in the liver of three patients were represented by only pharmacoangiographic CT, not by conventional CT and conventional CT angiography. In even peripheral and central areas of metastatic liver tumor, the lesions were enhanced after administration of AT-II.
CONCLUSION: These results support that high detection rate of liver metastasis revealed by pharmacoangiographic CT suggests the improvement of diagnosis on preoperative staging. Moreover, chemotherapy under AT-II induced hypertension may have a better effect on the treatment of metastatic liver tumors.
- Citation: Ishikawa T, Ushiki T, Kamimura H, Togashi T, Tsuchiya A, Watanabe K, Seki KI, Ohta H, Yoshida T, Takeda K, Kamimura T. Angiotensin-II administration is useful for the detection of liver metastasis from pancreatic cancer during pharmacoangiographic computed tomography. World J Gastroenterol 2007; 13(22): 3080-3083
- URL: https://www.wjgnet.com/1007-9327/full/v13/i22/3080.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i22.3080
Pancreatic cancer is a malignancy with a poor prognosis[1]. The cause of death in patients with advanced pancreatic cancer is primarily the local progression of cancer and distant metastases. At diagnosis pancreatic cancer is most commonly locally advanced and often accompanied by distant metastases to the liver and peritoneum[2]. In fact, liver metastasis is one of the major causes of cancer death after resection of pancreatic cancer. Foster reported that 73% of the patients who died of pancreatic cancer were found to have liver metastases at autopsy[3]. Therefore, liver metastasis appears to be an important prognostic factor. Ultrasonography and CT are the first line investigations to diagnose and localize the primary tumor and to detect metastatic lesions of pancreatic cancer[4,5]. However, some patients come to laparotomy with liver metastases not suspected or detected during these conventional preoperative examinations. To overcome this situation, some preoperative diagnostic procedures have been attempted. Computed tomography during arterial portography (CTAP) has been reported to be the most sensitive preoperative imaging modality for detecting and determining the location of hepatic lesions[6,7]. However, these results were reached mainly for the evaluation of hepatocellular carcinoma and metastatic tumor from colorectal cancer. Hence, angiotensin-II (AT-II) is the most potent vasoconstrictive agent. Ekelund et al reported the diagnostic value of phramacoangiography using AT-II[8]. Elevation of the arterial blood pressure by systemic infusion of AT resulted in a 5.7 fold selective increase in the blood flow of the tumor tissue without increasing the blood flow in normal tissue[9,10]. Sasaki et al[11] investigated the distribution of the hepatic blood flow using a short-lived radioisotope and demonstrated a 3.3 times increase in the tumor/non-tumor (T/N) ratio of the blood flow induced by intra-arterial infusion of AT-II. They also observed that intra-arterial infusion of AT-II increased the T/N ratio more than intravenous infusion did, with a smaller rise in the peripheral blood pressure. To our knowledge, there is no study of metastatic liver tumor from pancreatic cancer by means of CT angiography with/without administration of AT-II. We examined the efficacy of pharmacoangiographic CT with AT-II in patients with metastatic liver tumor from pancreatic cancer with/without administration of AT-II.
Between May 2002 and December 2005, we performed dynamic CT during celiac arteriography (hepatic arteriography) administrating with/without AT-II in 35 patients (10 men, 25 women) with liver metastasis from pancreatic cancer. Mean age was 64.3 years, with a range 43-83 years.
The celiac trunk catheter placement was done by using Seldinger’s approach through the right femoral artery with a 5.0-Fr or 3.2-Fr catheter. Celiac arteriography was performed before CT for the evaluation of the tumor feeding arteries. For small arteries, the coaxial technique was used by placing a 2.7 Fr microcatheter (Renegade Hi Fro catheter, Target Therapeutics, San Jose, CA, USA or Micro Ferret, Cook, Bloomington, IN, USA) in the appropriate feeding artery, depending on tumor location.
