Published online Apr 7, 2007. doi: 10.3748/wjg.v13.i13.1953
Revised: December 5, 2006
Accepted: January 9, 2007
Published online: April 7, 2007
AIM: To investigate the hypothesis that the protective effects of curcumin in hepatic warm ischemia/reperfusion (I/R) injury are associated with increasing heat shock protein 70 (Hsp70) expression and antioxidant enzyme activity.
METHODS: Sixty Sprague-Dawley male rats were randomly divided into sham, I/R, C + I/R groups. The model of reduced-size liver warm ischemia and reperfusion was used. Curcumin (50 mg/kg) was administered by injection through a branch of superior mesenteric vein at 30 min before ischemia in C + I/R group. Five rats were used to investigate the survival during 1 wk after operation in each group. Blood samples and liver tissues were obtained in the remaining animals after 3, 12, and 24 h of reperfusion to assess serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), liver tissue NO2- + NO3-, malondialdehyde (MDA) content, superoxide dismutase (SOD), catalase (CAT), nitricoxide synthase (NOS) and myeloperoxidase (MPO) activity, Hsp70 expression and apoptosis ratio.
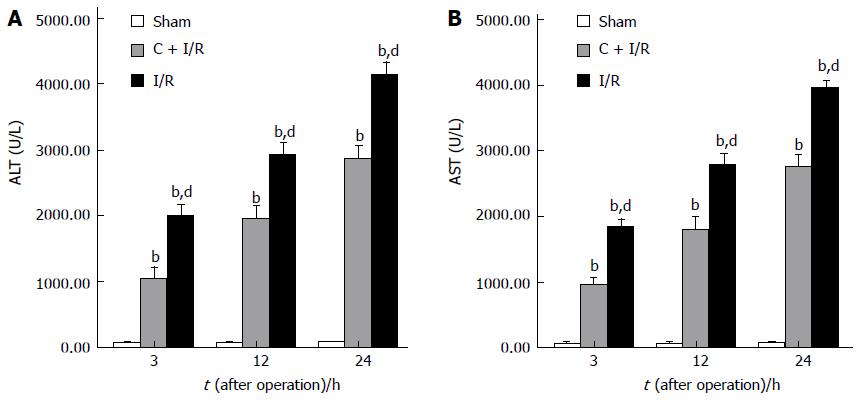
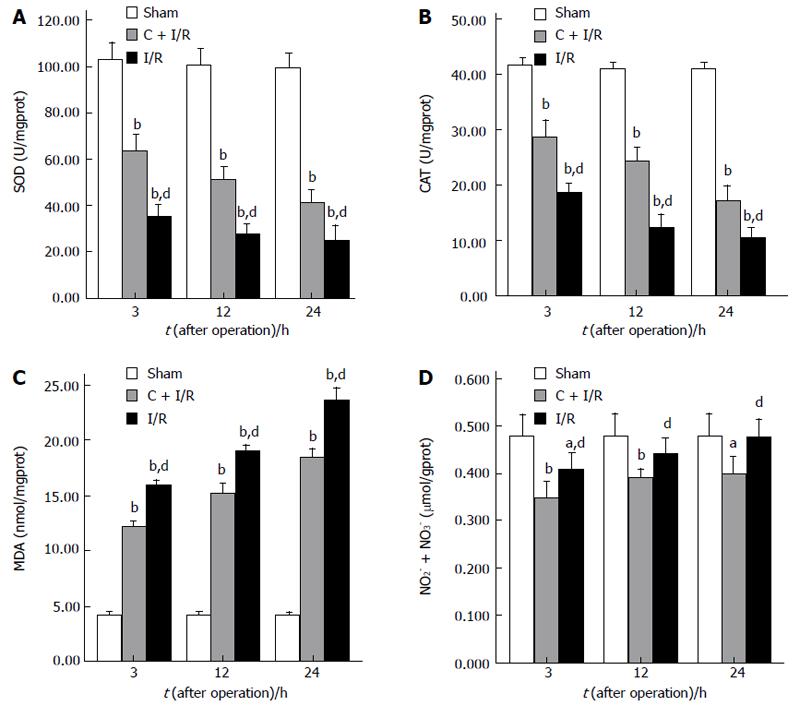
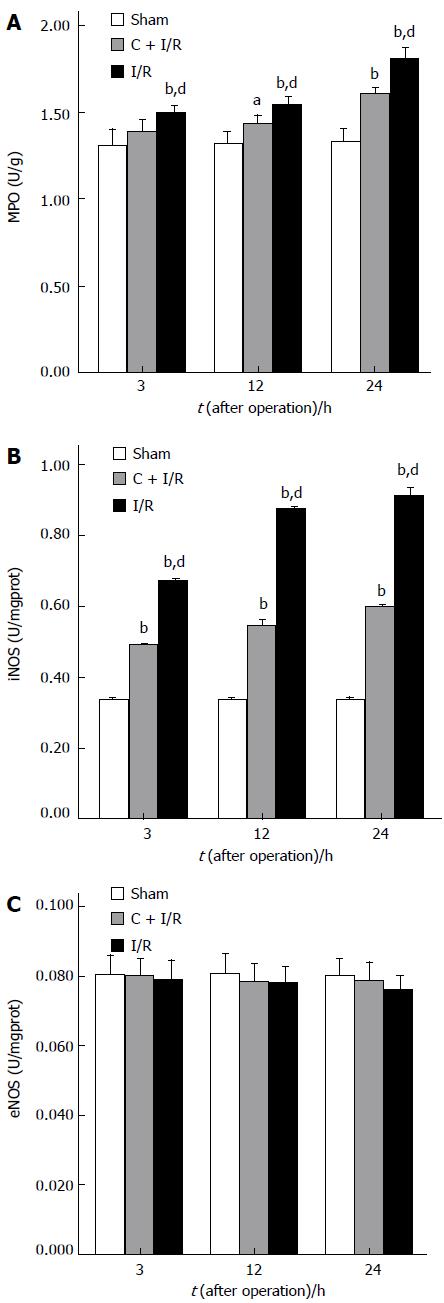
RESULTS: Compared with I/R group, curcumin pretreatment group showed less ischemia/reperfusion-induced injury. CAT and SOD activity and Hsp70 expression increased significantly. A higher rate of apoptosis was observed in I/R group than in C + I/R group, and a significant increase of MDA, NO2- + NO3- and MPO level in liver tissues and serum transaminase concentration was also observed in I/R group compared to C + I/R group. Curcumin also decreased the activity of inducible NO synthase (iNOS) in liver after reperfusion, but had no effect on the level of endothelial NO synthase (eNOS) after reperfusion in liver. The 7 d survival rate was significantly higher in C + I/R group than in I/R group.
CONCLUSION: Curcumin has protective effects against hepatic I/R injury. Its mechanism might be related to the overexpression of Hsp70 and antioxidant enzymes.
- Citation: Shen SQ, Zhang Y, Xiang JJ, Xiong CL. Protective effect of curcumin against liver warm ischemia/reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J Gastroenterol 2007; 13(13): 1953-1961
- URL: https://www.wjgnet.com/1007-9327/full/v13/i13/1953.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i13.1953
Liver warm ischemia/reperfusion (I/R) injury leads to liver cell (parenchymal and sinusoidal cells) damage, retards liver cell regeneration and predisposes to liver failure. Since I/R injury resulting in liver failure poses severe clinical problems, it is important for surgeons to prevent or ameliorate it.
