Published online Apr 21, 2007. doi: 10.3748/wjg.v13.i15.2243
Revised: January 10, 2007
Accepted: March 22, 2007
Published online: April 21, 2007
We recently encountered an unusual case of hilar cholangiocarcinoma in which a solitary recurrence in a mediastinal lymph node occurred two years after curative resection of the primary tumor. A 64-year old woman was admitted to our hospital with a complaint of right hypochondrial discomfort. After imaging studies demonstrated a hilar cholangiocarcinoma in the left hepatic duct, a curative resection of the tumor was performed, consisting of a left hepatic lobectomy along with caudate lobectomy, regional lymph node dissection, and resection of the extrahepatic bile duct. No nodal metastasis was observed histologically. Two years after surgery, the patient was found to have a nodule in the posterior mediastinum, which was thoracoscopically resected. No other swollen lymph nodes, local recurrence, or distant metastasis were noted. Histologically, the nodule proved to be a metastatic lymph node, and adjuvant chemoradiation therapy was initiated. The patient remained well for the four years following her first operation and had no evidence of disease recurrence 28 mo after her second operation. To our knowledge, this case is the first report of solitary recurrence in a mediastinal lymph node after curative resection of hilar cholangiocarcinoma.
- Citation: Ito Y, Tajima Y, Fujita F, Tsutsumi R, Kuroki T, Kanematsu T. Solitary recurrence of hilar cholangiocarcinoma in a mediastinal lymph node two years after curative resection. World J Gastroenterol 2007; 13(15): 2243-2246
- URL: https://www.wjgnet.com/1007-9327/full/v13/i15/2243.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i15.2243
Hilar cholangiocarcinoma, which comprises approximately 50% of all malignant bile duct tumors, is a cancer that is difficult to treat[1-3]. Although surgical resection is the best therapeutic strategy for this malignant disorder, long-term outcome after surgical intervention remains unfavorable because of frequent local and/or regional lymph node recurrences[4,5]. The most common sites of nodal recurrence are the hepatoduodenal ligament and the posterior pancreaticoduodenal area[6,7]. We recently encountered an unusual case of hilar cholangiocarcinoma in which a solitary nodal recurrence occurred in a mediastinal lymph node two years after curative resection of the primary tumor. Thoracoscopic removal of the lymph node definitively confirmed a diagnosis of nodal metastasis. The patient subsequently received adjuvant chemoradiation therapy and has had no further disease recurrence to date.
A 64-year old woman was admitted to our hospital in December 2001, with a complaint of right hypochondrial discomfort. Past medical history was not remarkable. She was not icteric and physical examination showed a soft abdomen without tenderness or guarding. Laboratory evaluation on admission revealed a normal white blood cell count, hemoglobin, total protein, total bilirubin, amylase, and creatinine. Hepatic and biliary enzymes were normal except for a γ-glutamyl transpeptitase of 114 IU/L (normal: 10-47 IU/L). Hepatitis B antigen and hepatitis C antibody were negative. Although serum levels of CEA and AFP were within normal limits, CA19-9 was elevated to 113.5 U/L (normal < 37 U/L).
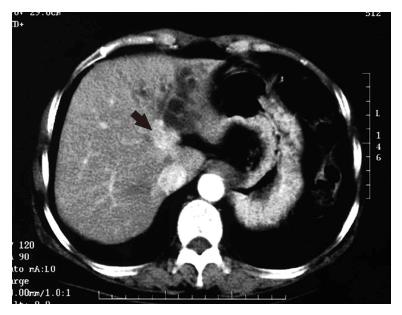
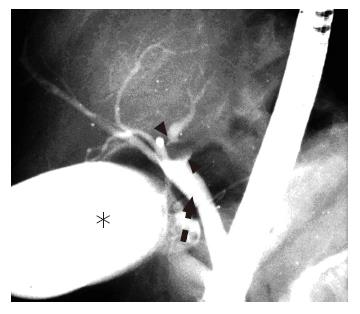
Abdominal ultrasonography demonstrated marked dilatation of the bile ducts in the left lobe of the liver. Abdominal computed tomography (CT) with intravenous contrast material showed a well-enhanced mass lesion on the left side of the hepatic hilum along with dilated left intrahepatic bile ducts (Figure 1). Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated a tumor protruding into the lumen of the left hepatic duct, resulting in complete obstruction of the duct (Figure 2). These findings were consistent with a diagnosis of hilar cholangiocarcinoma originating from the left hepatic duct.