CT arteriography was started 3 s after the initiation of a transcatheter hepatic arterial injection of 30 mL of nonionic contrast material (Omnipaque 100 mgI/mL, Dai-ichi, Osaka, Japan) through a catheter, using an automated power injector (Auto Enhance A-50; Nemoto Kyorindo. Tokyo). Injection rate ranged from 1.5 to 2.0 mL/s depending on the catheter tip location. CT imaging was performed on a helical CT scanner (HiSpeed Advantage; GE Medical Systems, Milwaukee, Wis) with the following technical parameters: 120 kVp, 200 mA, 1-second scan speed, 24-s scanning time, 6-s delay, 4-6 mm/s velocity of table feed, 3-5 mm section thickness, 12-cm field of view, 5-mm increment of reconstructed axial images and a standard body reconstruction algorithm.
After about 10 min. from conventional angiographic CT, AT-II was infused into the catheter at a rate of 5 μg in 5 mL normal saline for 10 s. Two min after administration of AT-II, repeat CT arteriography was carried out during the injection of contrast material with the same dose, injection rate, and technical parameters as at the conventional angiographic CT.
The mean attenuation values of regions of interest (ROIs) in the tumor and in the liver parenchyma were measured on the angiographic CT images obtained with and without AT-II.
Region of interest measurements of the normal liver tissue and central area of metastatic liver tumor were obtained at each time point. All ROIs were approximately 1 cm2. Attenuation (in Housefield Units) and enhancement (attenuation after injection minus attenuation before injection) were plotted as a function of time for each dose regimen and as a function of dose at each time point.
Radiologists evaluated the images independently; discrepancies in their interpretations were solved by consensus.
The contrast of the tumor to liver parenchyma in angiographic CT with/without AT-II infusion respectively were compared using the Wilcoxon paired test.
All patients gave their informed consent after being fully informed on this study. The study was approved by the Ethical Committee of our hospital and was conducted in accordance with the principles of the Declaration of Helsinki.
To evaluate the effect of AT-II on angiographic CT images, we divided the liver into two areas (normal parenchyma and central area of metastatic liver tumor). We calculated the number of enhanced areas on the images from the studies with and without AT-II.
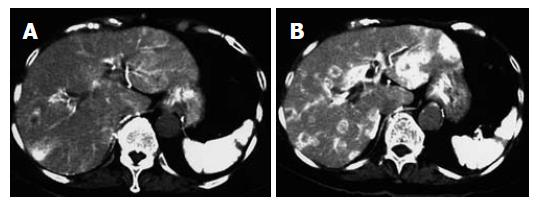
For instance, a 78 year old woman with liver metastasis from pancreatic cancer was examined with dynamic CT during celiac arteriography administrating with/without AT-II as described. Phamacoangiographic CT showed marked multiple liver metastasis enhancement (Figure 1).
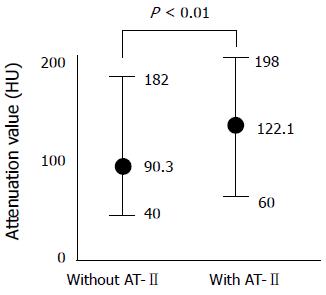
The attenuation value of the tumor was significantly increased after injection of AT-II at angiographic CT. The mean values of the tumor contrast at angiographic CT with and without AT-II were respectively 122.1 HU and 90.3 HU (P < 0.01) (Figure 2).
Furthermore, even central necrosis was well enhanced after injection of AT-II at pharmacoangiographic CT.
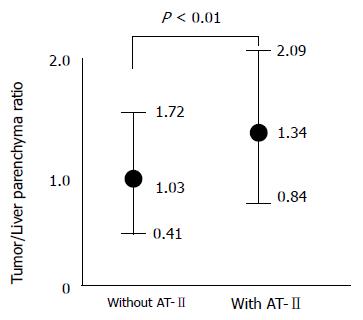
Although the attenuation value of tumors was also increased on images obtained after the injection of angio-tensin II, the tumor to liver contrast was significantly greater at pharmacoangiographic CT (Figure 3).
Pharmacoangiographic CT showed marked enhancement of the tumor surrounded by poorly enhanced parenchyma, although digital subtraction angiography revealed no tumor stain.
As expected, the hepatic arterial infusion of AT-II induced a modest rise in systemic blood pressure (an increase in the systolic pressure of up to 40 mmHg) which reached a peak at the end of the 100 s infusion, then gradually declined. No rebound hypotension was observed. These effects were accompanied by transient elevation of mean arterial pressure, but no change in pulse rate.