Several factors contribute to liver warm I/R injury. Hepatic ischemic injury is caused by oxygen deficiency. During reperfusion, hypoxia organ damage is aggravated following recovery of blood flow and oxygen delivery. During the ischemic period, hypoxia causes mitochondrial adenosine triphosphate depletion, alters H+, Na+, and Ca2+ homeostasis, activates hydrolytic enzymes, and impairs cell-volume regulation. After reperfusion, Kupffer cell activation is a principal pathophysiologic mechanism of early liver reperfusion injury. Activated Kupffer cells release reactive oxygen species (ROS), nitric oxide, and several proinflammatory cytokines. These cytokines, together with the increased expression of adhesion molecules by sinusoidal endothelial cells, promote liver neutrophil infiltration, thus contributing to the progression of parenchymal injury. Efforts to attenuate I/R injury have been focused on blocking the events associated with inconvertible liver damage.
Curcumin [1, 6-heptadiene, 3-5-dione, 1, 7-bis (4-hydroxy-3-methoxyphenyl) (C21H20O6)], a yellow-orange dye extracted from the spice tumeric, possessing both anti-inflammatory antioxidant and anti-carcinogenic effect[1], is a potent stimulator of the stress-induced expression of heat shock protein 70 (Hsp70). It was recently reported that administration of curcumin could ameliorate I/R injury in rat kidney, myocardium, and nervous tissue[2-6]. We aimed to study whether curcumin can ameliorate I/R injury, inhibit hepatocyte apoptosis, and induce Hsp over-expression in hepatic I/R injury.
Male Sprague-Dawley rats (8-10 wk old) weighing 220-300 g were purchased from the Center of Experimental Animals, Medical College of Wuhan University. This project was approved by the Committee of Experimental Animals of Wuhan University. All experimental procedures complied with the Guide for the Care and Use of Laboratory Animals.
Fasted (10-14 h) rats were anesthetized with 20% urethane solution (5 mL/kg). After midline laparotomy, liver warm ischemia was induced by clamping the left branches of both portal vein and hepatic artery to prevent portal congestion for 45 min (ischemia of about 70% liver). After 45 min of ischemia, the clip was released to allow reperfusion of the liver, and the non-ischemic lobes were immediately excised, leaving the remaining two reperfusion lobes. The abdominal cavity was closed, and the animals were allowed to recover with free access to food and water. The rats were divided into three groups: sham group in which the rats were laparotomized for 5 min without hepatectomy or vascular clamping, I/R group and C + I/R group (ischemia/reperfusion + curcumin infusion group) (20 animals per group). The rats in C + I/R group were injected with 50 mg/kg curcumin (Sigma, St. Louis, MO) (dissolved in 1 mL dimethyl sulfoxide solution) into a branch of superior mesenteric vein 30 min before the left branches of both portal vein and hepatic artery were clamped. The rats in I/R group were infused with only 1 mL dimethyl sulfoxide 30 min before the left branches of vessels were clamped. Five rats in each group were used to investigate the survival in 1 wk. Five rats in each group were killed at 3, 12, or 24 h after the start of reperfusion, and the left ischemic lobe of the liver was removed, weighed, snap frozen in liquid nitrogen and stored at -70°C for biochemical analysis or stored in 4% PBS-buffered paraformaldehyde for histopathological analysis. Blood samples were collected via the inferior vena cava.
Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) concentrations were measured using standard techniques with a serum analyzer (Olympus Automated Chemistry Analyzer AU2700, Japan).
Liver homogenate malondialdehyde (MDA) concentration was measured using the thiobarbituric acid (TBA) method. The amount of lipid peroxides (LPO) was measured as the production of MDA, which in combination with TBA forms a pink chromogen compound whose absorbance at 532 nm was measured. The result was expressed as nmol/mg protein.
Total superoxide dismutase (SOD) activity in hepatic homogenate was measured through the inhibition of nitroblue tetrazolium (NBT) reduction by O2- generated by the xanthine/xanthine oxidase system. One SOD activity unit was defined as the enzyme amount causing 50% inhibition in 1 mL reaction solution per milligram tissue protein and the result was expressed as U/mg protein.
Catalase (CAT) activity in liver homogenate was detected using ammonium molybdate method by measuring the intensity of a yellow complex formed by molybdate and H2O2 at 405 nm, after ammonium molybdate was added to terminate the H2O2 degradation reaction catalyzed by CAT. An enzyme activity unit was defined as the degradation of 1 mmol H2O2 per second per mg tissue protein and the enzyme activity was expressed as U/mg protein.
Liver homogenate NO products nitrite/nitrate (NO2- + NO3-) were detected using a colorimetric NO detection kit, which reflected the concentration of NO in the liver.
Total nitricoxide synthase (NOS), endothelial NO synthase (eNOS) and inducible NO synthase (iNOS) activity in hepatic homogenate was also detected using a spectrophotometric method assay kit.
Myeloperoxidase (MPO) activity in hepatice tissue was detected using a spectrophotometric method, reflecting the number of polymorphonuclear neutrophils (PMN) in the liver. This method uses 3, 3’, 5, 5’-tetramethyl benzidine (TMB) as an oxidizable dye, and the reaction was started by adding hydrogen peroxide (H2O2) in the medium.
Total protein concentrations in liver homogenate samples were determined with Coomassie blue method.
All assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Hsp70 mRNA was determined in liver tissues using semi-quantitative RT-PCR at 3, 12, and 24 h after surgery. Total RNA was isolated from liver tissue by using Trizol reagent (GIBCO BRL, Gaithersburg, MD). Five μg of RNA was reverse-transcribed to complementary DNA (cDNA) by using M-MuLV reverse transcriptase (Applied Biosystems, Foster City, CA) at 42°C for 90 min, and cDNA was then amplified by using the following primers for the rat Hsp70mRNA gene (sense: 5'-TGCCCGCCTACTTCAACGA-3' (614-632 bp) and anti-sense: 5'-CCGCGTGATGGACGTGTAG-3' (1074-1056 bp), product of the reaction was 461 bp. Thermal cycle conditions were 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s, for a total of 30 cycles. The final cycle was followed with 5 min incubation at 72°C. Amplification of a house keeping gene (β-actin) was performed by the same reaction program using the following primers for the rat β-actin mRNA gene (sense: 5’-TGGAGAAGATTTGGCACC-3’ (54-61 bp) and anti-sense: 5’-TACGACCAGAGGCATACAGG-3’ (222-241 bp) (all primers were synthesized by SBS Genetech, Shanghai, China), product of the reaction was 188bp. Seven point five μL of RT-PCR products was subjected to electrophoresis on 2% agarose gel containing 0.05% ethidium bromide and analyzed by densitometry using BIO-RAD Quantity One 4.4.0 software (BIO-RAD, Hercules, CA). Hsp70 mRNA expression was illustrated by determining the ratio of band intensity of Hsp70 and β-actin mRNA.
Hsp70 protein expression in liver tissue was detected using a commercial Hsp70 ELISA kit (Stressgen Bioreagents, Ann Arbor, MI). The protein was isolated from liver tissue homogenate by using protein extraction reagent. According to the assay procedure supplemented with the kit, Hsp70 standards and samples were added to the Hsp70 immunoassay plate, incubated and washed, then anti-Hsp70 biotin conjugate was added, incubated and washed, finally avidin-HRP conjugate was added, incubated and washed. The plant was developed with 3, 3', 5, 5' tetramethylbenzidine. We added acid solution to the plant, and measured its absorbance at 450 nm. Hsp70 in samples was calculated from a curve of absorbance vs log of the standards. Tissue total protein concentration was also measured with Coomassie blue method (assay kit was purchased from Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Excised liver specimens were fixed in 4% PBS-buffered paraformaldehyde and embedded in paraffin. Five-micrometer liver sections stained with hematoxylin and eosin were evaluated at × 200 magnification by a point-counting method for severity of hepatic injury using the ordinal scale: grade 0 = minimal or no evidence of injury; grade 1 = mild injury with cytoplasm vacuolization and focal nuclear pyknosis; grade 2 = moderate to severe injury with extensive nuclear pyknosis, loss of intercellular borders, and mild to moderate neutrophil infiltration; grade 3 = severe injury with disintegration of hepatic cords, hemorrhage, and severe PMN infiltration. Statistical evaluation was made using this scale. An average of 100 adjacent points on 1 mm2 grid was graded for each specimen.
Liver sample was fixed in 4% PBS-buffered paraforma-ldehyde and embedded in paraffin. Apoptosis was detected by using the terminal deoxynucleotidyl transferase-mediated biotin-dUTP nick end-labeling (TUNEL) technique with in situ apoptosis detection kit (Beijing Zhongshan Biotechnology Co. Ltd, Beijing, China) according to the manufacturer’s instructions. The number of TUNEL-positive hepatocytes was counted by 2 blinded observers under microscope (× 400 magnification). Five different visual fields were counted in each section. Hepatocytes with brown-stained nuclei were TUNEL-positive. The percentage of TUNEL-positive cells (apoptosis index) was calculated by the number of apoptotic cells divided by the total cells.
Fresh liver tissue samples were taken under aseptic conditions. Small vessels and connective tissue were removed. They were placed on a sterile 250 μm brass mesh over a conical tube, and gently teased with sterile forceps. Cells were rinsed through the brass mesh with pre-cold RPMI-1640 (Invitrogen, Carlsbad, CA), and washed twice at a concentration of 300 ×g for 4 min.
Apoptosis was detected using the Annexin V-FITC kit (BD-Pharmingen, San Diego, CA). Liver cell samples were washed twice with cold PBS and resuspended in 1 × binding buffer at a concentration of 1 × 106 cells/mL. One hundred μL of the cell suspension was incubated with Annexin V-FITC (5 μL) and propidium iodide (5 μL) in binding buffer on ice for 10 min in dark, then 150 μL of ice-cold (diluted in 1 × binding buffer) was added to the cell samples. Early apoptotic cells (Annexin V positive and propidium iodide negative) were quantitated by flow cytometry.
All values were expressed as mean ± SE. Statistical significance between two groups of parametric data was evaluated by ANOVA. Survival curves for 7 d were analyzed with the Kaplan-Meier method, and the differences in survival rates were evaluated by log-rank test. All statistical analyses were performed with the SPSS statistical package (SPSS 14.0 for Windows; SPSS, Inc, Chicago, IL). P < 0.05 was considered statistically significant.
Changes in serum AST and ALT concentrations after operation in each group are shown in Figure 1. Serum ALT and AST levels increased significantly after reperfusion in I/R group compared with sham group. Pretreatment with curcumin significantly decreased AST and AlT levels compared with I/R group.
The MDA content as an index of lipid peroxidation was significantly higher after reperfusion in I/R group than in sham group. Pretreatment with curcumin significantly decreased MDA content after 12 and 24 h of reperfusion (P < 0.05). CAT and SOD activity in hepatic tissue decreased markedly after reperfusion as compared with the sham group. In contrast, a significant decrease in the activity of CAT and SOD was observed in I/R group compared with curcumin pretreatment group (Figure 2).
Changes in nitric oxide of hepatic tissue measured as total NO2- + NO3- improved in the I/R group compared with the sham group after I/R. Pretreatment with curcumin significantly decreased total NO2- + NO3- levels when compared with I/R group without curcumin pretreatment (Figure 2).
Changes in iNOS, eNOS, and MPO activity after operation in each group are shown in Figure 3. MPO and iNOS activity increased in liver samples from animals subjected to ischemia-reperfusion as compared with the sham group. Curcumin pretreatment significantly reduced the MPO and iNOS activity compared with I/R group. No significant difference in eNOS activity was observed between curcumin pretreatment group and other groups after operation.
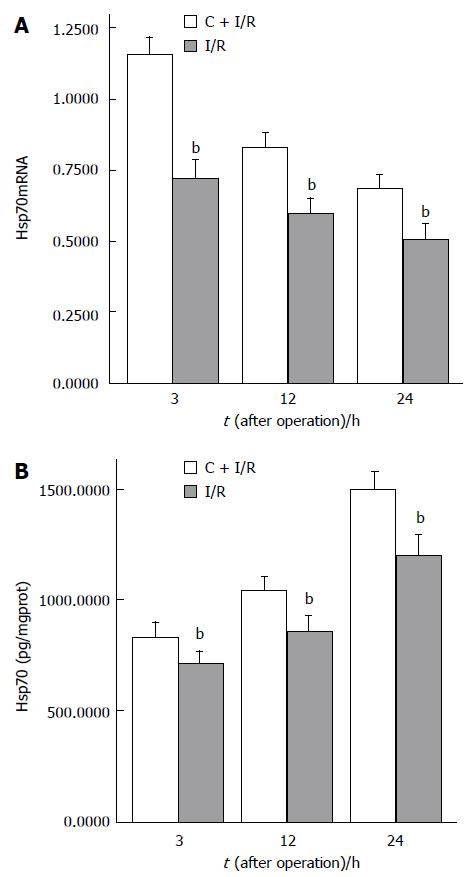
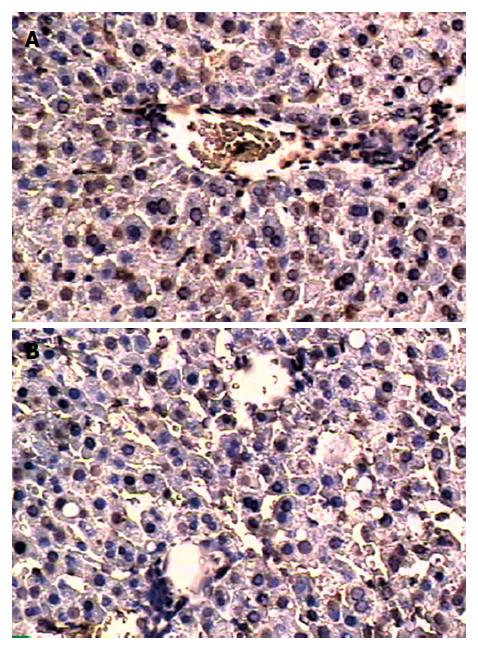
The expressions of β-actin mRNA and Hsp70mRNA are shown in Figure 4. Hsp70mRNA expression was not detected in sham group. No obvious alteration in β-actin mRNA expression was found in all groups. Hsp70mRNA expression in C + I/R group was significantly higher than that in I/R group after reperfusion. The expression of Hsp70 is shown in Figure 5.
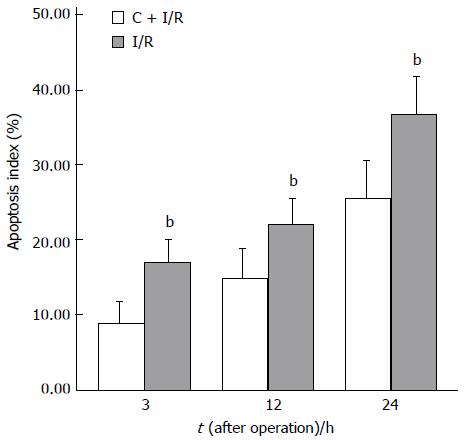
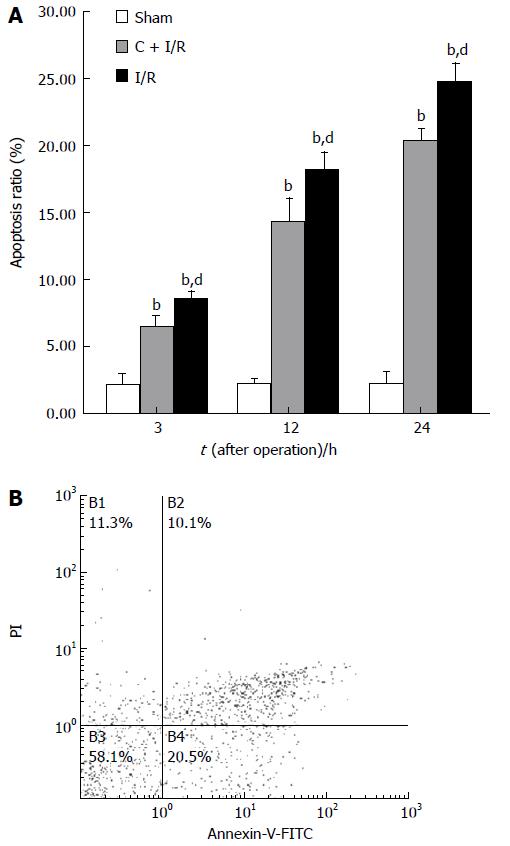
Analysis of apoptosis by TUNEL method showed that the apoptosis index was lower in C + I/R group than in I/R group. Very few TUNEL-positive hepatocytes were detected in sham group. TUNEL-positive hepatocytes remarkably increased to about 36% of total hepatocytes in I/R group at 24 h after reperfusion, the increase in TUNEL-positive hepatocytes was significantly inhibited by about 25% in the C + I/R group at the same time point (Figures 6 and 7). Flow cytometry showed a similar difference between C + I/R group and I/R group (Figure 8).
No significant changes were found in sham group at light microscopy. At 3 h after reperfusion, cytoplasmic vacuolation and focal nuclear pyknosis were seen in I/R group. At 12 and 24 h after reperfusion, extensive karyopycnosis, severe necrosis and loss of intercellular borders were seen in I/R group. At these time points, hepatocyte damage was slighter in curcumin treatment group than in I/R group (Table 1). These results indicated that hepatic injury induced by ischemia-reperfusion was much more severe in I/R group than in C + I/R group.
| Time after operation | Severity of hepatic injury | ||||
| G0 | G1 | G2 | G3 | ||
| 3 h | Sham group | 5 | |||
| I/R group | 4 | 1 | |||
| C + I/R group | 5 | ||||
| 12 h | Sham group | 5 | |||
| I/R group | 2 | 3 | |||
| C + I/R group | 3 | 2 | |||
| 24 h | Sham group | 5 | |||
| I/R group | 1 | 4 | |||
| C + I/R group | 3 | 2 | |||
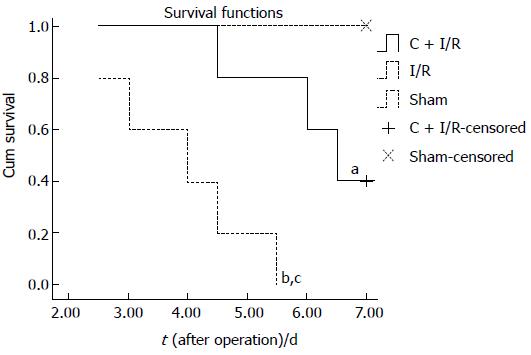
The 7 d survival was significantly longer in C + I/R group than in I/R group (Figure 9). No deaths occurred in sham group.
Heat-shock protein chaperones induced by stress are essential cellular protective machineries[7,8]. Hsp70 is an endogenous factor for protecting cell and tissue injury under various pathological conditions[9,10]. It has been well established that over-expression of Hsp70 could ameliorate various stress-induced injuries. Over-expression of Hsp70 can protect organs against I/R injury, stress-induced apoptosis, and toxic effects of nitric oxide[11-13]. Hsp70 over-expression induced by ischemic preconditioning or other pretreatment maneuvers can ameliorate tissue damage and improve resistance to I/R injury[14]. Michael et al[15] reported that stress-induced NO production in stressed cells is decreased by heat shock reaction through repression of iNOS gene transcription after heat shock pretreatment. Hsp70 and other heat shock proteins may play an essential role in mediating iNOS inhibition. Hsp70 overexpression could protect cells against stress-induced cell death and apoptosis[11,16]. Hsp70 could prevent apoptosis by affecting both upstream signaling (SAPK/JNK activation) and downstream effector caspases (caspase-3)[10,17-19], as well as cell death and apoptosis by interfering with the ability of cytochrome C and Apaf-1 to recruit procaspase-9[19-21]. Curcumin also directly blocks the I/R-mediated increase in JNK1 and c-Jun, thus decreasing I/R-induced cell death and apoptosis[22].
It was reported that curcumin, a potent stimulator of stress-induced Hsp70 expression, exhibits protective effects against some forms of stress, such as heat or toxicant by mechanisms distinct from the increased hepatic Hsp70 expression[23-25]. In this study, we explored whether curcumin pretreatment could increase the expression of Hsp70 in rat livers after warm I/R.
In the present study, Hsp70 over-expression in liver tissues was detected after ischemia-reperfusion. Compared with I/R group, Hsp70 and Hsp70mRNA expression increased significantly in curcumin pretreatment group. Curcumin pretreatment also attenuated the magnitude of hepatic injury and inhibited apoptosis and iNOS activity induced by ischemia-reperfusion compared with I/R group, suggesting that over-expression of Hsp70 after curcumin pretreatment could protect rat liver against I/R injury by reducing apoptosis and iNOS activity. Induction of Hsp70 expression is an important mechanism of curcumin against hepatic warm I/R injury.
Oxygen-derived free radicals formed after reperfusion also play an important role in hepatic I/R injury and contribute to regulation of signal transduction, oxidative damage to nucleic acids, proteins and lipids[26]. Reactions of oxygen-derived free radicals with biomolecules such as lipids-initiated chain reactions lead to tissue damage. LPO, also an important index for damage degree, results in structural and functional derangement and eventually cell death. It was reported that the protective effects of antioxidants against I/R injury in the heart, liver, intestine and kidney, play an important role of ROS in pathogenesis[27]. Superoxide dismutase (SOD), an oxygen radical scavenger which converts superoxide anion radicals present in the upper stream of reactive oxygen metabolism cascade, protects cells against damage. Catalase, an oxidoreductase that catalyzes the conversion of hydrogen peroxide to water and oxygen, also can protect cells from damage induced by ischemia/reperfusion through scavenging ROS. It is present in many animal cells. The efficacy of SOD on I/R injury of the liver has also been reported[28]. Previous studies have shown that curcumin could increase antioxidant enzyme expression and activity in tissue, inhibit neutrophil infiltration, and protect cell function under different stress conditions[29,30]. The results of the present study showed that pretreatment with curcumin could increase total SOD activity, and decrease MDA concentration and MPO activity, suggesting that curcumin pretreatment also increases antioxidative bioactive molecule expression in liver after I/R injury, and attenuates neutrophil infiltration and ROS injury in liver.
NO, a short-lived free radical, influences the physiolo-gical processes in almost all organs and tissues[31,32]. NO is generated by three known NO synthase isoforms: constitutively expressed endothelial NO synthase, neuronal NO synthase (type 1 NOS), and inducible NO synthase[33], which utilize semi-essential amino acid L-arginine, oxygen and reduced form of nicotinamide-adenine dinucleotide phosphate (NADPH) as substrates to produce NO and L-citrulline. Both cytoprotective and cytotoxic effects of NO have been demonstrated and NO produced from eNOS is beneficial to the liver[34,35], but NO produced from iNOS has opposite effects. Inducible NO synthase- generated NO not only directly contributes to tissue damage but also up-regulates the inflammatory response[34]. After hepatic warm I/R, iNOS is over-expressed, arginase also accumulates in liver tissue because of hypoxia. Since arginase and iNOS compete for L-arginine[36] and NADPH is deficient in live tissue because of oxygen deficiency, NO in liver tissue does not increase with iNOS over-expression at the early stage of liver warm I/R injury. At the late stage of liver I/R injury, NO in liver tissue may be impacted by other factors, such as activated Kupffer cells and infiltrated neutrophils.
Chan et al[37] reported that in vivo oral treatment of curcumin reduces iNOS mRNA expression in the liver of lipopolysaccharide (LPS)-injected mice by 50%-70%. It was reported that low concentrations of curcumin could inhibit NO production in activated macrophages, demonstrating that curcumin significantly reduces the levels of mRNA and 130-kDa protein of inducible NOS is expressed in activated macrophages[38,39].
The present study also showed that iNOS activity was significantly decreased in rats pretreated with curcumin after reperfusion. Curcumin pretreatment did not affect eNOS activity in hepatic tissues. However, the nitrate/nitrite levels were substantially decreased, thus significantly reducing organ damage in C + I/R group when compared with I/R group.
In summary, curcumin pretreatment protects liver from warm I/R injury through multiple pathways, regulates multiple bioactive molecule expression and activity, and inhibits cell apoptosis and neutrophil infiltration. Over-expression of Hsp70 and antioxidant enzymes after pretreatment with curcumin may play an essential role in protecting liver against warm I/R injury.
Liver warm ischemia-reperfusion (I/R) injury leads to liver cell (parenchymal and sinusoidal cells) damage, retards liver cell regeneration and predisposes to liver failure. Since I/R injury resulting in liver function failure poses significant clinical problems, it is important for surgeons to prevent or ameliorate I/R-induced liver injury. Curcumin [1, 6-heptadiene, 3-5-dione, 1, 7-bis (4-hydroxy-3-methoxyphenyl) (C21H20O6)], a yellow-orange dye extracted from spice tumeric, possessing both anti-inflammatory antioxidant and anti-carcinogenic effect, is a potent stimulator of stress-induced expression of heat shock protein 70 (Hsp70). It has been reported that administration of curcumin attenuates I/R injury in rat kidney, myocardium, and nervous tissue. In this study, we aimed to study whether curcumin could ameliorate I/R injury, inhibit hepatocyte apoptosis, and induce Hsp and antioxidant enzymes over-expression in hepatic I/R injury.
Curcumin has antioxidant and anti-carcinogenic properties. It has been reported that administration of curcumin attenuates I/R injury in rat kidney, myocardium, and nervous tissue, but no research has investigated its protective effects against liver warm I/R injury.
The objective of this study was to examine the hypothesis that the protective effects of curcumin against hepatic warm I/R injury are associated with increasing Hsp70 expression and antioxidant enzyme activity. Our data demonstrate that pretreatment with curcumin could protect rat liver from warm I/R injury through multiple pathways, such as expression of Hsps, decreased NO, enhanced antioxidant enzyme activities, and neutrophil infiltration.
Curcumin can be used in the treatment of hepatic warm I/R injury.
The authors demonstrated that curcumin treatment significantly reduced I-R-induced liver injury by modulating several pathways. These findings included several novel findings which are interested to readers in this field.
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH
| 1. | Barthelemy S, Vergnes L, Moynier M, Guyot D, Labidalle S, Bahraoui E. Curcumin and curcumin derivatives inhibit Tat-mediated transactivation of type 1 human immunodeficiency virus long terminal repeat. Res Virol. 1998;149:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Shahed AR, Jones E, Shoskes D. Quercetin and curcumin up-regulate antioxidant gene expression in rat kidney after ureteral obstruction or ischemia/reperfusion injury. Transplant Proc. 2001;33:2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Yeh CH, Chen TP, Wu YC, Lin YM, Jing Lin P. Inhibition of NFkappaB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J Surg Res. 2005;125:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Wang Q, Sun AY, Simonyi A, Jensen MD, Shelat PB, Rottinghaus GE, MacDonald RS, Miller DK, Lubahn DE, Weisman GA. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005;82:138-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 282] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Gaddipati JP, Sundar SV, Calemine J, Seth P, Sidhu GS, Maheshwari RK. Differential regulation of cytokines and transcription factors in liver by curcumin following hemorrhage/resuscitation. Shock. 2003;19:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2149] [Cited by in RCA: 2072] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 8. | Frydman J, Nimmesgern E, Ohtsuka K, Hartl FU. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994;370:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 498] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 9. | Tacchini L, Radice L, Pogliaghi G, Bernelli-Zazzera A. Differential activation of heat shock and nuclear factor kappaB transcription factors in postischemic reperfused rat liver. Hepatology. 1997;26:186-191. [PubMed] |
