INTRODUCTION
Although it is one of the most common malignant tumors in the digestive tract, the therapeutic tractability of colon cancer remains unsatisfying and the failure of treatment is mainly attributed to tumor metastasis to the liver. Previous studies demonstrated that 15%-35% of the patients with primary colon cancer had metastasis in their liver when diagnosed; even after surgical resection, distant metastases still occurred in 25% of patients[1]. Therefore, it is of utmost importance to both inhibit tumor growth and prevent metastasis to increase therapeutic efficacy and improve the prognosis of patients with colon cancer.
Hepatocyte growth factor (HGF), originally identified and cloned as a potent hepatocyte mitogen[2,3], is a heterodimer consisting of 728 amino acids with a 69-ku α-chain and a 34-ku β-chain linked by a pair of disulfide bonds. As a multi-functional growth factor derived from interstitial or matrix cells, HGF has been recognized as a mitogen, motogen, and morphogen through interaction with the transmembrane receptor c-Met, a product of the proto-oncogene c-Met and the only known receptor for HGF[4]. Several in vitro experiments have validated that HGF powerfully promotes angiogenesis through stimulating proliferation and motility of the endothelial cells, eventually resulting in vessel growth[5-7]. Accumulating evidence has shown that HGF plays an important role in tumor-stroma interactions[8-11], promotes motility of tumor cells, stimulates cancer cells escape through the extracellular matrix, thereby leading to invasion and metastasis of tumor cells in a variety of tumors[12-14]. Since over-expression of the c-Met receptor in many tumors derived from epithelial cells[15-17], including colon cancer[18], has been confirmed, studies on blocking the HGF/c-Met signaling pathway to inhibit growth, invasion and metastasis indicate that this is a promising direction in current tumor treatment investigations[19].
NK4, an antagonist of HGF discovered by Date et al[19] in 1997, is a fragment of the HGF molecule consisting of 447 residues. It comprises the N-terminal hairpin and subsequent four-kringle domains of HGF, but lacks the 16 amino acids at the C-terminus of the α-chain and the whole β-chain. NK4 binds to the c-Met receptor competitively with HGF by virtue of common interactions of the HGF binding domain, but does not activate tyrosine phosphorylation of c-Met because of the absence of the β-chain. Consequently, the competitive binding of NK4 to c-Met interrupts the HGF/c-Met signaling pathway, and thereby suppresses HGF-induced invasion and metastasis of tumor cells[20]. Moreover, as an HGF antagonist, NK4 can inhibit angiogenesis induced not only by HGF, but also by other pro-angiogenic factors, such as basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF)[21]. By such mechanisms, NK4 robustly inhibits tumors through suppression of invasion, metastasis, and angiogenesis. Many lines of evidence have demonstrated the tremendous potential of NK4 in the treatment of tumors, such as glioblastoma[22], pancreatic cancer[23], gastric cancer[24], gallbladder cancer[25], and liver cancer[26].
Gene therapy is a revolutionary approach in cancer therapy. To date, there are two types of adenoviral vectors-based gene therapy drugs have been licensed in the world. In order to exploit NK4 in the development of novel strategies against human colon tumors and based on previous in vitro experiments[27], we have chosen to employ rvAdCMV/NK4, an established non-replicating recombinant adenovirus containing the foreign NK4 gene, for gene therapy for human colon cancer cells in an athymic mouse model.
MATERIALS AND METHODS
Virus and cells culture
rvAdCMV/NK4 and Ad-LacZ [8.9 × 109 infection focus units (ifu)/mL)], the first generation of non-replicating recombinant adenoviruses expressing NK4 or β-galactosidase, were generated, verified, and propagated in HEK293 cells (American Type Culture Collection, Manassas, VA) as previously described[27,28]. The human colon cancer cell line LS174T, purchased from Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, was cultured in RPMI-1640 media (Invitrogen, Carsbad, CA) containing 100 mL/L heat-inactivated fetal bovine serum, 100 U/mL peniciliin, 100 μg/mL streptomycin, 2 mmol/L glutamine and 1.5 g/L sodium bicarbonate (Invitrogen) and incubated at 37°C in a humidified atmosphere consisting of 50 mL/L CO2 in air. The expression of c-Met in LS174T cells and NK4 transduction by the recombinant adenovirus were confirmed by Western blot as previously described[27].
Establishment of a human colon cancer model in athymic mouse
Four to six-week-old female athymic mice (BALB/c-nu/nu), weighing 18-22 g, were purchased from the Institute of Laboratory Animal Sciences, Chinese Academy of Medical Sciences (ILAS, CAMS) and were bred in homeothermic (25-27°C), constantly humidified (45%-50%), dust-free fresh air in a sterilized, specific pathogen-free (SPF) environment. All procedures for animal experiments were approved and supervised by the Animal Protection and Usage Committee of ILAS, CAMS and institutional guidelines for animal welfare and experimental conduct were followed.
LS174T cells in the logarithmic growth phase were treated with 2.5 g/L trypsin (Invitrogen), centrifuged, and resuspended in phosphate-buffered saline (PBS, pH7.4). After assessment for cell survival rate over 95% by trypan blue, the cell concentration was adjusted to 5 × 107/mL with PBS and 200 μL of the cell suspension (1 × 107 cells) was subcutaneously injected to the subscapular region of an athymic mouse using a 6-gauge needle. The tumors were not recovered until the diameter had increased to nearly 1 cm. After fixation in formalin, routine embedding in paraffin wax, cut into 5-μm thick sections, and staining with hematoxylin and eosin (HE), the tissue property of the tumors was confirmed by light microscopy.
In vivo tumor treatment
After the tumors were continuously passaged for 4 generations in mice, the athymic mice carrying productive tumors without ulcer were anesthetized by ethoxyethane and sacrificed. The tumors were removed under sterile conditions, put into sterile plates on ice, de-enveloped, and cut into pieces of about 1 mm3. The tumor pieces were then implanted into the right forelimb subscapular region of mice usin 18-gauge trochars. All 15 animals were randomly divided into 3 groups, each group containing 5 mice, when the tumor volume grew to approximately 100 mm3 (the 3rd d after implantation) as follows: PBS group (blank control); Ad-LacZ group (negative control); and rvAdCMV/NK4 group (treatment group). Subsequently, 100 μL of PBS alone, or a suspension of adenovirus (108 ifu) in 100 μL of PBS was administered directly into the tumor-bearing mice in each group every 6 d for a total of 5 injections per mouse.
Long diameter (a) and short diameter (b) were measured every 3 to 4 d and volumes (V) of the tumors were calculated according to the formula V = 1/2ab2 and a tumor growth curve was drawn. Inhibition of tumor growth was determined using the formula as follows: Tumor inhibition rate (%) = (volume of tumors in the control group-volume of tumors in the treatment group)/ volume of tumors in control group × 100%.
At d 3 following the final treatment, all 15 mice were sacrificed. The tumors were removed, fixed in 100 mL/L formalin for 24 h, embedded in paraffin wax, and serially sectioned (4-μm thick). Immunohistochemical staining was performed as described below.
Tumor proliferation assay
Proliferating tumor cells were detected by SP immu-nohistochemical method using an antibody against proliferating cell nuclear antigen (PCNA) according to the manufacturer’s (Maxin Biotech) instructions. Four sections were observed for each mouse. To determine the PCNA Labeling Index (PCNA LI), five representative PCNA-positive visual fields were selected under a low power microscope (× 100) in each section, then 200 tumor cells were counted in each visual field under a high power microscope (× 200), finally the percentage of labeled nuclei was calculated (%).
Evaluation of microvessel density
The microvessel density (MVD) in each tumor was evaluated by counting CD31-positive vessels. The expression of CD31 was detected by immunohistochemical methods as aforementioned. The primary antibody was a rat-anti-mouse CD31 monoclonal antibody (BD Biosciences, 1:200 dilution) and the secondary antibody was a biotinylated anti-rat IgG (Maxin Biotech, 1:100). For the microvessel counting, positive stainings for MVD in the 3 most highly vascularized areas (“hot spots”) in each slide, were counted in 200 × fields and MVD was expressed as the average of the microvessel count in these areas[29]. Any brown endothelial cell or cluster of endothelial cells were counted as one microvessel as long as it was separated from other neighboring microvessels, tumor cells, and connective tissues, while vessels with a thick muscular layer or diameter over 8 erythrocytes were not counted.
TUNEL assay
Apoptotic cells were detected using a terminal deoxynu-cleotidyl transferase (TdT)-mediated nick-end labeling (TUNEL) assay. The assay was performed according to the manufacturer’s instructions (Boehringer Mannheim). The apoptotic index (AI) was determined by selecting five representative TUNEL-positive fields under low power microscopy (× 100) in each section, then 200 tumor cells were counted in each field under high power microscopy (× 200). The number of positively labeled nuclei per total nuclei in those fields was expressed as the apoptotic index (AI). AI = (the number of apoptotic cells/total number of tumor cells) × 100%.
Treatment of peritoneal tumor dissemination
LS174T cells (1 × 107 in 200 μL PBS) were injected intraperitoneally (ip) into 15 mice at d 0, and mice were randomly divided into 3 groups (n = 5 in each group) as described above. Then 100 μL of PBS alone or a suspension of adenovirus (108 ifu) in 100 μL of PBS was ip injected in each group at an interval of 6 d for a total of two injections per mouse. All animals were sacrificed at d 15 to determine intraperitoneal tumor dissemination by direct visual inspection in terms of number, site and weight of the metastases.
Statistical analysis
Data were expressed as the mean ± SD. Statistical comparisons were made using the Student’s t test for the measurement data and Mann-Whitney U test for enumeration data. P < 0.05 was considered statistically significant.
RESULTS
Establishment of a human colon cancer model in athymic mice
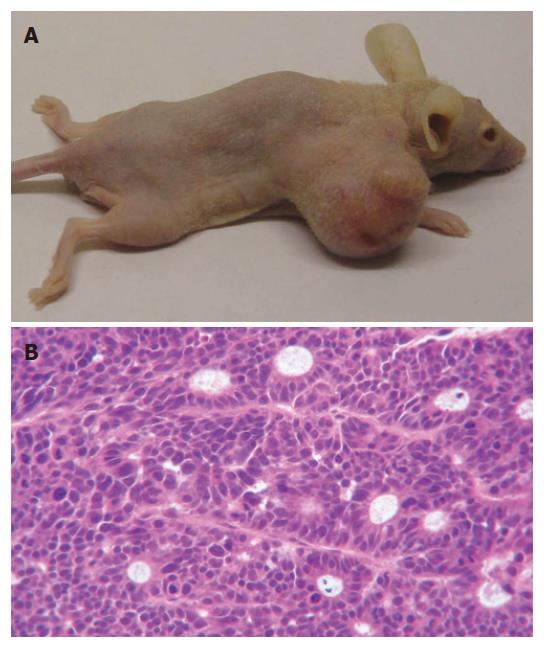
The human colon cancer cell line LS174T robustly formed tumors. Primary tumors growth was apparent in the subdermal tissue of athymic mice at 1 wk after implantation. The tumor grew rapidly into an oval shape with a smooth and glossy surface at early stages and an irregular shape with surface nodosity at later periods. Thus, it is an ideal tumor model for colon cancer research. Macroscopically, the subcutaneous tumors in athymic mice were silver-gray and looked like fish flesh when dissected. HE staining confirmed the tumors as colon adenocarcinoma, as expected. Microscopically, tumor cells were seen in a ball-like distribution with larger cell bodies and obvious heteromorphism; the nuclei were large and irregular and the nucleoli were obviously visible. Without necrotic changes, typical micro-cavity-like structures were seen separately and multi-nuclear giant cells were occasionally found in the tumor tissues. All of the aforementioned features are consistent with the characterization of a poorly differentiated human colon adenocarcinoma (Figure 1).
Figure 1 Tumors established in athymic BALB/c mice by subcutaneous injection of LS174T cells.
A: Photography of subcutaneous tumor of LS174T in athymic mice; B: The tumor sections (HE, × 200).
Inhibitory effect of rvAdCMV/NK4 on tumor growth
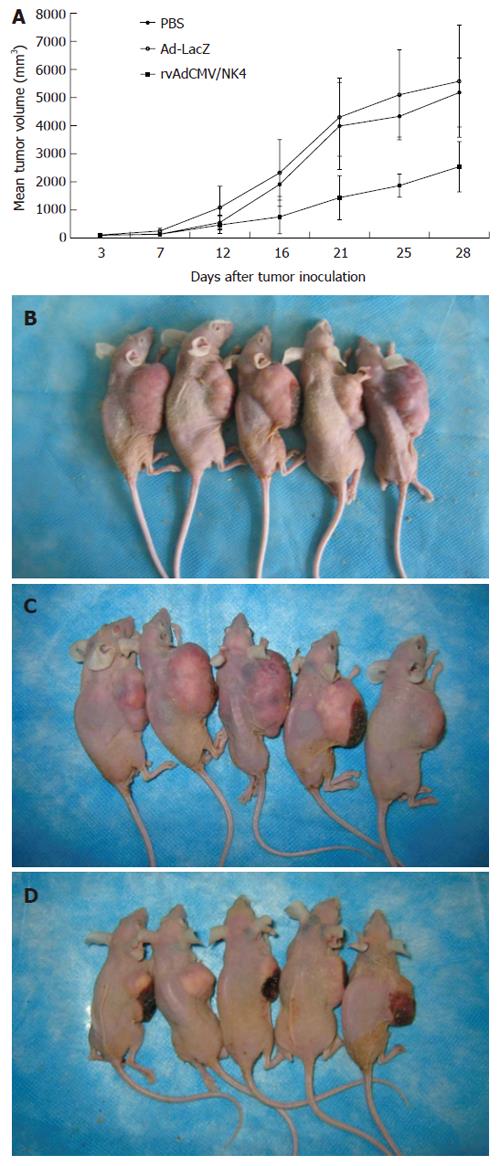
Subcutaneous injection of LS174T cells resulted in tumor formation in all 15 athymic mice (tumor formation rate 100%) and the tumors grew a large enough size to be treated with PBS, or Ad-LacZ, or rvAdCMV/NK4 from the 3rd d after tumor cell implantation. No animals died during the treatment and all of them grew well without dyscrasia. There was no significant difference in body weight among three groups before and after treatment (data not shown). No metastases in organs of the thoracic or abdominal cavity were found. Tumor growth curves showed that the average size of tumors in the rvAdCMV/NK4 group was significantly smaller compared to the two control groups (2537.4 ± 892.3 mm3 in rvAdCMV/NK4 group vs 5175.2 ± 1228.6 mm3 in PBS group or 5578.8 ± 1955.7 mm3 in Ad-LacZ group, P < 0.05) (Figure 2). The tumor growth inhibition rate was 51% in the rvAdCMV/NK4 group when compared with the Ad-LacZ group, while no significant difference was observed between the Ad-LacZ and PBS groups (5578.8 ± 1955.7 mm3vs 5175.2 ± 1228.6 mm3). These findings indicate that the rvAdCMV/NK4 treatment can inhibit tumor growth effectively.
Figure 2 Suppression of LS174T tumor growth in athymic mice by intratumoral administration of rvAdCMV/NK4.
A: Tumor growth curves. Data are presented as mean ± SD (n = 5/group); B: PBS control; C: Ad-LacZ; D: rvAdCMV/NK4.
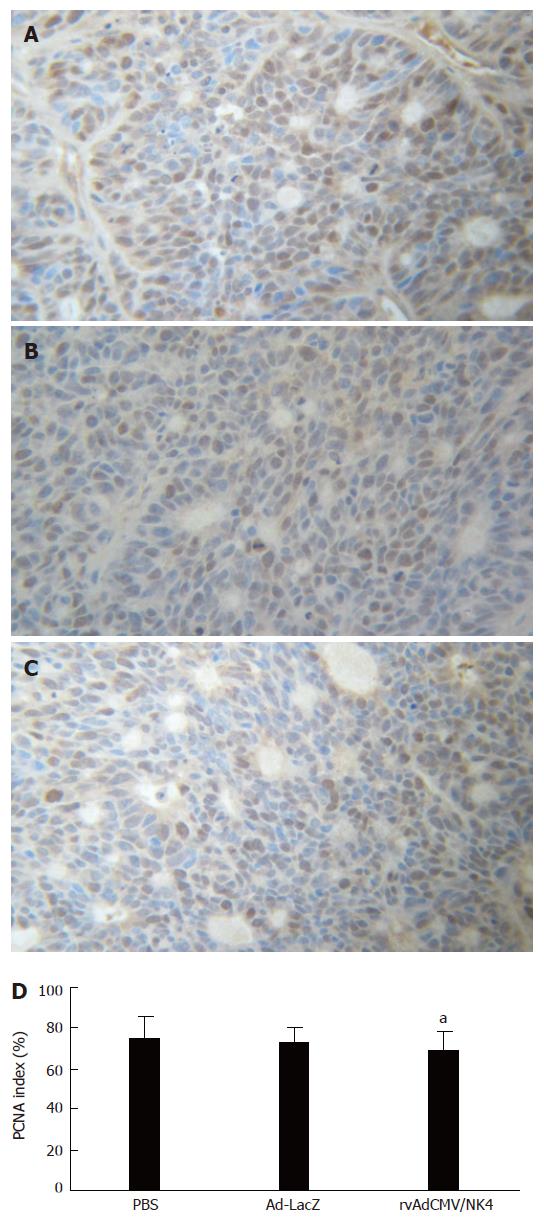
Because PCNA is mainly expressed during S, G1, and early G2 phase of the cell-cycle in proliferating cells, it is accepted as a marker of cell proliferation and malignancy of tumors[30]. In order to assess the inhibitory effects of the rvAdCMV/NK4 treatment on human colon cancer cells, we immunohistochemically examined the PCNA level in the tumors. The results showed that there was no significant difference in PCNA LI among the three groups (75.1% ± 11.2% in PBS group vs 72.8% ± 7.6% in Ad-LacZ group vs 69.3% ± 9.4% in rvAdCMV/NK4 group) (Figure 3), indicating that the rvAdCMV/NK4 treatment did not inhibit proliferation of tumor cells and the inhibitory effects on tumor growth clearly do not rely on direct inhibition of tumor cell proliferation. These results are also identical to those obtained in previous in vitro experiments and similar with the reports of others[27,31,32].
Figure 3 PCNA expression in LS174T tumors after rvAdCMV/NK4 treatment.
A, B, C: Immmunohistochemical staining of PCNA in tumor tissues (× 200), A: PBS; B: Ad-LacZ; C: rvAdCMV/NK4; D: PCNA labeling index in different groups (mean ± SD). aР > 0.05 vs PBS/Ad-LacZ.
Inhibition of angiogenesis in tumor by rvAdCMV/NK4
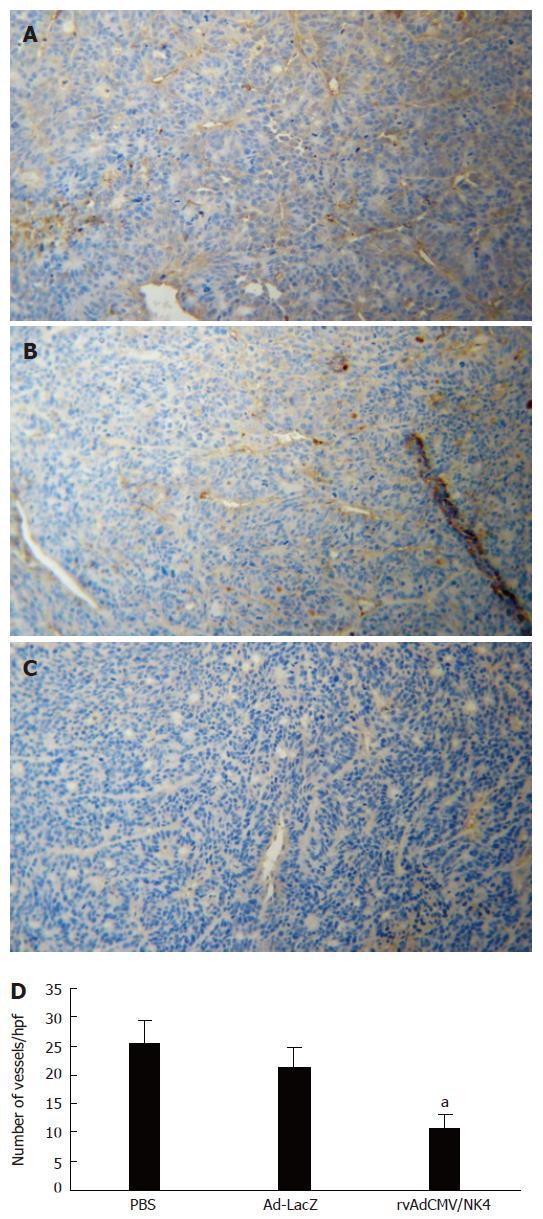
In order to demonstrate the inhibitory effect of rvAdCMV/NK4 treatment on tumor angiogenesis in the athymic mice model, tumor MVDs were quantified by immunohistochemical staining for CD31. The results indicated a marked reduction in MVD in the rvAdCMV/NK4-treated tumors (10.7 ± 2.4) compared to the PBS-treated (25.6 ± 3.8) and Ad-LacZ-treated (21.3 ± 3.5) tumors (P < 0.05) (Figure 4), indicating that NK4 may suppress tumor growth via inhibition of tumor angiogenesis.
Figure 4 Microvessel density of LS174T tumors in athymic mice after rvAdCMV/NK4 treatment.
A, B, C: Immmunohistochemical staining of CD31 in tumor tissues (× 100), A: PBS; B: Ad-LacZ; C: rvAdCMV/NK4; D: Number of vessels in tumor tissues after treatment (mean ± SD). aР < 0.05 vs PBS or Ad-LacZ.
rvAdCMV/NK4-induced apoptosis
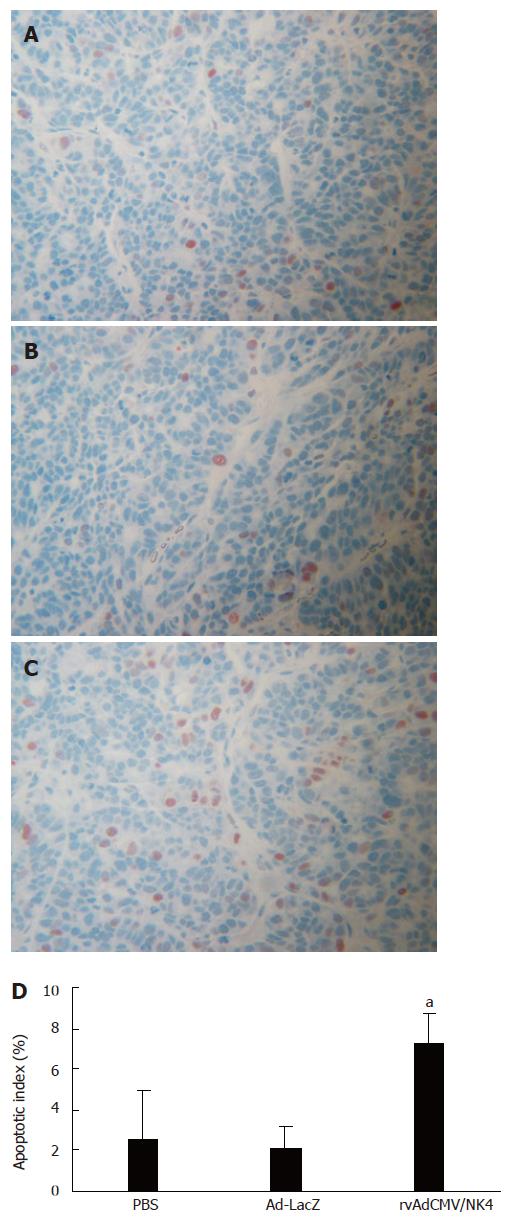
In order to further elucidate the mechanisms underlying the suppression of human colon cancer growth by adenovirus vector-mediated NK4-transduction, we applied the TUNEL assay to analyze apoptosis in the tumor tissues. We found a marked increase in apoptotic index in the rvAdCMV/NK4-treated tumors (7.3% ± 2.4%) compared to PBS-treated (2.6% ± 1.1%) or Ad-LacZ-treated tumors (2.1% ± 1.5%) (P < 0.05) (Figure 5), suggesting that NK4 transduction may result in an increased apoptosis of tumor cells, thereby leading to tumor growth inhibition.
Figure 5 Apoptosis in LS174T tumors in athymic mice after treatment.
A, B, C: Apototic cells stained by TUNEL assay (× 200), A: PBS; B: Ad-LacZ; C: rvAdCMV/NK4; D: Apoptotic index in different groups (mean ± SD). aР < 0.05 vs PBS or Ad-LacZ.
Suppression of tumor metastasis by rvAdCMV/NK4 treatment
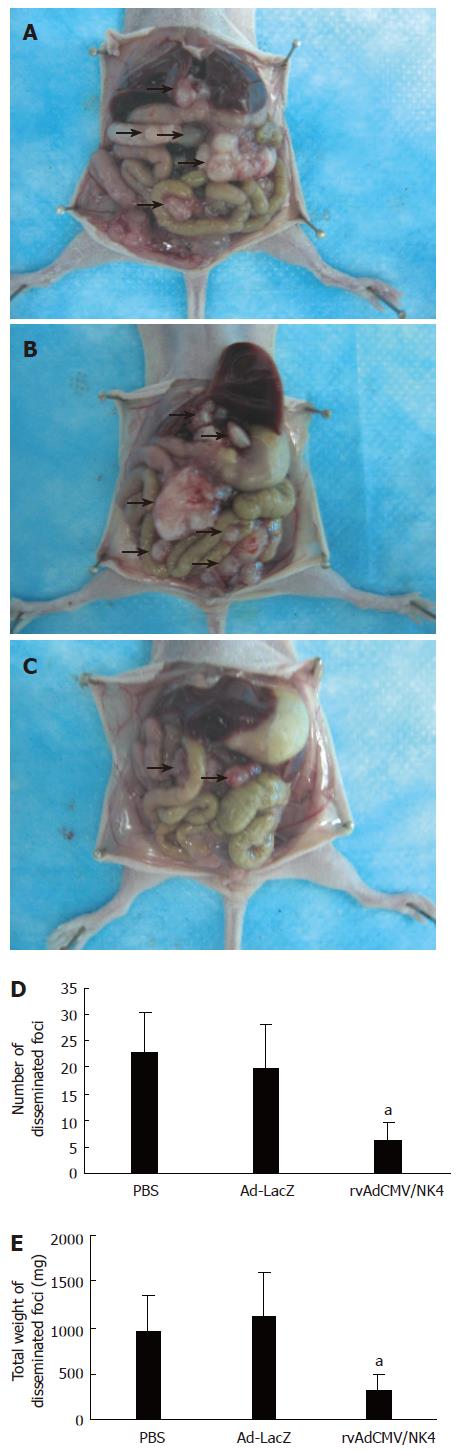
We investigated the consequences of ip injection with rvAdCMV/NK4 on the peritoneal dissemination of LS174T cells in vivo. Although the treatment was begun as early as the first day after LS174T cell implantation into the abdominal cavity, tumor dissemination in this cavity was found in all animals after only 2 wk, indicating the strong invasive ability and aggressive malignant nature of LS174T cells. The tumor dissemination was mainly observed in the omental cavity or on the mesentery and surface of the liver or peritoneum, but no ascites was found. After treatment, both the average number and the average weight of the disseminated tumors in the rvAdCMV/NK4 group were significantly less compared to the PBS and Ad-LacZ groups (tumor number: 6.2 ± 3.3 vs 22.9 ± 7.6 or 19.8 ± 8.5, P < 0.05; tumor weight: 324 ± 176 mg vs 962 ± 382 mg or 1116 ± 484 mg, P < 0.05) (Figure 6), demonstrating that adenovirus-mediated NK4 transduction can inhibit metastasis of LS174T tumors.
Figure 6 Suppression of peritoneal dissemination by intraperitoneal injection of rvAdCMV/NK4.
A, B, C: Macroscopic appearance of peritoneal dissemination on d 14 after the treatment following peritoneal implantation of 1 × 106 LS174T cells (n = 5/group), A: PBS; B: Ad-LacZ; C: rvAdCMV/NK4. Arrowheads indicate disseminated tumor nodules; D: Number of disseminated foci (mean ± SD); E: Total weight of disseminated foci (mean ± SD). aР < 0.05 vs PBS or Ad-LacZ.
DISCUSSION
More investigations have shown that HGF is associated with the malignant behavior of human colorectal cancer cells via enhancing invasion and metastasis[33,34], and our previous studies showed that recombinant adenovirus vector rvAdCMV/NK4-mediated NK4 transduction could attenuate HGF-induced tumor cell scattering, migration, and basement membrane invasion[27]. On this basis, we explore here the possibility of applying rvAdCMV/NK4 to gene therapy against a human colon cancer xenograft model in athymic mouse. Our results demonstrated that, compared with the control groups, rvAdCMV/NK4 treatment could significantly suppress the growth and metastasis of LS174T tumors. Furthermore, tumor suppression was associated with decreased angiogenesis and increased apoptosis in these tumors.
The viewpoint that tumor growth depends on angiogenesis was first proposed by Folkman in 1971[35]. Numerous investigations have shown that angiogenesis is indeed a primary condition for growth and metastasis of tumors over 1-2 mm3[36]. Thus, inhibition of tumor growth via suppression of tumor angiogenesis, rather than direct tumor cell killing, which is called “dormancy therapy”, has been a popular direction in tumor treatment in recent years[37]. To evaluate the suppressive effects of rvAdCMV/NK4 on angiogenesis, we assayed MVD in subcutaneously implanted tumors. We found that MVD in the rvAdCMV/NK4-treated group was significantly less than the control groups. Moreover, obvious diffuse tumor cell ischemia and necrosis due to blood supply deficiency could be seen in rvAdCMV/NK4-treated tumors (data not shown); while the basement membrane of new vessels was not integral and was deficient in mural cells in the PBS-treated or Ad-LacZ-treated tumors. This may promote tumor cell intravasation and, therefore, greater invasion and progression to metastasis. These findings suggest that ischemia and necrosis due to blood supply deficiency caused by rvAdCMV/NK4 treatment-induced anti-angiogenesis might be one of the primary reasons for tumor growth inhibition.
Apoptosis plays an important role in the inhibition of oncogenesis, tumor development and growth. Balance between proliferation and apoptosis drives a tumor to a silent or dormant state, which may inhibit clinical recurrence and metastasis caused by mini-tumors, placing the patients, especially those who received an operation, in a sub-clinical therapy state[38]. Thus, apoptosis in a tumor usually indicates a relatively good clinical prognosis. The majority of chemotherapeutic drugs at present suppress tumor growth through induction of apoptosis[39]. In our study, the AI of the rvAdCMV/NK4-treated group was much higher compared to the control groups, indicating its potential value for clinical therapy of cancer. The mechanism by which rvAdCMV/NK4 treatment promotes apoptosis in LS174T tumors is yet unknown. However, it is unlikely to be caused by direct suppression of cell proliferation, because the treatment did not lead to a significant decrease of PCNA LI. In consideration of the established linkage between inhibition of angiogenesis and tumor apoptosis, as well as the potent anti-angiogenic activity of NK4, it is reasonable to attribute the markedly increased apoptosis to the significantly inhibited angiogenesis. Hence, inhibition of angiogenesis may play a central role in the tumor suppression mediated by NK4.
At present, surgical resection remains the leading therapeutic approach for colon cancer. However, surgery has risks contributing to tumor cell dissemination around operation fields and metastasis in the abdominal cavity. In order to investigate whether rvAdCMV/NK4 may inhibit tumor dissemination, we established disseminating tumor models in athymic mice through injection of LS174T cells into the abdominal cavity. For modeling clinical patients who receive systemic therapy from the first day after the operation, we treated the animals from the first day after injection of LS174T cells. Our results suggest that rvAdCMV/NK4 may effectively inhibit tumor dissemination. Both the size and weight of tumors in rvAdCMV/NK4-treated group were lower than those in the control groups. Tumor implantation in the abdominal cavity is presently regarded to be caused by adherence between tumor cells and interstitial cells, allowing tumor cells to eventually break through the basement membrane. According to previous investigations, HGF released from fibroblasts and interstitial cells may cause the morphological changes of stromal cells and promote tumor cell invasion into the basal layer[40]. Thus, HGF may play a role in tumor dissemination, in addition to the effects of transforming growth factor-β (TGF-β), etc. In our study, rvAdCMV/NK4 inhibited the dissemination of LS174T cells in the abdominal cavity via HGF-antagonist activity of the NK4 protein, thereby inhibiting adherence between tumor cells and matrix. At the same time, the anti-angiogenic and pro-apoptotic activity of NK4 suppressed the growth of implanted tumors.
In summary, we have applied the first generation non-replicating recombinant adenovirus vector, rvAdCMV/NK4, to effectively treat human colon cancer in an in vivo athymic mouse model. The findings are in agreement with previous studies reported on various human cancers, such as pancreatic cancer[23], hepatocellular cancer[26], gastric cancer[41], etc. Although our preliminary results are encouraging, showing the relative efficacy of this type of treatment in human colon cancer cells, one must keep in mind that the efficacy of gene therapy as the sole treatment may be hampered by several factors, such as immunity against the viral vector, the duration of gene expression, and the specificity of cancer targeting, etc. Such therapy should be combined with currently available conventional therapies, including surgical resection, chemotherapy, and radiotherapy to achieve better therapeutic success. In future studies, we will examine the synergistic effects of combination with chemotherapy and/or radiotherapy, as well as any sensitizing effects on chemotherapy and/or radiotherapy.