The prognosis for pancreatic cancer is poor. Surgery is considered the only curative therapy. However, the 5 year survival rate after resection of pancreatic cancer is still very low, even when radical surgery is performed[12,13]. Most patients with pancreatic cancer have distant metastases at the time of diagnosis, particularly involving the liver. Even when small lesions (< 2 cm) can be detected, most of them are locally advanced and microscopically invasive. Some patients come to laparotomy with liver metastasis not suspected or detected during the conventional preoperative examinations. In 70% of patients who undergo pancreatectomy, occult liver metastasis that may already have existed at the time of surgery are one of the most common sites of treatment failure[14]. Patients with certain types of metastatic liver tumors such as colorectal cancer who undergo surgical resection of the tumor have improved long term survival rates compared with similar patients who do not undergo resection[15]. However, resection is useless and harmful for most of the patients with metastatic liver tumors from pancreatic cancer. Although Fortner[16] has attempted to resect such advanced tumors by using regional pancreatectomy, they failed to improve patient survival. The exact number of metastatic lesions is irrelevant in cases of pancreatic cancer, as opposed to metastatic colon cancer.
To improve the survival rate of pancreatic cancer, it is important to diagnose its resectability before operation. Furthermore, to improve the value of preoperative diagnosis in patients with pancreatic cancer in terms of evaluation of resectability, a modality other than conventional examination methods is needed. Therefore, we examined pharmacokinetic CTA.
Intratumoral blood vessels are immature, lacking both smooth muscle cells and immunoreactive nerves[17]. Therefore, tumor vessels are unable to react to vasocon-stricting agents[18,19].
AT-II causes arteriolar constriction in normal blood vessels. It is a powerful vasoconstrictor which has been shown to alter the distribution of blood flow in favor of intrahepatic tumor perfusion during short (3-4 min) intra-arterial infusions of the compound[11]. Increased tumor blood flow following AT-II infusion may increase the exposure of tumor to chemotherapeutic agents, and CT angiography may provide useful information about the potential of AT-II and other vasoconstrictors to enhance targeting precision.
It is reported that there is a temporary increase in the relative arterial perfusion of human hepatic tumors during an infusion of AT-II[11]. Furthermore, it was reported that the addition of AT-II to hepatic artery infusional chemotherapy, significantly improves the tumor/liver ratio of drug uptake in an experimental model of hepatic metastasis[20]. However, to our knowledge, there is no study of metastatic liver tumor from pancreatic cancer by means of CT angiography with/without administration of AT-II.
We quantitatively evaluated computed tomography during arterial angiography (CTA) with/without administration of AT-II by means of pharmacoangiographic CT in patients with metastatic pancreatic cancer for the preoperative evaluation of liver metastasis from pancreatic cancer.
The attenuation value of the liver metastasis was significantly increased in pharmacoangiographic CT. Furthermore, even central necrosis was well enhanced after injection of AT-II at pharmacoangiographic CT. The tumor to liver contrast was also significantly greater at pharmacoangiographic CT.
Pharmacoangiographic CT by AT-II showed marked enhancement of the tumor surrounded by poorly enhanced parenchyma, although digital subtraction angiography revealed no tumor stain.
Because the injection of AT-II into the celiac artery results in increased blood flow in the intrahepatic arteries, the tumor to liver contrast was increased significantly at angiographic CT. As shown in this study, prolonged enhancement of the tumor blood flow as compared with the liver suggests the usefulness of intra-arterial infusion of AT-II.
This finding described above was not recognized in the metastatic liver tumor from the cancer other than pancreas (data not shown). This result, therefore, may be a specific finding in metastatic liver tumor from pancreatic cancer.
Thus, CTA with/without administration of AT-II is useful for the preoperative evaluation of liver metastases from pancreatic cancer.
In terms of adverse effects, there were some accom-panying symptoms such as a sense of chest oppression and headache when the blood pressure was elevated, but they were not so severe and the administration of AT-II could be performed in nearly all the cases. But, careful attention must be paid to control blood pressure values to avoid excess elevation during the administration of AT-II.
AT-II administration may induce not only the high detection rate of liver metastasis from pancreatic cancer, but also the selective increase in tumor blood flow in drug delivery such as chemotherapy.
In conclusion, AT-II administration is useful for the detection of liver metastasis from pancreatic cancer during pharmacoangiographic computed tomography. This pharmacoangiographic CT is a useful technique for decisions in preoperative staging in pancreatic cancer.
