INTRODUCTION
Treatment of malignant tumours by radiotherapy is limited by the need to avoid acute and late damage to healthy tissue. Acute and late effects are increasingly being observed because of the introduction of new aggressive treatment protocols with combined modalities, specially radiotherapy plus chemotherapy protocols, or the application of new technologies allowing to escalate the dose, such as intensity modulated radiotherapy in prostate cancer[1].
Acute radiation damage is most prominent in tissues with rapid proliferating cells, such as skin or alimentary tract. Symptoms develop when functional cell are lost as a part of normal tissue turnover, and are not replaced because of the damage produced to the stem-cell compartment. Normally, there is a compensatory proliferation within the stem cell compartment followed by replacement of functional cells and a final recovery. Nevertheless, sometimes radiation can cause irreversible damage to the vital cellular components.
Vascular injury is also a key determinant of both acute and chronic organ dysfunction associated with irradiation of the gastrointestinal tract. Generally the acute vascular changes resolve, at least in part, but many eventually progress leading to obliterative endarteritis, producing intestinal ischemia and contributing to more extensive mucosal injury, ulceration and necrosis[2]. Progress in molecular and cellular biology has shown that normal tissue response to irradiation not only depends on the response of a single target cell type. Radiation dose delivery can affect the interactions between different cellular systems. This process relies on a dynamic equilibrium and radiation can result in the overproduction of a number of proinflammatory mediators and profibrotic cytokines which produce vascular injury and activation of the coagulation cascade[3].
The objective of this review is to define molecular implication in radiation-induced intestinal inflammation in order to improve therapeutic strategies to prevent radiation side effects.
REGULATION OF LEUKOCYTE-ENDOTHELIAL CELL INTERACTIONS
Multistep model of leukocyte recruitment
Intravital microscopy studies have documented an early inflammatory response, appearing only a few hours after irradiation, characterized by leukocyte infiltration into the irradiated organs[4,5]. This early inflammatory response has been implicated in the vascular alterations that result from radiation damage[6].
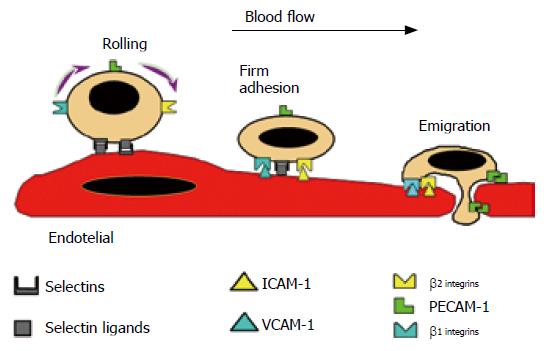
The development of an inflammatory response is a finely regulated process that involves sequential leukocyte-endothelial cell interactions composed of rolling, activation, adhesion and emigration phases. Adhesion molecules expressed on the surface of endothelial cells (postcapillary venules) and leukocytes, serve to ensure an ordered sequence of cell to cell interactions that sustain leukocyte adherence to vascular endothelium and subsequent transendothelial migration into the inflamed tissue. The initial event is a weak adhesive interaction that results in leukocyte rolling along the endothelial cell lining of postcapillary venules. Subsequently, there is a strengthening of these adhesive forces that lead leukocytes to become firmly attached to the endothelium and remain stationary (adherence). Finally, leukocytes migrate into the interstitium through spaces between adjacent endothelial cells (Figure 1). These adhesive interactions are regulated by sequential activation of different families of adhesion molecules expressed on the surface of leukocytes and endothelial cells.
Figure 1 Scheme showing the multistep model of leukocyte-endothelial cell interactions.
The leukocyte and endothelial cell receptors that contribute to the different steps (rolling, firm adhesion and emigration) are also illustrated. Reprinted with permission. (review Gastroenterology 1998).
Intravital microscopic studies of radiation-induced leukocyte-endothelial cell adhesion have revealed an increased number of rolling leukocyte in mesenteric and intestinal venules (Figure 2) 2 h after irradiation, with a marked increase in the number of firmly adherent leukocytes noted after 6 h and 24 h and decrease 14 d after irradiation[4,8,9]. An increase in oxygen radical production in the vascular wall has been documented as early as 2 h after irradiation with a more intense oxidant stress observed at 6 h, this second burst being produced mainly by infiltrating inflammatory cells.
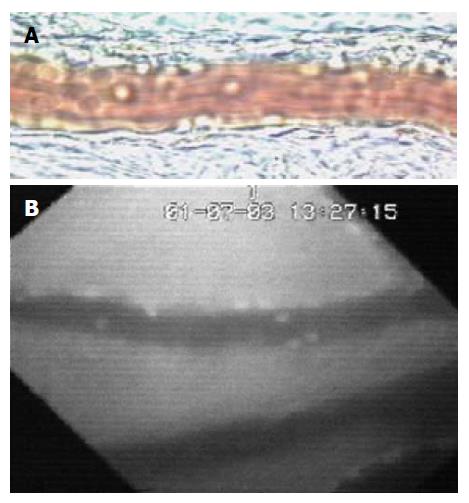
Figure 2 Image of mesenteric (A) and small bowel (B) venules was obtained by Intravital microscopic.
Rolling and firm adherent leukocytes can be easily identified by transillumination (A) or fluorescent staining (B).
An increase in the microvascular permeability of mesenteric venules has clearly been shown 6 h after abdominal irradiation. Furthermore, there is evidence indicating that the phenomena of leukocyte adherence plays an important role in mediating these increases in permeability because the immunoneutralization of β2-integrin (CD18) or ICAM-1 results in attenuation of radiation-induced leukocyte adherence and albumin leakage[8].
A possible explanation for the delayed recruitment of adherent and migrant leukocytes in postcapillary venules after radiation exposure is an increased expression of endothelial adhesion molecules. In addition to cytokines are proteins released by irradiated tissues and are implicated in the acute response phase to irradiation[10].
Molecular determinants of leukocyte-endothelial cell adhesion selectins
Selectins and their ligands mediate the phenomenon of leukocyte rolling. Selectines, designed as L-, P-, and E-selectins, represent a family of adhesive receptors expressed on leukocytes (L), platelets and endothelial cells (P), or endothelial cells alone (E). Formed P-selectin is stored in endothelium-specific storage granules called Weibel-Palade bodies and in the alpha granules of platelets. It is then redistributed to the cell surface of platelets and endothelial cells within minutes upon stimulation[11].
Several studies have involved E- and P-selectin in mediating leukocyte-endothelial cell interactions in late and acute inflammation[12,13].
Using P-selectin deficient mice, Mayadas et al[14] showed that the initial leukocyte recruitment to the peritoneal cavity in experimentally induced inflammation was entirely P-selectin dependent.
Different studies have reported that in vivo P-selectin expression increases after irradiation in lung tissue[15] and in intestinal tissue. Hallahan et al[16] showed in vitro that the lowest dose that induced translocation of P-selectin to the cell membrane was 2 Gy. Confirmatory evidence has been provided showing that P-selectin expression is significantly up-regulated in response to abdominal irradiation, and this regulation is dose- and time-dependent[6]. However, lack of P-selectin does not afford protection against radiation-induced inflammatory intestinal alterations probably because leukocyte adhesion is not solely a consequence of an increase in P-selectin mediated rolling; there is a release of proinflammatory mediators like PAF[5] or LTB4[17] that may directly affect firm leukocyte adhesion.
Nevertheless, the heterogeneity in constitutive and induced P-selectin expression in different vascular beds suggests that its role in acute inflammation may depend on the tissue in which damage occurs[18].
The role of E-selectin in radiation-induced intestinal inflammation has not been clearly defined. Hallahan et al[19] showed that thoracic irradiation increases E-selectin expression in the pulmonary vascular endothelium of mice. In contrast, another study did not detect expression of this molecule in wild-type or in P-selectin deficient mice after abdominal irradiation at different doses (4, 10 Gy) and time (2 h or 24 h) points[6]. Recent studies have suggested that E-selectin could be involved in the anti-inflammatory response after radiotherapy, mediating the drop in leukocyte adhesion after low dose irradiation (0.3- 0.6 Gy) in vitro[20].
Immunoglobulin superfamily
Extravasation of leukocytes from the vasculature requires the participation of molecules of the immunoglobulin superfamily (e.g., ICAM-1 and VCAM-1), which are expressed on the surface of endothelial cells and interact with their respective counter-receptors on leukocytes (integrins) to facilitate inflammatory cell extravasation[10].