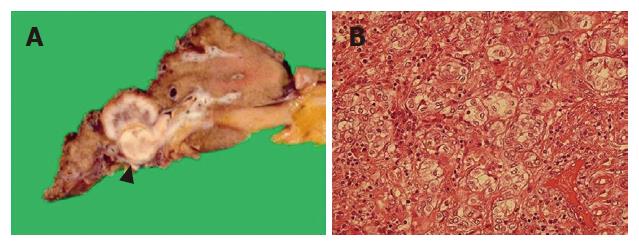
In December 2001, the patient underwent laparotomy. Since no evidence of ascites, peritoneal dissemination or liver metastasis was observed at the time of surgery, curative resection of the tumor was performed, consisting of a left hepatic lobectomy together with caudate lobectomy, lymphadenectomy along the hepatoduodenal ligament and the common hepatic artery, sampling of paraaortic lymph nodes, and resection of the extrahepatic bile duct. Macroscopically, the tumor evidently derived from the left hepatic duct and showed spread into the left intrahepatic bile ducts along with hepatic parenchymal invasion. The feature of tumor spread was expansive and well-demarcated (Figure 3A). Histological examination demonstrated a moderately differentiated adenocarcinoma (Figure 3B) with no nodal metastasis (pT3N0M0). However, the examination did find moderate lymphatic and venous involvement with cancer cells and an extensive invasion of cancer cells into hepatic parenchyma. After removal of the tumor, the serum level of CA19-9 decreased to the normal range, and the patient was discharged 66 d after the operation.
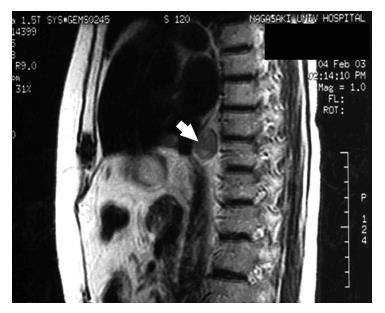
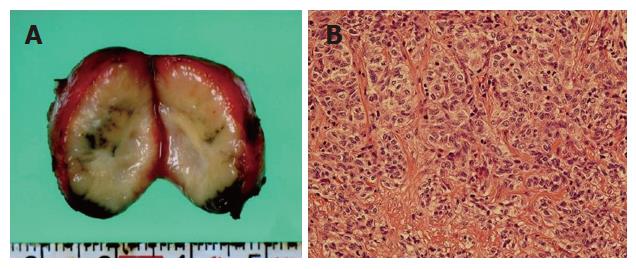
The patient was followed up abdominal CT scanning and tumor marker testing every six months on an outpatient basis. A follow-up abdominal CT examination two years after the operation detected a small nodule, measuring 2.2 cm in diameter, behind the inferior vena cava. Subsequent magnetic resonance imaging localized the nodule to the posterior mediastinum above the diaphragm (Figure 4). Reevaluation three months later with contrast-enhanced CT demonstrated a progressively enlarging nodule in the mediastinum. However, no other swollen lymph nodes, local recurrence, or distant metastasis was noted. Serum levels of CA19-9 and CEA were also normal. In February 2004, the patient underwent thoracoscopic removal of the mediastinal nodule, which had an appearance suspicious of a lymph node metastasis, to establish a definitive diagnosis. Intraoperative ultrasonography was useful for detecting the nodule and understanding the surrounding anatomy. The nodule was located adjacent to the aorta, vena cava, and esophagus. No other swollen lymph nodes were noted. The nodule was carefully removed by electrocautery. The cut surface of the surgical specimen revealed a well-encapsulated, whitish solid mass, measuring 2.2 cm × 2.0 cm (Figure 5A). Histologically, the nodule proved to be a metastatic lymph node containing moderately differentiated adenocarcinoma (Figure 5B) similar in appearance to the pathology of the primary tumor. The patient’s postoperative recovery was prompt and uneventful. Following the second operation, the patient received a total of 50 Gy of irradiation to the lower part of the periesophagus, including the cardiac portion of the stomach, the hepatic hilum, and the paraaortic area. The patient started on oral adjuvant chemotherapy with UFT capsule®, a combination of tegafur (a 5-fluorouarcil prodrug) and uracil in a 1:4 molar ratio, at a combined daily dose of 600 mg concomitant with the extracorporeal irradiation. However, the patient had to discontinue chemotherapy after three days because of nausea and vomiting. After finishing the radiation therapy, the patient started on oral adjuvant chemotherapy again. In June 2006, the patient did well during the four years following her first operation and had no evidence of recurrence 28 mo after her second operation.
