INTRODUCTION
Liver fibrosis and cirrhosis can lead to the development of hepatocellular carcinoma (HCC). The incidence of HCC in cirrhotic patients is 10-fold higher than among non-cirrhotic patients. Other risk factors for HCC are male gender, age above 50 years and elevated alpha-fetoprotein (AFP).
A relatively infrequent cause of cirrhosis is hereditary hemochromatosis (HHC), which belongs to a group of iron-storage diseases due to mutations in the HFE gene. All forms of HHC present with massive iron accumulation in hepatocytes, ultimately leading to cirrhosis. However, the clinical course of patients with HHC may vary considerably. HHC-induced hepatic iron overload may not only lead to cirrhosis, but may also trigger the manifestation of porphyria cutanea tarda (PCT). PCT is a metabolic disorder characterised by an increased amount of circulating porphyrins due to disrupted heme biosynthesis. The porphyrins accumulate particularly in the skin where they induce cytotoxic effects. PCT occurs both as hereditary (autosomal dominant inheritance) and acquired (sporadic) disease and can be triggered by iron overload, alcohol, estrogens and viral infections. The induction of PCT by iron overload also explains the association of this disease with hemochromatosis gene mutations.
We report about a patient who presented with HCC occurring in a fibrotic liver due to a hitherto unrecognized homozygous hemochromatosis and PCT.
CASE REPORT
A 68-year old male patient was referred to our clinic with a 5 cm mass in the left liver lobe detected by a CT-scan. The elevated AFP levels (434 μg/L) were highly suggestive of HCC. There were no morphological or biochemical signs of liver cirrhosis and preoperative laboratory values were normal (bilirubin 0.9 mg/dL, albumin 4.0 g/dL, INR 1.09, AST 52 IU/L). The patient presented in good clinical condition without fever, weight loss or pain. Additional diagnoses were atrial fibrillation, arterial hypertension, type 2 diabetes mellitus and hypothyroidism. Physical examination revealed vitiligous lesions on sun-exposed skin. The patient recalled them to be associated with “winegrowers’ disease” 25 years earlier.
An extended left hemihepatectomy was performed without surgical complications. The postoperative course was uneventful. Liver enzymes returned to normal values within the first week, but a persistent hyperbilirubinemia, with bilirubin levels around 3 mg/dL, was noticed. In order to rule out cholestasis or impaired liver perfusion, Doppler-ultrasound, CT-scan and MR-cholangiography were performed. These exams failed to reveal significant pathologies, apart from alterations in the MRI, suggesting an iron overload.
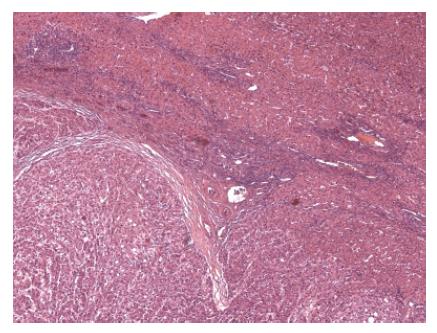
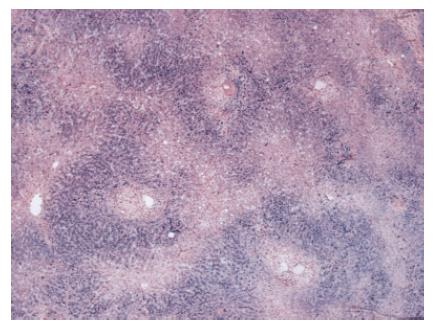
Histology confirmed a moderately to poorly differentiated HCC (Figure 1) and described a hitherto unrecognized periportal and initial bridging fibrosis with marked hemosiderosis (Figure 2). A transferrin saturation > 95% raised the suspicion of HHC. Molecular testing revealed a homozygous C282Y mutation within the HFE gene, confirming the diagnosis of HHC. Elevated urinary porphyrin levels (porphyrin 1638 μg/d, coproporphyrin 264 μg/d and uroporphyrin 624 μg/d) confirmed the diagnosis of PCT. Symptomatic therapy, including phlebotomies, resulted in normalization of transferrin saturation and iron levels. The hyperbilirubinemia resolved several weeks after discharge. Maintenance therapy with chloroquine has not been necessary so far. After one year follow-up no recurrence of HCC has been detected.
Figure 1 Histology of HCC with hemosiderosis, Hematoxylin and eosin staining.
Figure 2 Histology of hemosiderosis, Berlin blue staining.
DISCUSSION
We present the unhappy triad of HCC, HHC and PCT. HHC is one of the most frequent autosomal recessive hereditary diseases in European Caucasians with a prevalence of 1:200 to 1:500. Patients show extensive deregulation of their iron metabolism with elevated transferrin saturation and high ferritin levels. Later, they develop clinical symptoms due to the iron overload of internal organs. The mutation responsible for HHC is based on a 854G → A transition which leads to a cys282tyr substitution in the HFE gene on chromosome 6p21.3[1]. The specific effect of this mutation on the iron metabolism has not been entirely clarified. The favoured model describes the iron uptake at the basolateral membrane of duodenal crypt cells via the transferrin 1-receptor (TfR1) that needs the interaction with the wild-type HFE-molecule to guarantee the balance between absorption, storage and consumption[2,3]. Further allelic variants in the HFE gene have been described but seem to lack clinical relevance[4-6].