S- Editor Wang J L- Editor Roberts SE E- Editor Liu Y
| 1. | Gold EB, Cameron JL. Chronic pancreatitis and pancreatic cancer. N Engl J Med. 1993;328:1485-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Connolly MM, Dawson PJ, Michelassi F, Moossa AR, Lowenstein F. Survival in 1001 patients with carcinoma of the pancreas. Ann Surg. 1987;206:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 204] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Foster JH, Lundy J. Liver Metastases. Curr Probl Surg. 1981;18:157-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 76] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Lu DS, Vedantham S, Krasny RM, Kadell B, Berger WL, Reber HA. Two-phase helical CT for pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology. 1996;199:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 185] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 5. | O'Malley ME, Boland GW, Wood BJ, Fernandez-del Castillo C, Warshaw AL, Mueller PR. Adenocarcinoma of the head of the pancreas: determination of surgical unresectability with thin-section pancreatic-phase helical CT. AJR Am J Roentgenol. 1999;173:1513-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Matsui O, Takashima T, Kadoya M, Ida M, Suzuki M, Kitagawa K, Kamimura R, Inoue K, Konishi H, Itoh H. Dynamic computed tomography during arterial portography: the most sensitive examination for small hepatocellular carcinomas. J Comput Assist Tomogr. 1985;9:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Nelson RC, Chezmar JL, Sugarbaker PH, Murray DR, Bernardino ME. Preoperative localization of focal liver lesions to specific liver segments: utility of CT during arterial portography. Radiology. 1990;176:89-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 63] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Ekelund L, Lunderquist A. Pharmacoangiography with angiotensin. Radiology. 1974;110:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Sato H, Sato K, Sato Y, Asamura M, Kanamaru R, Sugiyama Z, Kitahara T, Mimata Y, Wakui A, Suzuki M. Induced hypertension chemotherapy of cancer patients by selective enhancement of drug delivery to tumor tissue with angiotensin II. Sci Rep Res Inst Tohoku Univ Med. 1981;28:32-44. [PubMed] |
| 10. | Suzuki M, Hori K, Abe I, Saito S, Sato H. A new approach to cancer chemotherapy: selective enhancement of tumor blood flow with angiotensin II. J Natl Cancer Inst. 1981;67:663-669. [PubMed] |
| 11. | Sasaki Y, Imaoka S, Hasegawa Y, Nakano S, Ishikawa O, Ohigashi H, Taniguchi K, Koyama H, Iwanaga T, Terasawa T. Changes in distribution of hepatic blood flow induced by intra-arterial infusion of angiotensin II in human hepatic cancer. Cancer. 1985;55:311-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | Cameron JL, Crist DW, Sitzmann JV, Hruban RH, Boitnott JK, Seidler AJ, Coleman J. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120-124; discussion 120-125;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 365] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 13. | Baumel H, Huguier M, Manderscheid JC, Fabre JM, Houry S, Fagot H. Results of resection for cancer of the exocrine pancreas: a study from the French Association of Surgery. Br J Surg. 1994;81:102-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 126] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Amikura K, Kobari M, Matsuno S. The time of occurrence of liver metastasis in carcinoma of the pancreas. Int J Pancreatol. 1995;17:139-146. [PubMed] |
| 15. | Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938-946. [PubMed] |
| 16. | Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla, and other related sites. Tumor staging and results. Ann Surg. 1984;199:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 183] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Ashraf S, Loizidou M, Crowe R, Turmaine M, Taylor I, Burnstock G. Blood vessels in liver metastases from both sarcoma and carcinoma lack perivascular innervation and smooth muscle cells. Clin Exp Metastasis. 1997;15:484-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Peterson HI, Mattson J. Vasoactive drugs and tumor blood flow. Biorheology. 1984;21:503-508. [PubMed] |
| 19. | Mattson J, Appelgren L, Karlsson L, Peterson HI. Influence of vasoactive drugs and ischaemia on intra-tumour blood flow distribution. Eur J Cancer. 1978;14:761-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Trezona T, Butler JA, Vargas H. Angiotensin alteration of drug uptake in an experimental model of hepatic metastases. J Surg Res. 1991;51:124-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |