Published online Apr 7, 2007. doi: 10.3748/wjg.v13.i13.1912
Revised: November 2, 2006
Accepted: November 14, 2007
Published online: April 7, 2007
Our goal is to provide a detailed review of veno-occlusive disease (VOD), Budd-Chiari syndrome (BCS), and congestive hepatopathy (CH), all of which results in hepatic venous outflow obstruction. This is the first article in which all three syndromes have been reviewed, enabling the reader to compare the characteristics of these disorders. The histological findings in VOD, BCS, and CH are almost identical: sinusoidal congestion and cell necrosis mostly in perivenular areas of hepatic acini which eventually leads to bridging fibrosis between adjacent central veins. Tender hepatomegaly with jaundice and ascites is common to all three conditions. However, the clinical presentation depends mostly on the extent and rapidity of the outflow obstruction. Although the etiology and treatment are completely different in VOD, BCS, and CH; the similarities in clinical manifestations and liver histology may suggest a common mechanism of hepatic injury and adaptation in response to increased sinusoidal pressure.
- Citation: Bayraktar UD, Seren S, Bayraktar Y. Hepatic venous outflow obstruction: Three similar syndromes. World J Gastroenterol 2007; 13(13): 1912-1927
- URL: https://www.wjgnet.com/1007-9327/full/v13/i13/1912.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i13.1912
Although the liver makes up < 3% of the total body weight, it receives one-quarter of the total cardiac output through the hepatic artery and portal vein[1]. Blood is drained from the hepatic acini via central veins into sublobular veins and then into the right, left, and middle hepatic veins, the inferior vena cava and the right atrium[2]. An obstruction to the blood flow out of the liver can result in a spectrum of clinical abnormalities ranging from acute hepatic failure to passive hepatic congestion, depending on the acuity and level of obstruction.
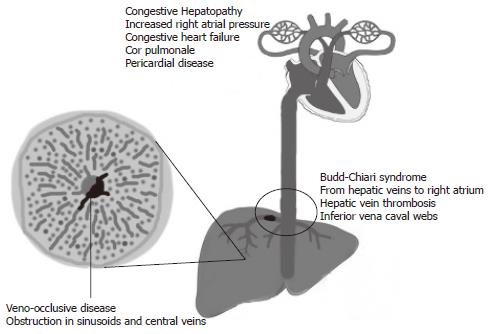
Hepatic venous outflow obstruction (HVOO) can be divided into three categories according to the level of obstruction: (1) Veno-occlusive disease (VOD): at the level of sinusoids and terminal venules, (2) Budd-Chiari syndrome (BCS): from hepatic veins to the superior end of inferior vena cava, and (3) Venous obstruction at the level of heart referred to as congestive hepatopathy (CH) (Figure 1).
The etiology of VOD, BCS, and CH are entirely different (Table 1). It should be noted that VOD develops within three weeks of an acute insult to the sinusoidal endothelial cells while BCS and CH may develop within a few days or may take several years of venous thrombosis and heart failure, respectively. The variations in the ‘acuteness’ of venous obstruction leads to subtle differences in the clinical presentations of VOD, BCS, and CH. Although patients with HVOO generally present with abdominal pain due to hepatomegaly, and jaundice and ascites due to portal hypertension; chronic BCS patients may first present with cirrhosis and its complications. BCS patients with inferior vena cava obstruction may also have leg edema and venous collaterals over the trunk. Additionally, signs and symptoms of heart failure such as jugular venous distention, leg edema, and dyspnea may be seen in patients with CH. Nonetheless, the histological findings in all three syndromes are almost identical and include sinusoidal congestion and hepatocyte necrosis predominating in perivenular areas of hepatic acini which eventually leads to bridging fibrosis between adjacent central veins (Table 1)[1,3,4].
| VOD | BCS | CH | |
| Site of venous obstruction | Hepatic sinusoids and terminal venules | From hepatic veins to the superior end of IVC | Heart |
| Etiology | Sinusoidal endothelial injury due to HSCT, chemotherapy, abdominal radiotherapy, and pyrrolizidine alkaloids | Hepatic vein thrombosis, IVC webs, compression of hepatic veins or IVC by tumor, cyst, or abscess | Increased right atrial pressure due to CHF (CAD, cardiomyopathies, valve abnormalities), cor pulmonale (COPD, ILD, pulmonary HTN), and pericardial disease (constrictive pericarditis, pericardial tamponade) |
| Histology | Changes predominantly in perivenular areas | Predominantly in perivenular areas except in presence of concomitant PVT. | Predominantly in perivenular areas Sinusoidal congestion and hepatocellular necrosis |
| Gaps in SEC barrier leading to subendothelial edema | Sinusoidal congestion followed by ischemic cell necrosis and bridging fibrosis between central veins | Bridging fibrosis between central veins leading to cardiac fibrosis in chronic cases | |
| Narrowing of central veins and sinusoids with sinusoidal congestion and hepatocellular necrosis | Caudate lobe hypertrophy, with fibrosis and atrophy in the rest of liver | ||
| Collagen accumulation in sinusoids and veins leading to bridging fibrosis between central veins |
The laboratory findings in VOD, BCS, and CH are also very similar. Hyperbilirubinemia is universal in HVOO except in patients with constrictive pericarditis[5], and is believed to be due to hepatocellular dysfunction, hemolysis, and biliary canalicular obstruction secondary to distended hepatic veins[6]. Serum aminotransferase levels may be mildly elevated except in fulminant BCS and CH with severe cardiac output impairment causing hepatic ischemia when the levels may exceed 1000 U/L. Alkaline phosphatase levels may also be mildly elevated. New markers for diagnosis of VOD such as plasminogen activator inhibitor-1 are under investigation.
The gold standard for the diagnosis of VOD is liver biopsy. However, because of the risks of liver biopsy in thrombocytopenic patients, the diagnosis is primarily based on clinical criteria. CH is usually diagnosed by routine laboratory tests in patients with symptomatic heart failure. However, physicians may miss the diagnosis of CH in patients with constrictive pericarditis who do not have overt symptoms. Echocardiogram and invasive cardiac hemodynamic evaluation may be necessary in patients with a strong suspicion of CH. Diagnosis of BCS, unlike VOD and CH, is greatly dependant on radiological studies, although a careful history and physical examination is crucial in order not to overlook this protean syndrome. Doppler sonogram followed by venogram are the first line tests in patients with suspected BCS (Table 2).
| VOD | BCS | CH | |
| Radiological findings | Ultrasonography to rule out other liver disorders | Doppler: Abnormal flow in a hepatic vein; large intrahepatic collateral vessels; e nlarged, stenotic, or tortuous hepatic veins | Dilatation of all three hepatic veins on sonogram |
| Doppler may show reverse blood flow in the portal vein | MRI: Large intrahepatic comma shaped c ollaterals. Hepatic venography: Spider web venous network pattern | ECHO: Increased pulmonary artery pressure, dilatation of right side of heart, TR, abnormal diastolic ventricular filling due to pericardial disease | |
| Treatment | (1) Prevention: UDCA, heparin, LMWH, and defibrotide | (1) Prevention of thrombus extension: Anticoagulation with heparin and warfarin | Treatment of the underlying heart disease |
| (2) Treatment: Symptomatic care, defibrotide, tPA, AT-III concentrate | (2) Restoration of blood flow: Thrombolytic therapy, percutaneous, angioplasty, TIPS, or shunt surgery | Pericardiectomy in constrictive pericarditis | |
| (3)TIPS and liver transplantation in selected cases | (3) Liver transplantation | ||
| Prognosis | Mortality rate between 9% to 98% depending on the severity | Five-year survival rate 42% to 89% in hepatic vein thrombosis and 25% in IVC obstruction | Liver disease rarely contributes to mortality in these patients |
The treatment and prognosis of VOD, BCS, and CH are summarized in Table 2. It should be noted that despite new promising therapies, prevention is still the mainstay of VOD management because of its high mortality and the limited efficacy of current therapeutic modalities. Treatment of BCS depends on the site and extent of the obstruction, and varies from sole anticoagulation to TIPS and liver transplantation. Treatment of the underlying heart disease is the basis of the management of CH.
Following are the detailed reviews of VOD, BCS, and CH.
Hepatic VOD is characterized by tender hepatomegaly, fluid retention, weight gain, and jaundice. This condition is seen typically after hematopoietic stem cell transplantation (HSCT)[7], high dose abdominal radiation therapy[8], use of certain chemotherapeutic agents[9], ingestion of pyrrolizidine alkaloids[10,11], and liver transplantation[12]. In 1954, Bras et al[13] first described veno-occlusive disease in Jamaican children who developed occlusion of the tributaries of the hepatic vein with subsequent centrilobular or non-portal fibrosis, associated with poisoning with Senecio type alkaloids. The term, sinusoidal obstruction syndrome was introduced in 2002 by deLeve et al[14] to replace VOD based on their studies identifying the primary site of injury as the sinusoidal endothelial cells, which results in fibrosis and obstruction of hepatic venous outflow. VOD after HSCT will be the focus of our review since it is the most common and studied form of VOD.
The reported incidence of VOD after HSCT varies greatly from 5% to 50% largely due to differences in the chemotherapeutic conditioning regimens[15]. Most patients develop signs and symptoms of VOD within the first three weeks after HSCT. The initial signs are sudden weight gain and development of hepatomegaly[16]. Weight gain is believed to be due to renal retention of water and sodium. Hyperbilirubinemia is seen after a few days, sometimes followed by increase in serum transaminase and alkaline phosphatase levels. The first symptom and generally the only one reported by patients with VOD is right upper quadrant pain, likely due to acute congestion of liver[17]. Physical exam reveals tender hepatomegaly and ascites in 83% and 39% patients with histologically proven VOD respectively[18].
Patients with VOD generally require more platelet transfusions than patients without liver disease[19]. This might be because of ongoing thrombotic process in the liver sinusoids or increased splenic sequestration due to portal hypertension[17]. Patients with VOD may develop renal failure as a result of hepatorenal syndrome. The prevalence of renal, cardiac, and pulmonary failure was noted to be higher in patients with VOD than in those without VOD in a report of 355 patients who underwent HSCT[16]. Coagulation factor deficiencies and prolonged prothrombin time can be encountered as the hepatic function declines.
The overall mortality is reported to be between 3% and 67%[20,21]. The severity of VOD can be classified as mild (requiring no treatment and with complete resolution), moderate (requiring treatment with diuretics and pain medications and with complete resolution), and severe (requiring treatment and no resolution before death or by transplant d 100). In one large series, the mortality rate was reported to be 98%, 23%, and 9% in patients with severe, moderate, and mild VOD, respectively[16]. Patients with severe VOD differed from those with mild or moderate VOD in the amount of weight gained, increment speed of total serum bilirubin level, and frequency of edema and ascites. One drawback of this classification is that it can be used only for research purposes because of its retrospective assessment. Therefore, in 1994 Bearman et al[22] formulated a mathematical model to predict the severity of VOD based on alterations in weight and serum bilirubin level.
Several pretransplant and transplant-associated risk factors have been identified in different studies.
One of the most important independent risk factors of VOD is the presence of liver injury prior to HSCT. In a cohort of 1652 patients, the relative risk of VOD in the presence of pre-transplant elevated AST levels and prior radiotherapy to the abdomen were 2.4 and 2.9, respectively[15]. In addition, the presence of hepatic metastases of solid tumors was associated with an increased risk of VOD[21]. Although hepatitis C virus (HCV) infection was reported to increase the risk of VOD in one study[23], in another study on 355 patients the risk for VOD was found not to be increased unless the pretransplant AST levels were elevated[24].
Advanced age[25] and previous HSCT may be associated with higher incidence of VOD[16,17]. Additionally, Factor V Leiden and prothrombin G20210A mutation may be associated with VOD[26,27].
Medications used before, during or after HSCT may increase the risk of VOD. Prior therapy with gemtuzumab ozogamicin, a calicheamicin-conjugated monoclonal CD33 antibody used in the treatment of acute myelogenous leukemia, significantly increases the risk of VOD, regardless of the dose and serum AST levels[28]. The use of vancomycin or acyclovir in the pre-transplant period has been associated with the development of VOD. However, it is unclear whether the increased risk of VOD is due to antibiotics or the underlying infections that necessitated their use. Additionally, women who received norethisterone to prevent menstruation during HSCT have been found to have an increased incidence of VOD[29,30].
One of the most important risk factors for the development of VOD is the type and dose of the conditioning regimens of HSCT. The incidence of VOD was found to be 3 times higher in patients who received cyclophosphamide in combination with busulfan or total body irradiation (TBI) (> 12 Gy) or carmustine and etoposide[15]. The mechanism is thought to involve acrolein, the inactive toxic metabolite of cyclophosphamide[31] which is detoxified by conjugation with glutathione[32]. Hepatic glutathione reserve is decreased by busulfan, carmustine and TBI, thus explaining the increased risk of VOD by these drug combinations.
It is widely believed that the incidence of VOD is higher after allogeneic stem cell transplantation than after autologous stem cell transplantation. However, some studies have failed to show any difference[16,33]. Furthermore, the differences seen in other studies[15] may be due to higher intensity conditioning regimens used in allogeneic transplantation. Additionally, patients receiving stem cells from unrelated or mismatched donors have higher risk of developing VOD[16].
The gold standard for the diagnosis of VOD is liver histology. However, because of the risks of liver biopsy in thrombocytopenic patients, diagnosis is primarily based on clinical findings. The Seattle and Baltimore clinical criteria (Table 3) are generally used for the diagnosis of VOD[33,34]. Carreras et al[18] reported that the diagnosis of VOD could be confirmed histologically in 41%, 91%, and 91% of patients satisfying two and three features of Seattle criteria, and Baltimore criteria, respectively. Sensitivity of latter two was 56%.
| Seattle criteria | |
| Development of at least 2 of the following 3 clinical features before d 30 after transplantation | |
| Jaundice | |
| Hepatomegaly with right upper quadrant pain | |
| Ascites and/or unexplained weight gain | |
| Baltimore criteria | |
| Development of hyperbilirubinemia with serum bilirubin > 2 mg/dL within 21 d after transplantation and at least 2 of the following clinical signs and symptoms | |
| Hepatomegaly, which may be painful | |
| Weight gain > 5% from baseline | |
| Ascites | |
Liver specimens for histological examination can be obtained through percutaneous, laparoscopic, and transvenous (transjugular or femoral) biopsies. The latter method has the advantage of lower risk of bleeding and the opportunity to measure wedge hepatic venous pressures at the same time. Although transvenous liver biopsy specimens tend to be small, with modern techniques and equipment a representative sample can be obtained[35]. Transfemoral approach often results in crush artifact in the tissues obtained so that the transjugular method is generally preferred. It should be noted that the histological abnormalities may be patchy, especially in the early stages and could lead to false negative biopsy results. In one series, 2 of the 29 patients (7%) died due to intraperitoneal bleeding after transvenous liver biopsies[35] whereas Carreras et al[18,36] reported no procedure-related deaths in two series of 71 and 30 patients. Hepatic venous pressure gradient of > 10 mmHg was reported to have > 80 % sensitivity and specificity in these series.
Ultrasonography is useful in excluding other disorders that mimic VOD. Yoshimoto et al[37] suggested that detection of reverse blood flow in a segment of portal vein by color-Doppler sonogram is useful for early diagnosis of VOD.
Plasma plasminogen activator inhibitor-1 (PAI-1) level above 100 ng/mL was found to be 100% sensitive and specific for VOD in a series of 31 posttransplant patients with bilirubin levels > 3 mg/dL[38]. Additionally, serum N-terminal peptide of type III procollagen and hyaluronic acid levels may also have a diagnostic role[39,40].
A variety of clinical conditions can mimic VOD (Table 4) and it is difficult to differentiate these from VOD in the early stages. The main causes of intrahepatic cholestasis after HSCT are acute graft-versus-host disease (GVHD), cholangitis lenta (sepsis related cholestasis), cyclosporine-induced cholestasis, and VOD. Compared to VOD, GVHD generally develops at a later stage after the appearance of intestinal and cutaneous manifestations and moreover does not cause hepatomegaly or ascites. Although hyperbilirubinemia and increased alkaline phosphatase levels can be seen in sepsis, other features of VOD are rare[17,41,42].
| Cholangitis lenta (sepsis-related cholangitis) |
| Drug/parenteral nutrition induced hepatotoxicity/cholestasis |
| Acute graft-versus-host disease |
| Fungal infection |
| Viral hepatitis |
| Congestive heart failure |
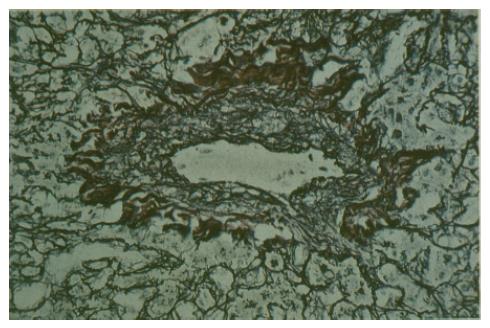
DeLeve et al[43] reported that the initial histological change in murine models of VOD was the loss of sinusoidal endothelial cell (SEC) fenestrae and appearance of gaps in SEC barrier. Other early histological findings were narrowing of the sublobular and central veins due to subendothelial edema, likely secondary to disruption of SEC barrier and congestion of hepatic sinusoids surrounded by pale necrotic hepatocytes[3]. Fragmented red blood cells, fibrinogen, and Factor VIII/von Willebrand factor can be demonstrated in the subendothelial space of central veins and perivenular zones of hepatic acini[44]. In the later stages, sinusoidal and venous lumen become obliterated by typeI, III, and IV collagen accompanied by an increase in the stellate cells that line the sinusoids[44,45] (Figure 2). In some cases, fibrous bridges between central venules were observed[14].
One hypothesis for the pathogenesis of VOD is depletion of liver glutathione reserve resulting in diminished ability to detoxify acrolein, an inactive but hepatotoxic metabolite of cyclophosphamide. SECs are especially prone to toxic effects of acrolein because of their lower glutathione level compared to hepatocytes[31,46]. Most of the drug metabolism in liver occurs in zone 3 hepatocytes which are rich in p-450 enzymes, explaining why the initial morphological changes are seen in SECs in zone 3. Several findings support this hypothesis. Continuous infusion of glutathione into the portal vein prevents VOD in murine models that were injected with monocrotaline to induce VOD[47]. Additionally, polymorphisms of glutathione S-transferase M1 gene were associated with increased risk of VOD in thalassemic patients undergoing HSCT[48]. Glutathione may also suppress expression of matrix metalloproteinase-9 that was shown to increase in VOD and whose inhibition completely prevents VOD in murine models[14].
Presence of fibrinogen, Factor VIII/von Willebrand factor in the subendothelial space and the finding of low plasma levels of Protein C and antithrombin III suggest involvement of the coagulation system in the pathogenesis of VOD[49]. However, it is unclear if coagulation plays a primary role or is a secondary event. Finally, an immunologic mechanism may have a pathogenetic a role in VOD. Increased risk of VOD in mismatched and unrelated transplants, and decreased risk in T-cell depleted HSCT supports this hypothesis[42,50].
In the absence of an established effective therapy, prevention of VOD is a priority. Non-myeloablative conditioning regimens may be used in eligible patients with a high risk of VOD. In patients who require myeloablation, several measures that may protect against the development of VOD have been recommended. These include: administering busulfan intravenously and adjusting its dose, increasing the interval between cytotoxic drugs and TBI, reducing the total dose and dose-rate of TBI, shielding the liver during TBI, fractionating TBI and carmustine dose, and administering busulfan after the other agents have been given[42].
Administration of ursodeoxycholic acid (UDCA) prior and during the HSCT has been shown to decrease the incidence of VOD in two randomized, prospective, placebo-controlled studies[51,52]. However, two more recent studies showed no decrease in the incidence of VOD in UDCA-treated patients, although one of them reported lower incidence of grade III and IV acute graft-versus-host disease. UDCA is well tolerated, and therefore is used in many transplantation centers for prophylaxis.
Low-dose continuous infusion of heparin is associated with decreased incidence of VOD in a few trials[53,54]. However, other studies have failed to show any beneficial effect[15,29,55]; additionally heparin may increase the risk of bleeding. Low-molecular weight heparin is safer and easier to administer, and may have a preventive role in VOD[56,57], and moreover may accelerate platelet engraftment[58]. However, well designed, randomized controlled studies are needed to confirm its efficacy.
Other agents that have been used in the prevention of VOD with varying results are: glutamine infusion, prostaglandin-E1, and pentoxyfilline[59-63]. Defibrotide, is another promising agent found to be effective in the prevention of VOD[64,65].
In most patients the only treatment needed is the use of diuretics and sodium restriction for the management of water and sodium balance. Repeated paracenteses may be required if ascites causes abdominal discomfort or pulmonary compromise. Renal perfusion and intravascular volume should be maintained while avoiding extravascular fluid accumulation[42]. Hepatotoxic drugs should be avoided and infections should be identified and treated promptly. Further treatment approaches vary greatly in different institutions, both in the choice of intervention and choice of patients to be treated.
A modest response to recombinant human tissue plasminogen activator (γ-tPA) and continuous heparin infusion has been reported in several studies[66,67]. However, thrombolytic therapy is limited by its high risk of fatal bleeding[68,69]. In a series of 42 patients, 12 patients responded to γ-TPA and heparin while 10 patients experienced severe bleeding, at least three of which were fatal[70].
Administration of anti-thrombin III (AT-III) concentrate might decrease the mortality in patients with severe VOD, without any associated serious side effects[71,72]. In one report of 10 patients with severe VOD, clinical improvement was seen in all patients after 5 d of AT-III infusion[73].
There are several reports suggesting a beneficial effect of prostaglandin E1 administration combined with heparin infusion in severe VOD[74-76]. However severe adverse effects limits its routine use. There are anecdotal reports on the successful use of charcoal hemofiltration[77], N-acetylcysteine[78] and glutamine/vitamin E[79,80].
Defibrotide, the most promising drug currently available for the treatment of VOD, is a single-stranded polydeoxyribonucleotide with anti-ischemic, anti-thrombotic and thrombolytic properties. It lacks systemic anticoagulant activity, an advantage in severely thrombocytopenic patients after HSCT[81]. Its mechanism of action is poorly understood but likely involves adenosine receptors[82]. Richardson et al[83] reported that 32 (36%) of 88 patients with VOD with a predicted risk of ≥ 30% of developing severe disease had a complete response to defibrotide treatment. Thirty-one of the 32 patients survived beyond 100 d. Adverse effects were limited to nausea, transient systolic hypotension, fever, abdominal cramping, and vasomotor symptoms such as hot flushes. In another clinical trial of defibrotide, complete response was achieved in 22 (55%) of 40 VOD patients[84].
Isolated case reports have shown benefit of transjugular intrahepatic portosystemic shunt (TIPS) in patients with VOD[85-87]. Although it can control portal hypertension, it is not clear whether TIPS can alter the course of the disease. Orthotopic liver transplantation has been attempted successfully in a few cases, however few institutions are capable of performing it on patients undergoing HSCT[88-90].
Budd-Chiari Syndrome (BCS) is an uncommon but potentially life-threatening disorder caused by obstruction of the hepatic venous outflow at any level from the small hepatic veins to the junction of the inferior vena cava (IVC) with the right atrium[91].
BCS can be classified as primary (due to intrinsic intraluminal thrombosis or webs) or secondary (due to intraluminal invasion by a parasite or malignant tumor or due to extraluminal compression by an abscess, cyst, or solid tumor)[92]. Intravascular thrombosis, mostly seen in primary myeloproliferative disorders, is the most common mechanism leading to the obstruction of the hepatic venous system. At least one hereditary or acquired procoagulative disorder can be identified in 75% of patients with BCS (Table 5)[93]. Polycythemia vera is found in between 10%-40% of patients, whereas essential thrombocythemia and myelofibrosis are less prevalent causes[94,95]. Of note, hepatic vein thrombosis occurs in up to 12% of patients with paroxysmal nocturnal hemoglobinuria and is the leading cause of mortality in this disorder[96,97]. As many as 30% of all cases of BCS are found to have factor V Leiden mutation which is present in the majority of pregnancy- or oral contraceptive-related cases of hepatic vein thrombosis[98,99]. Protein C, Protein S and antithrombin levels may be decreased nonspecifically due to impaired hepatic synthesis in patients with BCS, but levels below 20% of normal suggest inherited deficiency of these proteins[100]. Patients with BCS, as well as their relatives, should be counseled and investigated for hereditary thrombophilias.
| Common causes |
| Hypercoagulable states |
| Inherited thrombophilic disorders |
| Antithrombin III deficiency |
| Protein C deficiency |
| Protein S deficiency |
| Factor V Leiden mutation |
| Prothrombin gene mutation |
| Acquired procoagulative disorders |
| Myeloproliferative disorders (overt and occult) |
| Paroxysmal nocturnal hemoglobinuria |
| Antiphospholipid syndrome |
| Cancer |
| Pregnancy |
| Use of oral contraceptives |
| Uncommon causes |
| Tumoral invasion |
| Hepatocellular carcinoma |
| Renal cell carcinoma |
| Adrenal carcinoma |
| Miscellaneous |
| Aspergillosis |
| Behcet’s syndrome |
| Inferior vena caval webs |
| Trauma |
| Inflammatory bowel syndrome |
| Dacarbazine therapy |
| Idiopathic |
Although less common in western countries, primary membranous obstruction of the inferior vena cava (IVC) is the most common cause of BCS in South Africa and Asia, and is thought to be a consequence of IVC thrombosis[101]. For unknown reasons, 45-50% patients with known membranous occlusion ultimately develop hepatocellular carcinoma (HCC), even in the absence of cirrhosis[102]. On the other hand, HCC has not been reported to be a complication of hepatic vein thrombosis, except in Behcet’s disease-associated BCS[103].
In about 10% of patients with BCS, an underlying etiology cannot be identified[95]. Recently, endothelial dysfunction and decreased fibrinolytic activity have been suggested as contributing factors in patients with idiopathic BCS[104].
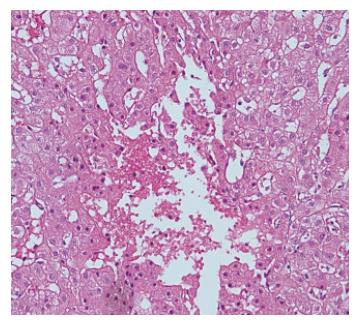
The parenchymal hepatic damage and the histologic abnormalities are variable, depending on the acuity and extent of hepatic venous outflow obstruction. Rapid occlusion of all 3 hepatic veins or 2 veins including the right hepatic vein leads to diffuse hepatic congestion and enlargement. The result is ischemic necrosis followed by fibrosis, predominantly in the perivenular areas (Figure 3). However, in patients with concomitant portal vein thrombosis, fibrosis prevails in the periportal zone[6,105]. In chronic disease, direct blood flow from the caudate lobe to the IVC compensates for hepatic outflow obstruction. Over time, the caudate lobe becomes hypertrophic while cirrhosis and atrophy develops in the rest of the liver[106,107].
Compensatory nodular regenerative hyperplasia is common in areas of hepatic parenchyma that have an adequate blood supply, with progression to fibrosis and cirrhosis at a later stage of the disease[6,108]. Also, in the advanced stages, it is common to see areas of infarction secondary to concomitant thrombosis of the intrahepatic and extrahepatic portal veins[109].
There is a wide clinical spectrum, ranging from a fulminant picture to an asymptomatic condition, depending on the location, extent, and rapidity of the obstructive process and on whether the portal vein is thrombosed or not. Obstruction of a single main hepatic vein is clinically silent[110], whereas sudden occlusion of all major hepatic veins may result in fulminant hepatic failure.
BCS is more common in women, and usually presents in the third or fourth decade of life[111]. The most common signs and symptoms are ascites, hepatomegaly, and abdominal pain. In a series of 237 patients, obstruction was found in hepatic veins, IVC or both in 62%, 7%, and 31% patients respectively[112].
For descriptive purposes only, the syndrome can be classified as asymptomatic, fulminant, acute, subacute, or chronic[113]. However, it should be stressed that the correlation between clinical history and the duration of the occlusive process is weak and this classification’s prognostic significance is not clear[114].
Patients with the asymptomatic variant are usually discovered on investigation of abnormal liver function tests. These patients constitute between 5%-20% of BCS cases[112,113]. Absence of ascites and abdominal pain may be attributed to large intrahepatic and portosystemic venous collaterals or patency of one large hepatic vein[93].
Patients with acute BCS develop severe RUQ abdominal pain, hepatomegaly, jaundice, and intractable ascites within a few weeks. The acute form is observed in 20% of BCS patients[113]. Serum aminotransferase levels are elevated due to ischemic hepatocellular damage. Serum alkaline phosphatase level is elevated, usually between 300 and 400 IU/L. Liver functions can deteriorate quickly leading to fulminant hepatic failure.
Fortunately, the fulminant form is uncommon and typically follows rapid and complete occlusion of all major hepatic veins. Patients rapidly develop hepatic encephalopathy, renal failure and coagulopathy. Serum aminotransferase levels are markedly elevated. Unlike the findings in fulminant viral hepatitis, the liver is enlarged and tender[93].
The subacute form has a more insidious presentation, spread over several months. Patients may have minimal ascites, splenomegaly, hepatomegaly, and vague RUQ discomfort. Jaundice is either absent or mild.
The chronic form is seen in 60% of patients with BCS. Patients have signs or symptoms for more than six months and generally present with complications of cirrhosis including variceal bleeding, encephalopathy, coagulopathy, and hepatorenal syndrome. Additionally, hepatopulmonary syndrome has been described in up to 28% patients[115]. Renal impairment is present in one-half of the cases[93].
There is currently no consensus on the association of disease severity with disease duration. Furthermore, the duration of symptoms do not correlate with the degree of histologic damage to the liver. It has been observed that 58% of patients with an acute clinical onset may have marked liver fibrosis, suggesting a long preclinical course. These findings can be explained by the development of recent thrombosis superimposed on previous lesions[110,116].
IVC compression or thrombosis generally presents with less severe symptoms compared with hepatic vein thrombosis and is characterized by leg edema or venous collaterals over the trunk in addition to hepatomegaly, ascites, and abdominal pain. The clinical course is chronic, with repeated acute episodes eventually leading to congestive cirrhosis[117,118].
Portal venous system is usually affected in patients with BCS because: 1) increased sinusoidal pressure decreases flow through the portal system, 2) hypertrophied caudate lobe may compress the main portal branches and the intrahepatic portal venules. These changes can result in stasis and vulnerability to thrombosis in the portal vein, further depleting liver perfusion[119]. However, in our experience the clinical picture in patients with both hepatic and portal vein thrombosis is better compared to that in patients with hepatic vein thrombosis only. This may be due to decreased blood flow to liver resulting in reduced hepatic congestion.
The natural history of BCS is poorly understood, mainly because most patients receive some form of treatment. The spectrum of clinical outcome ranges from progressive deterioration of the general condition to a few reports of spontaneous regression of the acute manifestations[120]. After the institution of anticoagulation therapy and early recognition of asymptomatic cases, mortality rates have decreased over time[113]. In a cohort of 120 patients, survival rates of 77%, 65% and 57% were found at 1, 5 and 10 years, respectively[121]. Murad et al[112] developed a model predicting survival in BCS, classifying patients into three categories with statistically different 5-year survival rates of 89%, 74%, and 42% based on the presence of ascites, encephalopathy, and prothrombin time and serum bilirubin levels.
Histologic abnormalities of the liver were not found to determine the prognosis[112,121,122] except in one study in which advanced fibrosis was associated with increased mortality[123]. One reason for this discrepancy can be explained by biopsy specimens being not representative of the whole liver due to uneven distribution of the hepatic lesions.
IVC obstruction has a good short term prognosis but there is limited data regarding long term prognosis. In a study from Japan, there was a 25% mortality rate over 15 years in patients with obliterative cavopathy. The main causes of death were liver failure, variceal bleeding and HCC[101,124].
In a series of 237 patients, portal vein thrombosis (PVT) with and without spleno-mesenteric vein thrombosis was identified in 18 (9%) and 15 (7%) patients respectively (total 33 patients, 16%). The five-year survival was 59% in patients with BCS and PVT and 85% in isolated BCS[125].
The diagnosis of BCS requires a high index of suspicion. It should be considered in all patients presenting with ascites, hepatomegaly and RUQ abdominal pain or when intractable ascites occurs in the presence of mildly altered liver function tests. Standard laboratory investigations are rarely helpful in establishing the diagnosis, because abnormal liver function tests are not specific for BCS. Edema of the lower extremities and presence of venous collaterals on the back suggests IVC occlusion. Ascitic fluid analysis adds little specificity to the diagnosis and ascitic fluid albumin content can differ according to the stage of the BCS[126]. Thus, the diagnosis is largely made by imaging studies.
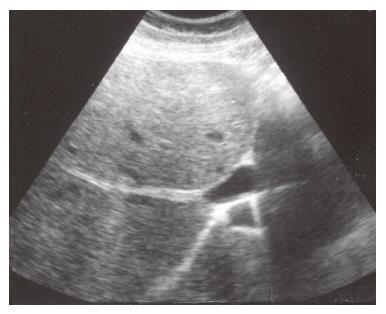
Real-time and Doppler sonography: Real-time or color and pulsed Doppler should be the initial investigation for BCS, with a sensitivity and specificity of nearly 85%[127]. Specific findings for hepatic vein obstruction are abnormal flow in a hepatic vein, large intrahepatic or subcapsular collateral vessels and inability to visualize the junction of major hepatic veins with IVC. Moreover, the presence of intrahepatic venous to venous spider web collaterals is highly suggestive of BCS[128,129] (Figure 4) Acute venous occlusion is identified by enlarged, stenotic, or tortuous hepatic veins, whereas in patients with chronic disease the major hepatic veins may not be readily visualized. Additional findings include: compression of IVC by hypertrophic caudate lobe or obstruction by thrombus, tumor, or membranes[130,131].
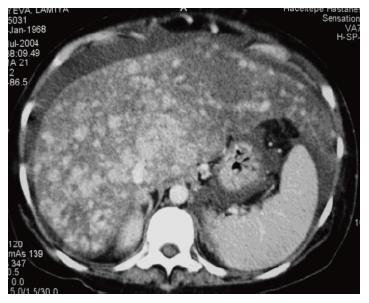
Computed tomography (CT): Failure to visualize the hepatic veins or IVC is suggestive of BCS. Contrast enhancement may be seen more centrally than peripherally resulting in a patchy flea-bitten appearance (Figure 5). CT also allows assessment of the extent of hepatic parenchymal disease, ascites, and splenomegaly[132]. However, the role of CT scan is limited because of false-positive and indeterminate results in almost one-half of the patients[128].
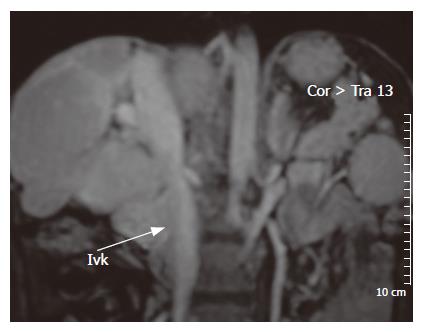
Magnetic resonance imaging (MRI): In patients with an unremarkable ultrasound examination but in whom the suspicion for BCS is high, MRI with intravenous gadolinium is a reasonable option (Figure 6). It allows excellent visualization of hepatic veins and IVC. Large intrahepatic, comma-shaped collaterals can be seen in patients with chronic obstruction. The major drawback of MRI is that, unlike ultrasonography, it does not provide the direction of hepatic venous blood flow[133,134].
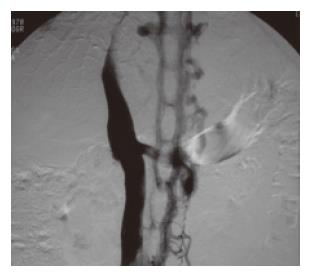
Hepatic venography: Although not considered essential for the diagnosis of BCS, venography provides useful information such as the extent of the thrombosis and caval pressures which help in determining the optimal therapy. Iodinated contrast can be injected into the hepatic veins either by transhepatic puncture or retrograde cannulation through the IVC (Figure 7). Abnormalities specific for BCS include a “spider-web” venous network, a coarse network of collaterals arching outward from the catheter tip, and a patent vein upstream from a stricture. A distorted appearance of hepatic veins that is observed in cirrhosis should not be confused with BCS. Another advantage of venography is that the portal vein can be assessed at the same sitting and if found to be occluded can be dilated with or without stenting in order to decompress the liver. Pressure measurements obtained during venography provide useful information before attempting surgical decompression[135,136]. Also, pressure measurements are useful in the post-surgical follow-up of these patients.
Liver biopsy: Liver biopsy may demonstrate congestion, hepatocyte necrosis, and fibrosis in the centrilobular area, findings that are similar to those seen in congestive hepatopathy. Venular thrombosis is usually not evident in biopsy specimens. All patients under consideration for surgery should undergo liver biopsy in conjunction with angiography to determine whether the patient will benefit more from a shunt procedure or a liver transplant[135,137].
The goals of treatment are to alleviate venous obstruction, prevent extension of thrombosis in the hepatic veins and preserve hepatic function by decreasing the centrilobular congestion. Diagnostic workup to identify the underlying cause should be considered at the time of the initial diagnosis.
Medical therapy: Medical therapy for BCS consists of efforts to control ascites, to prevent the extension of thrombosis with anticoagulation therapy, to dissolve the clot and relieve the hepatic congestion with thrombolysis and to treat the complications. Additionally, the underlying cause of BCS should be investigated and treated.
Ascites is managed by low-sodium diet, diuretics, and therapeutic paracentesis when needed. The type and duration of optimal anticoagulation therapy has not been established, however most centers give lifelong anticoagulation, initially with heparin followed by warfarin titrated to maintain the INR between 2 and 3[100,126]. During active variceal bleeding, balloon tamponade or endoscopic therapy is preferred to vasoconstrictor agents because the reduction in splanchnic blood flow induced by vasoconstrictors can theoretically precipitate mesenteric and portal vein thrombosis[138].
Medical therapy alone can be a good option for patients in whom there is no ongoing hepatic necrosis, as indicated by the presence of minimal symptoms, relatively normal liver function results, and ascites that is easily controlled[139]. Patients on medical therapy alone should be monitored closely for disease progression and to detect progressive necrosis by serial upper endoscopies and liver biopsies.
Restoration of hepatic blood flow: Relief of venous obstruction can be attempted by thrombolytic therapy, percutaneous angioplasty, transjugular intrahepatic portosystemic shunt (TIPS), or shunt surgery. In patients with an acute presentation, especially when angiography shows presence of a fresh thrombus, thrombolytic therapy can be administered systemically or directly into the thrombosed hepatic vein. The best results are obtained when the thrombolytic agent is administered early directly into the vein, although promising data has been reported with thrombolysis carried out two to three weeks after the onset of symptoms[140,141].
Balloon angioplasty has been used to relieve hepatic outflow obstruction secondary to caval webs or short-segment hepatic vein stenosis[142]. Although short-term results are excellent, the sustained patency rate is only 50% at two years after the procedure[143]. However, the use of intraluminal stents has been shown to increase the long-term patency rates to nearly 90%[144-146]. Once inserted, the stents cannot be removed, and placement of a stent above the intrahepatic IVC may interfere with liver transplantation. Failure of angioplasty or stenting, should prompt consideration for a surgical portosystemic shunt or TIPS[147].
TIPS decompresses the liver by creating an alternative venous outflow tract. It is particularly useful, either alone or as a bridge to liver transplantation, in patients with an acute presentation such as those with variceal bleeding, and patients with fulminant hepatic failure in whom thrombolysis and angioplasty were unsuccessful[148,149]. TIPS may be preferred over surgical shunting because it avoids laparotomy and has less periprocedure mortality and morbidity, its efficacy is not affected by caudate lobe hypertrophy, and it can be done in patients with PVT[150-152]. Although long-term patency rate is only about 50%, patients in whom stent stenosis occurs do not generally worsen, perhaps because the shunt allows time for collateral circulation to develop[153]. The recently introduced, polytetrafluoroethylene-covered stents may improve the patency rates[154]. However, extension of the stent into the suprahepatic IVC may preclude liver transplantation and should be avoided.
Surgical shunts decompress the liver by creating hepatofugal flow through the portal vein. Patients with a non-fulminant presentation and those who have a chronic presentation without significant hepatic fibrosis should be considered for surgical shunting, providing the portal vein is patent[150]. The five-year survival rate after surgical shunting ranges between 57% and 94% and absence of IVC occlusion is associated with better outcomes[155-157].
A pressure difference of at least 10 mmHg between the portal vein and intrahepatic IVC is essential for portocaval and mesocaval shunts to function[123]. Acute hepatic decompensation may occur postoperatively, therefore shunt operations should be performed in centers where rescue liver transplantation can take place.
The type of surgical shunt depends on the anatomy of the obstruction. Side-to-side portacaval shunts have the highest patency rate but may make subsequent liver transplantation more difficult. Side-to-side mesocaval shunts are technically less demanding and do not interfere with liver transplantation but when placed with a synthetic graft, have a higher thrombosis rate than a side-to-side portacaval shunt[147]. Mesoatrial shunt should be considered if there is IVC compression resulting in low portocaval pressure gradient. Mesoatrial shunt has a high rate of thrombosis due to relatively low flow rate in the prosthetic graft[126]. Side-to-side portocaval shunt and cavoatrial shunt have been described for patients with both caval and hepatic venous thrombosis[136,157,158].
In addition to shunts, other surgical procedures can also provide adequate decompression in selected patients. Transatrial membranotomy can be effective in patients with IVC membranes[136,157,159,160]. Dorsocranial resection of the liver is the only feasible procedure if there is both portal and superior mesenteric vein obstruction[161].
Liver transplantation: Patients who have cirrhosis, fulminant hepatic failure or biochemical evidence of advanced liver dysfunction are best managed with liver transplantation[157]. Long term survival rates range from 50% to 95%[155,162-164]. Development of postoperative portal vein or hepatic artery thrombosis occurs in about 12% of patients. Liver transplantation can cure almost all hereditary thrombophilias, however thrombosis can still occur and anticoagulation is necessary[150].
Congestive hepatopathy refers to hepatic manifestations attributable to passive hepatic congestion resulting from right-sided heart failure of any cause including and not limited to constrictive pericarditis (CP), tricuspid regurgitation, cor pulmonale, and cardiomyopathies. Development of hepatic ischemia and infarction secondary to left-sided heart failure which usually coincides with congestive hepatopathy will not be discussed here. However, it should be kept in mind that the clinical picture in congestive hepatopathy varies greatly depending on both the degree and acuity of congestion, and presence or absence of hepatic ischemia and infarction[2].
Liver dysfunction in congestive hepatopathy is usually mild and asymptomatic and often detected incidentally on routine biochemical testing. Symptomatic patients usually present with mild jaundice which is characteristically absent in patients with CP. In patients with severe heart failure, jaundice may be so deep as to suggest cholestasis[165]. Right upper quadrant discomfort due to stretching of liver capsule and ascites may also be present.
Occasionally, the clinical picture may resemble that of acute viral hepatitis, when jaundice is accompanied by elevated aminotransferase levels due to acute hepatic ischemia[166,167]. Several cases of fulminant hepatic failure resulting in death have been reported due to congestive heart failure. However, most of these patients had characteristics of both hepatic congestion and ischemia[165,168,169].
On physical examination, patients may have tender hepatomegaly, sometimes massive, with a firm and smooth liver edge. Splenomegaly is uncommon, and like ascites is due to transmitted elevated central venous pressure. Jugular venous distention and hepatojugular reflux may be present and are helpful in differentiating congestive hepatopathy from BCS and primary liver diseases. Liver may be pulsatile especially in patients with tricuspid regurgitation. Loss of hepatic pulsatility suggests progression to cardiac cirrhosis.
The most common laboratory finding is elevation of total serum bilirubin level, most of which is unconjugated. Hyperbilirubinemia occurs in up to 70% of patients with congestive hepatopathy[170]. Severe hyperbilirubinemia may develop in patients with severe, usually acute, right-sided heart failure. Serum bilirubin level shows a correlation with right atrial pressure but not with cardiac output[6]. Even in the presence of deep jaundice, serum alkaline phosphatase level is generally only mildly elevated, which helps to distinguish congestive hepatopathy from obstructive jaundice. Serum aminotransferase levels also show a mild elevation unless cardiac output is impaired. In patients with severe acute heart failure, aminotransferase levels may be extremely high secondary to hepatic ischemia and the degree of elevation correlates with the extent of necrosis as seen on liver biopsy specimens. Additionally, the prothrombin time may be mildly abnormal[171], albumin level may be decreased[172], and serum ammonia level may be elevated[173].
Increasing specialization in medicine has caused physicians to overlook diseases that cross the boundaries of two specialties. Congestive hepatopathy may be missed in patients with heart failure and mild hepatic congestion, and in patients with overt hepatic congestion and vague cardiac symptoms[174,175]. Physicians should consider right-sided heart failure in patients with hepatomegaly with or without jaundice. CP is especially difficult to differentiate from primary liver cirrhosis and BCS because of its relatively nonspecific clinical features.
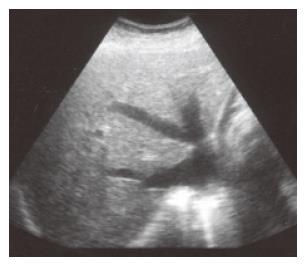
A careful history and a thorough physical examination are crucial in the diagnosis of congestive hepatopathy. Symptoms such as exertional dyspnea, orthopnea, and angina, and physical findings like jugular venous distention, heart murmurs, and rales may help physicians to differentiate congestive hepatopathy from primary liver diseases. In addition to liver biochemical testing, viral hepatitis serology, and abdominal ultrasound with Doppler studies of the liver; EKG and echocardiogram should be performed when congestive hepatopathy is suspected. However, a normal echocardiogram does not always rule out congestive hepatopathy. Upon the finding of dilatation of all three main hepatic veins on abdominal sonogram (Figure 8), physicians should look for a cardiac cause such as constrictive pericarditis more vigorously with cardiac angiogram or other radiological methods.
Diagnostic paracentesis in patients with congestive hepatopathy usually reveals a high ascitic fluid protein content and a serum to ascites albumin gradient > 1.1 g/dL reflecting the contribution of “hepatic lymph” and portal hypertension to the ascites[176].
Improvement in liver biochemical tests with treatment of the underlying cardiac disease supports the diagnosis. Liver biopsy may help confirm the diagnosis in equivocal cases.
The characteristic gross appearance of liver in congestive hepatopathy is referred to as the nutmeg liver. This appearance is the result of contrasting areas of red caused by sinusoidal congestion and bleeding in the necrotic regions surrounding the enlarged hepatic veins and yellow due to the normal or fatty liver tissue. Histologically, sinusoidal engorgement and hemorrhagic necrosis is apparent in perivenular areas of hepatic acini. Variable degrees of cholestasis with occasional bile thrombi in the canaliculi may also be apparent. With chronic heart failure, fibrosis develops in perivenular areas, ultimately causing bridging fibrosis between adjacent central veins. This process results in cardiac fibrosis, inappropriately referred as cardiac cirrhosis, which is distinct from primary liver cirrhosis in which fibrous bands tend to link adjacent portal areas. The regeneration of periportal hepatocytes in cardiac fibrosis may result in nodular regenerative hyperplasia. If heart failure is treated successfully, the early histologic changes of congestive hepatopathy may resolve, and even cardiac fibrosis may regress histologically and clinically[2].
Treatment of the underlying heart disease is fundamental to the management of congestive hepatopathy and is outside the scope of this review. Jaundice and ascites usually respond significantly to diuresis. However, excessive diuresis may decrease cardiac output and result in hepatic ischemia in patients with severe congestive heart failure.
Most patients with congestive hepatopathy die of cardiac causes and liver disease rarely contributes to the morbidity or mortality in these patients. Unlike patients with liver cirrhosis, those with cardiac fibrosis rarely develop serious complications such as variceal bleeding[177]. Hepatocellular carcinoma may rarely complicate cardiac fibrosis[178]. However, the incidence of hepatocellular carcinoma and liver failure due to congestive hepatopathy is likely to increase as survival is prolonged with advances in the treatment of heart failure.
CP deserves mention because its diagnosis can be easily missed and its clinical manifestations are different from those seen in congestive hepatopathy due to other causes. CP is the result of scarring and loss of elasticity of the pericardial sac which restricts cardiac filling. Tuberculosis, cardiac surgery, radiation therapy, and connective tissue disorders are among common causes, however most of the cases are idiopathic or viral[179-181].
Patients with CP may present with symptoms of fluid overload such as edema and ascites, or symptoms of diminished cardiac output like exertional dyspnea and fatigue. Hepatomegaly, massive ascites, and peripheral edema are common findings. For reasons that are unclear, patients with CP typically do not develop jaundice[5] and very rarely may develop chylous ascites[182-183]. Because its manifestations are protean, it is easy to mistake CP for BCS and liver cirrhosis[184]. Jugular venous distention which was noted in 93% patients in a large series is a critical finding in the diagnosis of CP[180]. Although rarely observed, Kussmaul’s sign, pericardial knock, and pulsus paradoxus may offer additional clues to the diagnosis.
EKG may show a low voltage and nonspecific ST and T wave changes. Chest X-ray may demonstrate pericardial calcification. Echocardiography is essential in the diagnosis of CP, but right and left heart catheterization with hemodynamic evaluation may be required to confirm the diagnosis[185-187].
Cardiac fibrosis develops more frequently and rapidly in CP than in other causes of right-sided heart failure, perhaps due to higher hepatic venous pressures leading to severe zone 3 congestion and necrosis[2]. Pericardiectomy is the standard of treatment and curative if performed early.
The clinical manifestations and liver histology in VOD, BCS, and congestive hepatopathy are similar because of the common underlying mechanism of hepatic injury and adaptation in response to increased sinusoidal pressure. Further animal and in-vitro studies are needed to better understand the pathways involved in sinusoidal injury in response to increased venous pressure. The severity of clinical picture depends mostly on the rapidity and extent of the obstructive process. Physicians should consider congestive hepatopathy and BCS in all patients with tender hepatomegaly and ascites. Presence of jugular venous distention is an invaluable clue to differentiate congestive hepatopathy from BCS and other primary liver diseases. Veno-occlusive disease should be kept in mind in patients who rapidly develop jaundice and ascites with hepatomegaly after HSCT. The advent of interventional radiology and new medical therapies has improved survival in patients with hepatic venous outflow obstruction, although prevention is still mainstay in the management of VOD.
S- Editor Wang J L- Editor Anand BS E- Editor Che YB
| 1. | Lautt WW, Greenway CV. Conceptual review of the hepatic vascular bed. Hepatology. 1987;7:952-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 233] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Rosenberg PM, Friedman LS. The liver in circulatory failure. Schiff's diseases of the liver. 9th ed. Philadelphia: Lippincott Williams & Wilkins 2004; 1327-1340. |
| 3. | Shulman HM, McDonald GB, Matthews D, Doney KC, Kopecky KJ, Gauvreau JM, Thomas ED. An analysis of hepatic venocclusive disease and centrilobular hepatic degeneration following bone marrow transplantation. Gastroenterology. 1980;79:1178-1191. [PubMed] |
| 4. | Tanaka M, Wanless IR. Pathology of the liver in Budd-Chiari syndrome: portal vein thrombosis and the histogenesis of veno-centric cirrhosis, veno-portal cirrhosis, and large regenerative nodules. Hepatology. 1998;27:488-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 146] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 5. | Arora A, Tandon N, Sharma MP, Acharya SK. Constrictive pericarditis masquerading as Budd-Chiari syndrome. J Clin Gastroenterol. 1991;13:178-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Sherlock S. The liver in heart failure; relation of anatomical, functional, and circulatory changes. Br Heart J. 1951;13:273-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Berk PD, Popper H, Krueger GR, Decter J, Herzig G, Graw RG. Veno-occlusive disease of the liver after allogeneic bone marrow transplantation: possible association with graft-versus-host disease. Ann Intern Med. 1979;90:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 65] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Fajardo LF, Colby TV. Pathogenesis of veno-occlusive liver disease after radiation. Arch Pathol Lab Med. 1980;104:584-588. [PubMed] |
| 9. | King PD, Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001;6:162-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 323] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Tandon BN, Tandon HD, Tandon RK, Narndranathan M, Joshi YK. An epidemic of veno-occlusive disease of liver in central India. Lancet. 1976;2:271-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 135] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Mohabbat O, Younos MS, Merzad AA, Srivastava RN, Sediq GG, Aram GN. An outbreak of hepatic veno-occlusive disease in north-western Afghanistan. Lancet. 1976;2:269-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 149] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Sebagh M, Debette M, Samuel D, Emile JF, Falissard B, Cailliez V, Shouval D, Bismuth H, Reynès M. "Silent" presentation of veno-occlusive disease after liver transplantation as part of the process of cellular rejection with endothelial predilection. Hepatology. 1999;30:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Bras G, Jelliffe DB, Stuart KL. Veno-occlusive disease of liver with nonportal type of cirrhosis, occurring in Jamaica. AMA Arch Pathol. 1954;57:285-300. [PubMed] |
| 14. | DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis. 2002;22:27-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 427] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 15. | Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H, Bandini G, Esperou H, Russell J, de la Rubia J. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood. 1998;92:3599-3604. [PubMed] |
| 16. | McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, Shulman HM, Clift RA. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 829] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 17. | Kumar S, DeLeve LD, Kamath PS, Tefferi A. Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clin Proc. 2003;78:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Carreras E, Grañena A, Navasa M, Bruguera M, Marco V, Sierra J, Tassies MD, García-Pagán JC, Martí JM, Bosch J. On the reliability of clinical criteria for the diagnosis of hepatic veno-occlusive disease. Ann Hematol. 1993;66:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Rio B, Andreu G, Nicod A, Arrago JP, Dutrillaux F, Samama M, Zittoun R. Thrombocytopenia in venocclusive disease after bone marrow transplantation or chemotherapy. Blood. 1986;67:1773-1776. [PubMed] |
| 20. | Méresse V, Hartmann O, Vassal G, Benhamou E, Valteau-Couanet D, Brugieres L, Lemerle J. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant. 1992;10:135-141. [PubMed] |
| 21. | Ayash LJ, Hunt M, Antman K, Nadler L, Wheeler C, Takvorian T, Elias A, Antin JH, Greenough T, Eder JP. Hepatic venoocclusive disease in autologous bone marrow transplantation of solid tumors and lymphomas. J Clin Oncol. 1990;8:1699-1706. [PubMed] |
| 22. | Bearman SI, Anderson GL, Mori M, Hinds MS, Shulman HM, McDonald GB. Venoocclusive disease of the liver: development of a model for predicting fatal outcome after marrow transplantation. J Clin Oncol. 1993;11:1729-1736. [PubMed] |
| 23. | Frickhofen N, Wiesneth M, Jainta C, Hertenstein B, Heymer B, Bianchi L, Dienes HP, Koerner K, Bunjes D, Arnold R. Hepatitis C virus infection is a risk factor for liver failure from veno-occlusive disease after bone marrow transplantation. Blood. 1994;83:1998-2004. [PubMed] |
| 24. | Strasser SI, Myerson D, Spurgeon CL, Sullivan KM, Storer B, Schoch HG, Kim S, Flowers ME, McDonald GB. Hepatitis C virus infection and bone marrow transplantation: a cohort study with 10-year follow-up. Hepatology. 1999;29:1893-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Rozman C, Carreras E, Qian C, Gale RP, Bortin MM, Rowlings PA, Ash RC, Champlin RE, Henslee-Downey PJ, Herzig RH. Risk factors for hepatic veno-occlusive disease following HLA-identical sibling bone marrow transplants for leukemia. Bone Marrow Transplant. 1996;17:75-80. [PubMed] |
| 26. | Ertem M, Akar N. Factor V Leiden mutation as a predisposing factor for veno-occlusive disease following BMT. Bone Marrow Transplant. 2000;25:1110-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Duggan C, Schmidt M, Lawler M, White B, Cusack S, McCann S, Smith O. The prothrombin gene variant G20210A but not factor V leiden may be associated with veno-occlusive disease following BMT. Bone Marrow Transplant. 1999;24:693-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Wadleigh M, Richardson PG, Zahrieh D, Lee SJ, Cutler C, Ho V, Alyea EP, Antin JH, Stone RM, Soiffer RJ. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood. 2003;102:1578-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 232] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Hägglund H, Remberger M, Klaesson S, Lönnqvist B, Ljungman P, Ringdén O. Norethisterone treatment, a major risk-factor for veno-occlusive disease in the liver after allogeneic bone marrow transplantation. Blood. 1998;92:4568-4572. [PubMed] |
| 30. | Kalayoglu-Besisik S, Yenerel MN, Caliskan Y, Ozturk S, Besisik F, Sargin D. Time-related changes in the incidence, severity, and clinical outcome of hepatic veno-occlusive disease in hematopoietic stem cell transplantation patients during the past 10 years. Transplant Proc. 2005;37:2285-2289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | DeLeve LD. Cellular target of cyclophosphamide toxicity in the murine liver: role of glutathione and site of metabolic activation. Hepatology. 1996;24:830-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 129] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Shulman HM, Hinterberger W. Hepatic veno-occlusive disease--liver toxicity syndrome after bone marrow transplantation. Bone Marrow Transplant. 1992;10:197-214. [PubMed] |
| 33. | McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 582] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 34. | Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, Sensenbrenner LL, Santos GW, Saral R. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 667] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 35. | Shulman HM, Gooley T, Dudley MD, Kofler T, Feldman R, Dwyer D, McDonald GB. Utility of transvenous liver biopsies and wedged hepatic venous pressure measurements in sixty marrow transplant recipients. Transplantation. 1995;59:1015-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 89] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Carreras E, Grañena A, Navasa M, Bruguera M, Marco V, Sierra J, Tassies MD, García-Pagán JC, Martí JM, Bosch J. Transjugular liver biopsy in BMT. Bone Marrow Transplant. 1993;11:21-26. [PubMed] |
| 37. | Yoshimoto K, Ono N, Okamura T, Sata M. Recent progress in the diagnosis and therapy for veno-occlusive disease of the liver. Leuk Lymphoma. 2003;44:229-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Salat C, Holler E, Kolb HJ, Reinhardt B, Pihusch R, Wilmanns W, Hiller E. Plasminogen activator inhibitor-1 confirms the diagnosis of hepatic veno-occlusive disease in patients with hyperbilirubinemia after bone marrow transplantation. Blood. 1997;89:2184-2188. [PubMed] |
| 39. | Rio B, Bauduer F, Arrago JP, Zittoun R. N-terminal peptide of type III procollagen: a marker for the development of hepatic veno-occlusive disease after BMT and a basis for determining the timing of prophylactic heparin. Bone Marrow Transplant. 1993;11:471-472. [PubMed] |
| 40. | Fried MW, Duncan A, Soroka S, Connaghan DG, Farrand A, Peter J, Strauss RM, Boyer TD, McDonald GB. Serum hyaluronic acid in patients with veno-occlusive disease following bone marrow transplantation. Bone Marrow Transplant. 2001;27:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Strasser SI, McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation. Schiff's diseases of the liver. 9th ed. Philadelphia: Lippincott Williams & Wilkins 2004; 1635-1658. |
| 42. | Carreras E. Veno-occlusive disease of the liver after hemopoietic cell transplantation. Eur J Haematol. 2000;64:281-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | DeLeve LD, McCuskey RS, Wang X, Hu L, McCuskey MK, Epstein RB, Kanel GC. Characterization of a reproducible rat model of hepatic veno-occlusive disease. Hepatology. 1999;29:1779-1791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 237] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 44. | Shulman HM, Gown AM, Nugent DJ. Hepatic veno-occlusive disease after bone marrow transplantation. Immunohistochemical identification of the material within occluded central venules. Am J Pathol. 1987;127:549-558. [PubMed] |
| 45. | Sato Y, Asada Y, Hara S, Marutsuka K, Tamura K, Hayashi T, Sumiyoshi A. Hepatic stellate cells (Ito cells) in veno-occlusive disease of the liver after allogeneic bone marrow transplantation. Histopathology. 1999;34:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | DeLeve LD, Wang X, Kuhlenkamp JF, Kaplowitz N. Toxicity of azathioprine and monocrotaline in murine sinusoidal endothelial cells and hepatocytes: the role of glutathione and relevance to hepatic venoocclusive disease. Hepatology. 1996;23:589-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 173] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Wang X, Kanel GC, DeLeve LD. Support of sinusoidal endothelial cell glutathione prevents hepatic veno-occlusive disease in the rat. Hepatology. 2000;31:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Srivastava A, Poonkuzhali B, Shaji RV, George B, Mathews V, Chandy M, Krishnamoorthy R. Glutathione S-transferase M1 polymorphism: a risk factor for hepatic venoocclusive disease in bone marrow transplantation. Blood. 2004;104:1574-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 49. | Lee JH, Lee KH, Kim S, Lee JS, Kim WK, Park CJ, Chi HS, Kim SH. Relevance of proteins C and S, antithrombin III, von Willebrand factor, and factor VIII for the development of hepatic veno-occlusive disease in patients undergoing allogeneic bone marrow transplantation: a prospective study. Bone Marrow Transplant. 1998;22:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Soiffer RJ, Dear K, Rabinowe SN, Anderson KC, Freedman AS, Murray C, Tarbell NJ, Mauch P, Nadler LM, Ritz J. Hepatic dysfunction following T-cell-depleted allogeneic bone marrow transplantation. Transplantation. 1991;52:1014-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Ohashi K, Tanabe J, Watanabe R, Tanaka T, Sakamaki H, Maruta A, Okamoto S, Aotsuka N, Saito K, Nishimura M. The Japanese multicenter open randomized trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am J Hematol. 2000;64:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 52. | Essell JH, Schroeder MT, Harman GS, Halvorson R, Lew V, Callander N, Snyder M, Lewis SK, Allerton JP, Thompson JM. Ursodiol prophylaxis against hepatic complications of allogeneic bone marrow transplantation. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1998;128:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 127] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 53. | Attal M, Huguet F, Rubie H, Huynh A, Charlet JP, Payen JL, Voigt JJ, Brousset P, Selves J, Muller C. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective, randomized trial. Blood. 1992;79:2834-2840. [PubMed] |
| 54. | Rosenthal J, Sender L, Secola R, Killen R, Millerick M, Murphy L, Cairo MS. Phase II trial of heparin prophylaxis for veno-occlusive disease of the liver in children undergoing bone marrow transplantation. Bone Marrow Transplant. 1996;18:185-191. [PubMed] |
| 55. | Marsa-Vila L, Gorin NC, Laporte JP, Labopin M, Dupuy-Montbrun MC, Fouillard L, Isnard F, Najman A. Prophylactic heparin does not prevent liver veno-occlusive disease following autologous bone marrow transplantation. Eur J Haematol. 1991;47:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 60] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 56. | Or R, Nagler A, Shpilberg O, Elad S, Naparstek E, Kapelushnik J, Cass Y, Gillis S, Chetrit A, Slavin S. Low molecular weight heparin for the prevention of veno-occlusive disease of the liver in bone marrow transplantation patients. Transplantation. 1996;61:1067-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 71] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Forrest DL, Thompson K, Dorcas VG, Couban SH, Pierce R. Low molecular weight heparin for the prevention of hepatic veno-occlusive disease (VOD) after hematopoietic stem cell transplantation: a prospective phase II study. Bone Marrow Transplant. 2003;31:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Or R, Elad S, Shpilberg O, Eldor A. Low molecular weight heparin stimulates megakaryocytopoiesis in bone-marrow transplantation patients. Am J Hematol. 1996;53:46-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Brown SA, Goringe A, Fegan C, Davies SV, Giddings J, Whittaker JA, Burnett AK, Poynton CH. Parenteral glutamine protects hepatic function during bone marrow transplantation. Bone Marrow Transplant. 1998;22:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Gluckman E, Jolivet I, Scrobohaci ML, Devergie A, Traineau R, Bourdeau-Esperou H, Lehn P, Faure P, Drouet L. Use of prostaglandin E1 for prevention of liver veno-occlusive disease in leukaemic patients treated by allogeneic bone marrow transplantation. Br J Haematol. 1990;74:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Bearman SI, Shen DD, Hinds MS, Hill HA, McDonald GB. A phase I/II study of prostaglandin E1 for the prevention of hepatic venocclusive disease after bone marrow transplantation. Br J Haematol. 1993;84:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Attal M, Huguet F, Rubie H, Charlet JP, Schlaifer D, Huynh A, Laurent G, Pris J. Prevention of regimen-related toxicities after bone marrow transplantation by pentoxifylline: a prospective, randomized trial. Blood. 1993;82:732-736. [PubMed] |
| 63. | Clift RA, Bianco JA, Appelbaum FR, Buckner CD, Singer JW, Bakke L, Bensinger WI, Bowden RA, McDonald GB, Schubert M. A randomized controlled trial of pentoxifylline for the prevention of regimen-related toxicities in patients undergoing allogeneic marrow transplantation. Blood. 1993;82:2025-2030. [PubMed] |
| 64. | Chalandon Y, Roosnek E, Mermillod B, Newton A, Ozsahin H, Wacker P, Helg C, Chapuis B. Prevention of veno-occlusive disease with defibrotide after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2004;10:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Versluys B, Bhattacharaya R, Steward C, Cornish J, Oakhill A, Goulden N. Prophylaxis with defibrotide prevents veno-occlusive disease in stem cell transplantation after gemtuzumab ozogamicin exposure. Blood. 2004;103:1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Bearman SI, Shuhart MC, Hinds MS, McDonald GB. Recombinant human tissue plasminogen activator for the treatment of established severe venocclusive disease of the liver after bone marrow transplantation. Blood. 1992;80:2458-2462. [PubMed] |
| 67. | Leahey AM, Bunin NJ. Recombinant human tissue plasminogen activator for the treatment of severe hepatic veno-occlusive disease in pediatric bone marrow transplant patients. Bone Marrow Transplant. 1996;17:1101-1104. [PubMed] |
| 68. | Ringdén O, Wennberg L, Ericzon BG, Kallman R, Aström M, Duraj F, Söderdahl G, Tydén G, Groth CG. Alteplase for hepatic veno-occlusive disease after bone marrow transplantation. Lancet. 1992;340:546-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Hágglund H, Ringdén O, Ericzon BG, Duraj F, Ljungman P, Lönnqvist B, Winiarski J, Tydén G. Treatment of hepatic venoocclusive disease with recombinant human tissue plasminogen activator or orthotopic liver transplantation after allogeneic bone marrow transplantation. Transplantation. 1996;62:1076-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Bearman SI, Lee JL, Barón AE, McDonald GB. Treatment of hepatic venocclusive disease with recombinant human tissue plasminogen activator and heparin in 42 marrow transplant patients. Blood. 1997;89:1501-1506. [PubMed] |
| 71. | Ibrahim RB, Peres E, Dansey R, Abidi MH, Abella EM, Klein J. Anti-thrombin III in the management of hematopoietic stem-cell transplantation-associated toxicity. Ann Pharmacother. 2004;38:1053-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Mertens R, Brost H, Granzen B, Nowak-Göttl U. Antithrombin treatment of severe hepatic veno-occlusive disease in children with cancer. Eur J Pediatr. 1999;158 Suppl 3:S154-S158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | Morris JD, Harris RE, Hashmi R, Sambrano JE, Gruppo RA, Becker AT, Morris CL. Antithrombin-III for the treatment of chemotherapy-induced organ dysfunction following bone marrow transplantation. Bone Marrow Transplant. 1997;20:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Higashigawa M, Watanabe M, Nishihara H, Tabata N, Azuma E, Ido M, Ito M, Sakurai M. Successful treatment of an infant with veno-occlusive disease developed after allogeneic bone marrow transplantation by tissue plasminogen activator, heparin and prostaglandin E1. Leuk Res. 1995;19:477-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 75. | Ibrahim A, Pico JL, Maraninchi D, Zambon E, Attal M, Brault P, Tilly H, Blaise D, Hayat M. Hepatic veno-occlusive disease following bone marrow transplantation treated by prostaglandin E1. Bone Marrow Transplant. 1991;7 Suppl 2:53. [PubMed] |