A consistent body of evidence indicates[21,22] that ICAM-1 expression is up-regulated following irradiation. Studies performed in experimental models of radiation enteritis[23] support the view that ICAM-1 plays a pivotal role in determining leukocyte adhesion to intestinal venular endothelium in early time points after irradiation; these results were confirmed by two approaches used to test ICAM-1 function by monoclonal antibodies and ICAM-1 deficient mice[9]. Even though the role of a particular determinant of leukocyte recruitment in response to an inflammatory stimulus varies among different organs, the important role of ICAM-1 has been also documented in irradiated lung[22,24]. The molecular determinants of late radiation-induced inflammation seems to differ from the acute condition. It has been shown that at late time points (14 d) ICAM-1 expression returns to baseline levels and blockade of ICAM-1 by monoclonal antibodies has no longer an effect on leukocyte adhesion in intestinal normal tissue. By contrast, VCAM-1 seems to be a key determinant of leukocyte infiltration in the irradiated intestine at late time points, because expression of this adhesion molecule is upregulated late after irradiation, and the increase in leukocyte adhesion is abrogated by VCAM-1 immunoblockade[9]. In other chronic inflammatory models like experimental colitis, such as inflammatory bowel disease, VCAM-1 has also been shown to play a key role as a determinant in leukocyte recruitment in intestinal tissue[25]. These findings support the hypothesis that ICAM-1 and VCAM-1 induction by ionizing radiation mediated in leukocyte recruitment. Pharmaceuticals that block these molecules may prevent radiation-mediated inflammation in normal tissue.
Regulation of endothelial adhesion molecular expression
Regulation of adhesion molecules is determined in part by activation of transcription factors. Of the many transcription factors that have been described, NF-κB and activation protein-1 (AP-1) seem to be particularly relevant to the regulation of endothelial cell adhesion molecules[26-28]. NF-κB is believed to play a pivotal role in the inducible expression of many genes, including cytokines in gut immune and inflammatory response. In vitro[29,30] and in vivo[31], irradiation induced a cascade of inflammatory responses that involved the transcription factor NF-κB.
Activation of NF-κB in nuclear extracts of intestinal samples in irradiated rats was detectable 30 min after irradiation and was maximal at 60 min, with a progressive decline in the amount of this transcription factor in nuclear extracts over the following 60 min[17]. The radiation-induced inflammatory response is preceded by the NF-κB transcription factor that up-regulated ICAM-1 expression on endothelial cells[32,33]. These findings provide the rationale for manipulation of NF-κB system as a mean of controlling transcription-dependent cellular events that are involved in radiation-induced inflammatory response.
Other important mechanisms that seem to regulate endothelial cells expression was the transcription factor AP-1. The studies suggest that oxidative stress affect AP-1 and NF-κB differently and can be explained by the differential binding of AP-1 and NF-κB to the IL-8 promoter[34]. These cell specific activation signals in endothelial cells may influence the leukocyte recruitment and inflammatory reactions.
CONCLUSIONS
Many factors come into play in the development of radiation induced intestinal damage.
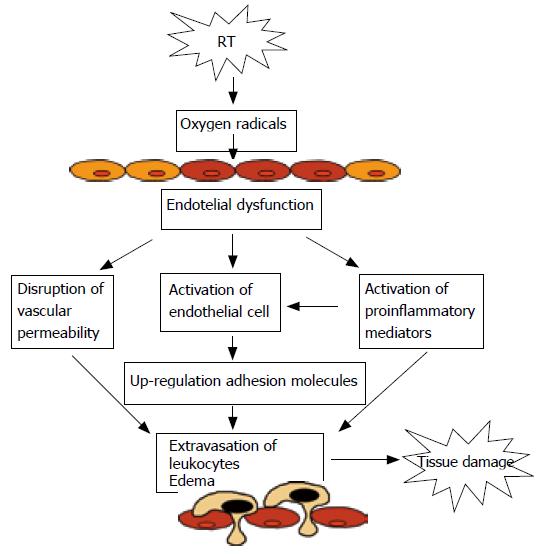
The data derived from in vivo and in vitro models of radiation-induced intestinal inflammation are generally consistent with the notion that activated endothelial cells produce inflammatory mediators and induce the up-regulation of CD11/CD18 on leukocytes, which along with the oxidant stress cause an NF-κB-dependent increase in expression of endothelial cell adhesion molecules. This results in firm leukocyte adhesion to the vascular endothelium and subsequent extravasation of inflammatory cells into the inflamed tissue (Figure 3). Leukocyte infiltration of irradiated tissues is one the initial histological changes of radiation-induced organ damage. The recognition that leukocyte recruitment is so critical in the pathogenesis of radiation-induced acute and chronic organ damage has resulted in an intensive effort to define the molecular mechanisms that underlie these cell- cell adhesive interactions. Therapeutic potential of adhesion molecules inhibition provides the opportunity to develop therapeutic strategies that can prevent inflammatory response.
Figure 3 Mechanism proposed to explain leukocyte-cell adhesion and tissue damage caused by irradiation.