Our patient presented with an additional hereditary disease, PCT. PCT belongs to the non-acute porphyric diseases that are mostly hereditary and only rarely sporadic. Most porphyrias become clinically apparent after exposure to triggering factors such as alcohol, UV radiation, hormones, viral hepatitis and porphyrogenic drugs. The prominent feature of non-acute porphyrias is an increased photosensitivity with lesions on sun-exposed skin. Our patient’s cutaneous lesions led to hypothesize a non-acute hepatic porphyria. The so-called “winegrowers’ disease”, he erroneously recalled, may have been caused by arsenic poisoning. The German word is “Winzer” disease, which sounds like the word “Windsor” disease, a term that was used synonymously in spoken language for acute intermittent porphyria, a hereditary defect in the British royal family. PCT is the most common porphyric disease worldwide. It is caused by a mutation of the uroporphyrinogen-decarboxylase gene (URO-D) on chromosome 1p34[7]. URO-D is the fifth enzyme of heme biosynthesis and decarboxylates uroporphyrinogen to coproporphyrinogen[8].
About 80% of all PCT patients carry the sporadic mutation that is singularly associated with a 50% decrease in URO-D activity of the liver[9]. Only approximately 20% of all patients suffer from the autosomal dominantly inherited form of PCT that causes a 50% reduction of the URO-D-activity in all tissues[10]. Due to the malfunctioning URO-D, oxidized uroporphyrinogen accumulates in the liver, is excreted in urine and feces and becomes deposited in skin. Here, the photoactivation of the porphyrines by UV radiation leads to the typical lesions with blisters, erosions and scarring. URO-D function is also influenced by iron. Iron directly blocks URO-D and produces peroxides and free radicals that oxidize uroporphyrinogen to porphyrin, which by itself blocks URO-D function. As soon as the iron content of the liver is substantially decreased by phlebotomy, the URO-D activity is partially restored[11].
The latter interaction constitutes the link between porphyrias and hemochromatosis. HHC, as an iron storage disease, precipitates the clinical manifestation of porphyrias through various mechanisms. The excessive amount of iron, on the one hand, catalyses the formation of reactive oxygen-species that oxidize uroporphyrinogen to uroporphyrin. On the other hand, iron inhibits URO-D indirectly with oxidized metabolites that block the enzyme. Additionally, induction of the δ-ALA-synthase increases the amount of δ-aminolevulinic acid, the primary product of the porphyrin synthesis pathway, thus accumulating intermediate products which provoke the symptoms of PCT. The high frequency of homo- and heterozygous allele carriers for the C282Y- and H63D-mutations of HHC among patients with sporadic PCT, as opposed to healthy controls, was demonstrated in several countries, such as Germany[12], Great-Britain[13], the Netherlands[14] and Australia[15]. Further studies confirmed the HFE mutations as a risk factor for PCT as well as additional risk factors like alcohol consumption, smoking and hepatitis C virus infection[16-18].
HHC and PCT may have triggered the development of HCC in our patient. HCC is one of the few cancers with well-defined risk factors. Among these, liver cirrhosis is believed to be the strongest predisposing factor[19] and also fibrotic changes seem to induce carcinogenesis. Other risk factors differ regionally, such as hepatitis B virus and aflatoxin B1 exposure in Asia and Africa[20]. Further predisposing factors are male gender, age above 50 years, elevated AFP and alcohol[21]. Concerning HHC, only few data exist. Homozygosity for the C282Y mutation seems to increase the risk for HCC in cirrhotic patients, but neither heterozygous nor compound heterozygous (C282Y/H63D) mutations are unequivocally identified risk factors[22,23]. In 2001, PCT was described to be a risk factor for the development of malignant liver tumours, confirming similar observations reported over 30 years earlier[24-26].
Combining these statements we describe the unu-sual combination of HCC in a non-cirrhotic patient with two rather rare conditions, HHC and PCT. Both hereditary diseases are representing minor risk factors for carcinogenesis, but, taken together, may synergistically increase the risk of developing HCC. HHC, as iron-storage disease, on the one hand, promoted the symptoms of PCT, but, on the other hand, may also have triggered carcinogenesis, even with only minimal parenchymal destruction. PCT itself, via the iron overload of HHC, should be considered as carcinogenic risk factor of equal importance, although only few reports underlined this fact so far. We state that both conditions in our case must be considered equally important for the development of HCC, as no additional risk factors could be identified.
In conclusion, the case presented here with HCC, HHC and PCT is special in several ways. Histology revealed a fibrosis with pronounced hemosiderosis suggesting a HHC, which was confirmed by genetic assay. Nevertheless, this constituted an unusual risk profile for the development of HCC, as approximately 80% of all HCCs develop in cirrhosis due to HCV infection and alcoholism, while HHC only ranks as cofactor of carcinogenesis. However, the hemochromatosis amplified the manifestations of a hitherto unrecognized PCT, another risk factor for carcinogenesis.
To the best of our knowledge, this is the first report ever of the unhappy triad of HCC in a fibrotic liver with PCT and homozygous HHC. It may shed light on the importance of both risk factors, HHC and PCT, for the carcinogenesis of HCC and certainly has to be taken into account in the medical care of these patients.