| 10. | Lin YH, Chiu JH, Tung HH, Tsou MT, Lui WY, Wu CW. Preconditioning somatothermal stimulation on right seventh intercostal nerve territory increases hepatic heat shock protein 70 and protects the liver from ischemia-reperfusion injury in rats. J Surg Res. 2001;99:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Jäättelä M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124-6134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 522] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 12. | Jaattela M. Heat shock proteins as cellular lifeguards. Ann Med. 1999;31:261-271. [RCA] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 358] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Mosser DD, Caron AW, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317-5327. [PubMed] |
| 14. | Schoeniger LO, Andreoni KA, Ott GR, Risby TH, Bulkley GB, Udelsman R, Burdick JF, Buchman TG. Induction of heat-shock gene expression in postischemic pig liver depends on superoxide generation. Gastroenterology. 1994;106:177-184. [PubMed] |
| 15. | de Vera ME, Kim YM, Wong HR, Wang Q, Billiar TR, Geller DA. Heat shock response inhibits cytokine-inducible nitric oxide synthase expression in rat hepatocytes. Hepatology. 1996;24:1238-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146-7159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 511] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 17. | Nylandsted J, Gyrd-Hansen M, Danielewicz A, Fehrenbacher N, Lademann U, Høyer-Hansen M, Weber E, Multhoff G, Rohde M, Jäättelä M. Heat shock protein 70 promotes cell survival by inhibiting lysosomal membrane permeabilization. J Exp Med. 2004;200:425-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 432] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 18. | Musch MW, Sugi K, Straus D, Chang EB. Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology. 1999;117:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat Cell Biol. 2000;2:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1104] [Cited by in RCA: 1124] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 20. | Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat Cell Biol. 2000;2:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 621] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 21. | Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, Zamzami N, Mak T, Jäättelä M, Penninger JM, Garrido C. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 645] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 22. | Sato M, Cordis GA, Maulik N, Das DK. SAPKs regulation of ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2000;279:H901-H907. [PubMed] |
| 23. | Ishida T, Taketoh J, Nakatsune E, Kan-o S, Naito E, Takeda S, Mutoh J, Ishii Y, and Yamada H. Curcumin anticipates the suppressed body weight gain with 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice. J Health Sci. 2004;50:474-482. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Sood A, Mathew R, Trachtman H. Cytoprotective effect of curcumin in human proximal tubule epithelial cells exposed to shiga toxin. Biochem Biophys Res Commun. 2001;283:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Kato K, Ito H, Kamei K, Iwamoto I. Stimulation of the stress-induced expression of stress proteins by curcumin in cultured cells and in rat tissues in vivo. Cell Stress Chaperones. 1998;3:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Rauen U, Viebahn R, Lauchart W, de Groot H. The potential role of reactive oxygen species in liver ischemia/reperfusion injury following liver surgery. Hepatogastroenterology. 1994;41:333-336. [PubMed] |
| 27. | Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 82] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH, Li X. Modulation of liver oxidant-antioxidant system by ischemic preconditioning during ischemia/reperfusion injury in rats. World J Gastroenterol. 2005;11:1825-1828. [PubMed] |
| 29. | Ghoneim AI, Abdel-Naim AB, Khalifa AE, El-Denshary ES. Protective effects of curcumin against ischaemia/reperfusion insult in rat forebrain. Pharmacol Res. 2002;46:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 30. | Madan B, Ghosh B. Diferuloylmethane inhibits neutrophil infiltration and improves survival of mice in high-dose endotoxin shock. Shock. 2003;19:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Taylor BS, Alarcon LH, Billiar TR. Inducible nitric oxide synthase in the liver: regulation and function. Biochemistry (Mosc). 1998;63:766-781. [PubMed] |
| 32. | Taylor BS, Geller DA. Molecular regulation of the human inducible nitric oxide synthase (iNOS) gene. Shock. 2000;13:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 128] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Billiar TR, Curran RD, Harbrecht BG, Stuehr DJ, Demetris AJ, Simmons RL. Modulation of nitrogen oxide synthesis in vivo: NG-monomethyl-L-arginine inhibits endotoxin-induced nitrate/nitrate biosynthesis while promoting hepatic damage. J Leukoc Biol. 1990;48:565-569. [PubMed] |
| 34. | Harbrecht BG, Wu B, Watkins SC, Marshall HP, Peitzman AB, Billiar TR. Inhibition of nitric oxide synthase during hemorrhagic shock increases hepatic injury. Shock. 1995;4:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Li J, Billiar TR. Nitric Oxide. IV. Determinants of nitric oxide protection and toxicity in liver. Am J Physiol. 1999;276:G1069-G1073. [PubMed] |
| 36. | Hecker M, Nematollahi H, Hey C, Busse R, Racké K. Inhibition of arginase by NG-hydroxy-L-arginine in alveolar macrophages: implications for the utilization of L-arginine for nitric oxide synthesis. FEBS Lett. 1995;359:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Chan MM, Huang HI, Fenton MR, Fong D. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 285] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | Brouet I, Ohshima H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem Biophys Res Commun. 1995;206:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 337] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 39. | Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol. 2000;60:1665-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 262] [Article Influence: 10.5] [Reference Citation Analysis (0)] |