Cholangiocarcinoma is an adenocarcinoma arising from the bile duct epithelium and is the second most common primary hepatobiliary cancer. It includes intrahepatic, hilar, and distal extrahepatic tumors of the bile ducts. The hepatic duct bifurcation is the most frequently involved site, and approximately 50% of cholangiocarcinomas are encountered in this region[1-3].
Despite recent advances in diagnostic modalities and intensive management, hilar cholangiocarcinoma still has a dismal prognosis because of an extensive progression and a high frequency of recurrence after surgery. Jarnagin et al[7] have reported a recurrence rate of 68% for hilar cholangiocarcinoma after removal of the primary tumor, and found that most recurrences occur locally and at the following sites: retroperitoneal lymph nodes, peritoneum, liver, and lung.
Pericholedochal lymph nodes in the hepatoduodenal ligament are the most common sites of nodal metastasis in hilar cholangiocarcinoma. These nodes appear to be representative key stations for lymphatic spread of cancer cells towards peripancreatic and more distant lymph nodes[6]. Periportal, common hepatic, and posterior pancreaticoduodenal nodes also show a high incidence of metastatic involvement[6,8]. Thus, two major lymphatic flow pathways have been associated with hilar cholangiocarcinoma: a lymphatic pathway from the hepatoduodenal ligament to the superior border of the pancreas or retropancreatic area, and a pathway to the celiac trunk via the common hepatic area and along the gastric lesser curvature[9].
Our patient, however, developed a solitary nodal recurrence in a posterior mediastinum lymph node 2 years after a curative resection of hilar cholangiocarcinoma despite the absence of nodal metastasis in the hepatoduodenal ligament, around the common hepatic artery, or in the paraaortic area at the time of the first operation. In a study of 13 patients with intrahepatic cholangiocarcinoma in the left hepatic lobe of the liver, Okami et al[10] identified a second metastatic pathway via lymph nodes around the cardiac portion of the stomach or along the gastric lesser curvature in addition to the well-known metastatic pathway via lymph nodes in the hepatoduodenal ligament. In a study employing a contrast material staining technique, Kitazume and Okudaira[11] demonstrated lymph-flow from the intrahepatic lymph vessels to the lower anterior mediastinum and posterior mediastinal lymph nodes (periesophageal lymph nodes). In the present case, histological involvement of the liver parenchyma in the left hepatic lobe with cancer cells was evident along with lymphatic and venous invasion. Thus, we presume that the lymphatic metastasis in our patient arose by direct spread towards the posterior mediastinal lymph node or via the cardiac pathway, as has been reported previously in patients with intrahepatic cholangiocarcinoma in the left lobe of the liver.
We delayed performing the second operation for three months following the detection of a doubtful nodal recurrence in the mediastinum because metastasis after surgical resection of hilar cholangiocarcinoma usually shows a wide spread and because a solitary nodal recurrence in a mediastinal lymph node has not been previously reported. We successfully removed the mediastinal lymph node thoracoscopically, enabling us to make a definitive diagnosis of nodal metastasis with a minimally invasive procedure. Histological examination revealed that the lymph node contained tubular structures and was similar in appearance to the pathology of the primary tumor. Finally we diagnosed the metastasis of hilar cholangiocarcinoma.
Adjuvant therapy for biliary carcinoma utilizes one of the following three alternative approaches: chemotherapy, radiation therapy, or chemoradiation therapy. Based upon a study by Pederson et al[12] reporting that 5-FU prodrug therapy had a radiosensitizing effect on external beam irradiation of cholangiocarcinoma cells, we performed adjuvant radiation therapy combined with UFT capsule® for our patient. To cover the well-known and potential pathways for lymphatic spread mentioned above, the irradiation area included the lower part of the periesophagus, the cardiac portion of the stomach, the hepatic hilum, and the paraaortic area. The patient had to discontinue chemotherapy after three days because of its adverse effects. After finishing the radiation therapy, the patient started on oral adjuvant chemotherapy again. The patient has been doing well since June 2006, with no evidence of recurrence 28 mo after the second surgery.
In conclusion, this case report describes a delayed, solitary nodal recurrence in a posterior mediastinal lymph node in a patient who has previously undergone curative resection of hilar cholangiocarcinoma of the left hepatic duct. Hilar cholangiocarcinoma of the left hepatic duct may thus have an ability to spread to mediastinal lymph nodes via an unusual pathway when the tumor involves hepatic parenchyma along with lymphatic and venous invasion.
S- Editor Wang J L- Editor Wang XL E- Editor Chen GJ
| 1. | Tompkins RK, Saunders K, Roslyn JJ, Longmire WP. Changing patterns in diagnosis and management of bile duct cancer. Ann Surg. 1990;211:614-620; discussion 620-621. [PubMed] |
| 2. | Schoenthaler R, Phillips TL, Castro J, Efird JT, Better A, Way LW. Carcinoma of the extrahepatic bile ducts. The University of California at San Francisco experience. Ann Surg. 1994;219:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Nakeeb A, Lipsett PA, Lillemoe KD, Fox-Talbot MK, Coleman J, Cameron JL, Pitt HA. Biliary carcinoembryonic antigen levels are a marker for cholangiocarcinoma. Am J Surg. 1996;171:147-152; discussion 152-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Kurosaki I, Hatakeyama K, Tsukada K. Long-term survival of patients with biliary tract cancers with lymph node involvement. J Hepatobiliary Pancreat Surg. 1999;6:399-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Todoroki T, Kawamoto T, Koike N, Takahashi H, Yoshida S, Kashiwagi H, Takada Y, Otsuka M, Fukao K. Radical resection of hilar bile duct carcinoma and predictors of survival. Br J Surg. 2000;87:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Kitagawa Y, Nagino M, Kamiya J, Uesaka K, Sano T, Yamamoto H, Hayakawa N, Nimura Y. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 222] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Jarnagin WR, Ruo L, Little SA, Klimstra D, D'Angelica M, DeMatteo RP, Wagman R, Blumgart LH, Fong Y. Patterns of initial disease recurrence after resection of gallbladder carcinoma and hilar cholangiocarcinoma: implications for adjuvant therapeutic strategies. Cancer. 2003;98:1689-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 337] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Kayahara M, Nagakawa T, Ueno K, Ohta T, Takeda T, Miyazaki I. Lymphatic flow in carcinoma of the distal bile duct based on a clinicopathologic study. Cancer. 1993;72:2112-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Yoshida T, Shibata K, Yokoyama H, Morii Y, Matsumoto T, Sasaki A, Kitano S. Patterns of lymph node metastasis in carcinoma of the distal bile duct. Hepatogastroenterology. 1999;46:1595-1598. [PubMed] |
| 10. | Okami J, Dono K, Sakon M, Tsujie M, Hayashi N, Fujiwara Y, Nagano H, Umeshita K, Nakamori S, Monden M. Patterns of regional lymph node involvement in intrahepatic cholangiocarcinoma of the left lobe. J Gastrointest Surg. 2003;7:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Pederson LC, Buchsbaum DJ, Vickers SM, Kancharla SR, Mayo MS, Curiel DT, Stackhouse MA. Molecular chemotherapy combined with radiation therapy enhances killing of cholangiocarcinoma cells in vitro and in vivo. Cancer Res. 1997;57:4325-4332. [PubMed] |