| 76. | Schlegel PG, Haber HP, Beck J, Krümpelmann S, Handgretinger R, Bader P, Bierings M, Niethammer D, Klingebiel T. Hepatic veno-occlusive disease in pediatric stem cell recipients: successful treatment with continuous infusion of prostaglandin E1 and low-dose heparin. Ann Hematol. 1998;76:37-41. [PubMed] |
| 77. | Tefferi A, Kumar S, Wolf RC, Lacy MQ, Inwards DJ, Gloor JM, Albright RC, Kamath PS, Litzow MR. Charcoal hemofiltration for hepatic veno-occlusive disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28:997-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 78. | Ringdén O, Remberger M, Lehmann S, Hentschke P, Mattsson J, Klaesson S, Aschan J. N-acetylcysteine for hepatic veno-occlusive disease after allogeneic stem cell transplantation. Bone Marrow Transplant. 2000;25:993-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 79. | Goringe AP, Brown S, O'Callaghan U, Rees J, Jebb S, Elia M, Poynton CH. Glutamine and vitamin E in the treatment of hepatic veno-occlusive disease following high-dose chemotherapy. Bone Marrow Transplant. 1998;21:829-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Nattakom TV, Charlton A, Wilmore DW. Use of vitamin E and glutamine in the successful treatment of severe veno-occlusive disease following bone marrow transplantation. Nutr Clin Pract. 1995;10:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 81. | Palmer KJ, Goa KL. Defibrotide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in vascular disorders. Drugs. 1993;45:259-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Bianchi G, Barone D, Lanzarotti E, Tettamanti R, Porta R, Moltrasio D, Cedro A, Salvetti L, Mantovani M, Prino G. Defibrotide, a single-stranded polydeoxyribonucleotide acting as an adenosine receptor agonist. Eur J Pharmacol. 1993;238:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 83. | Richardson PG, Murakami C, Jin Z, Warren D, Momtaz P, Hoppensteadt D, Elias AD, Antin JH, Soiffer R, Spitzer T. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100:4337-4343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 210] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 84. | Chopra R, Eaton JD, Grassi A, Potter M, Shaw B, Salat C, Neumeister P, Finazzi G, Iacobelli M, Bowyer K. Defibrotide for the treatment of hepatic veno-occlusive disease: results of the European compassionate-use study. Br J Haematol. 2000;111:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Fried MW, Connaghan DG, Sharma S, Martin LG, Devine S, Holland K, Zuckerman A, Kaufman S, Wingard J, Boyer TD. Transjugular intrahepatic portosystemic shunt for the management of severe venoocclusive disease following bone marrow transplantation. Hepatology. 1996;24:588-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Azoulay D, Castaing D, Lemoine A, Hargreaves GM, Bismuth H. Transjugular intrahepatic portosystemic shunt (TIPS) for severe veno-occlusive disease of the liver following bone marrow transplantation. Bone Marrow Transplant. 2000;25:987-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 65] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Annaloro C, Robbiolo L, Pozzoli E, Ibatici A, Nicolini A, Salerno F, Deliliers GL. Four-year survival after trans-jugular intrahepatic porto-systemic shunt for veno-occlusive disease following autologous bone marrow transplantation. Leuk Lymphoma. 2004;45:1485-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 88. | Nimer SD, Milewicz AL, Champlin RE, Busuttil RW. Successful treatment of hepatic venoocclusive disease in a bone marrow transplant patient with orthotopic liver transplantation. Transplantation. 1990;49:819-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 89. | Rapoport AP, Doyle HR, Starzl T, Rowe JM, Doeblin T, DiPersio JF. Orthotopic liver transplantation for life-threatening veno-occlusive disease of the liver after allogeneic bone marrow transplant. Bone Marrow Transplant. 1991;8:421-424. [PubMed] |
| 90. | Kim ID, Egawa H, Marui Y, Kaihara S, Haga H, Lin YW, Kudoh K, Kiuchi T, Uemoto S, Tanaka K. A successful liver transplantation for refractory hepatic veno-occlusive disease originating from cord blood transplantation. Am J Transplant. 2002;2:796-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 91. | Okuda K, Kage M, Shrestha SM. Proposal of a new nomenclature for Budd-Chiari syndrome: hepatic vein thrombosis versus thrombosis of the inferior vena cava at its hepatic portion. Hepatology. 1998;28:1191-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 115] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Bogin V, Marcos A, Shaw-Stiffel T. Budd-Chiari syndrome: in evolution. Eur J Gastroenterol Hepatol. 2005;17:33-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | Valla DC. Hepatic vein thrombosis (Budd-Chiari syndrome). Semin Liver Dis. 2002;22:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Valla D, Casadevall N, Lacombe C, Varet B, Goldwasser E, Franco D, Maillard JN, Pariente EA, Leporrier M, Rueff B. Primary myeloproliferative disorder and hepatic vein thrombosis. A prospective study of erythroid colony formation in vitro in 20 patients with Budd-Chiari syndrome. Ann Intern Med. 1985;103:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 193] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | Denninger MH, Chaït Y, Casadevall N, Hillaire S, Guillin MC, Bezeaud A, Erlinger S, Briere J, Valla D. Cause of portal or hepatic venous thrombosis in adults: the role of multiple concurrent factors. Hepatology. 2000;31:587-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 451] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 96. | Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 592] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 97. | Socié G, Mary JY, de Gramont A, Rio B, Leporrier M, Rose C, Heudier P, Rochant H, Cahn JY, Gluckman E. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet. 1996;348:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 329] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 98. | Deltenre P, Denninger MH, Hillaire S, Guillin MC, Casadevall N, Brière J, Erlinger S, Valla DC. Factor V Leiden related Budd-Chiari syndrome. Gut. 2001;48:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 99. | Mahmoud AE, Elias E, Beauchamp N, Wilde JT. Prevalence of the factor V Leiden mutation in hepatic and portal vein thrombosis. Gut. 1997;40:798-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 87] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 100. | Valla DC. The diagnosis and management of the Budd-Chiari syndrome: consensus and controversies. Hepatology. 2003;38:793-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 101. | Okuda K. Inferior vena cava thrombosis at its hepatic portion (obliterative hepatocavopathy). Semin Liver Dis. 2002;22:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 61] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 102. | Simson IW. Membranous obstruction of the inferior vena cava and hepatocellular carcinoma in South Africa. Gastroenterology. 1982;82:171-178. [PubMed] |
| 103. | Bayraktar Y, Egesel T, Sağlam F, Balkanci F, Van Thiel DH. Does hepatic vein outflow obstruction contribute to the pathogenesis of hepatocellular carcinoma. J Clin Gastroenterol. 1998;27:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 104. | Harmanci O, Buyukasik Y, Kirazli S, Balkanci F, Bayraktar Y. Does endothelium agree with the concept of idiopathic hepatic vessel thrombosis. World J Gastroenterol. 2006;12:1273-1277. [PubMed] |
| 105. | Henrion J. Ischemia/reperfusion injury of the liver: pathophysiologic hypotheses and potential relevance to human hypoxic hepatitis. Acta Gastroenterol Belg. 2000;63:336-347. [PubMed] |
| 106. | Olzinski AT, Sanyal AJ. Treating Budd-Chiari Syndrome: making rational choices from a myriad of options. J Clin Gastroenterol. 2000;30:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 107. | Tavill AS, Wood EJ, Kreel L, Jones EA, Gregory M, Sherlock S. The Budd-Chiari syndrome: correlation between hepatic scintigraphy and the clinical, radiological, and pathological findings in nineteen cases of hepatic venous outflow obstruction. Gastroenterology. 1975;68:509-518. [PubMed] |
| 108. | Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11:787-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 406] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 109. | Cazals-Hatem D, Vilgrain V, Genin P, Denninger MH, Durand F, Belghiti J, Valla D, Degott C. Arterial and portal circulation and parenchymal changes in Budd-Chiari syndrome: a study in 17 explanted livers. Hepatology. 2003;37:510-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 132] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Parker RG. Occlusion of the hepatic veins in man. Medicine (Baltimore). 1959;38:369-402. [PubMed] |
| 111. | Mahmoud AE, Mendoza A, Meshikhes AN, Olliff S, West R, Neuberger J, Buckels J, Wilde J, Elias E. Clinical spectrum, investigations and treatment of Budd-Chiari syndrome. QJM. 1996;89:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 112. | Darwish Murad S, Valla DC, de Groen PC, Zeitoun G, Hopmans JA, Haagsma EB, van Hoek B, Hansen BE, Rosendaal FR, Janssen HL. Determinants of survival and the effect of portosystemic shunting in patients with Budd-Chiari syndrome. Hepatology. 2004;39:500-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 224] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 113. | Hadengue A, Poliquin M, Vilgrain V, Belghiti J, Degott C, Erlinger S, Benhamou JP. The changing scene of hepatic vein thrombosis: recognition of asymptomatic cases. Gastroenterology. 1994;106:1042-1047. [PubMed] |
| 114. | Dilawari JB, Bambery P, Chawla Y, Kaur U, Bhusnurmath SR, Malhotra HS, Sood GK, Mitra SK, Khanna SK, Walia BS. Hepatic outflow obstruction (Budd-Chiari syndrome). Experience with 177 patients and a review of the literature. Medicine (Baltimore). 1994;73:21-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 217] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 115. | De BK, Sen S, Biswas PK, Mandal SK, Das D, Das U, Guru S, Bandyopadhyay K. Occurrence of hepatopulmonary syndrome in Budd-Chiari syndrome and the role of venous decompression. Gastroenterology. 2002;122:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Singh V, Sinha SK, Nain CK, Bambery P, Kaur U, Verma S, Chawla YK, Singh K. Budd-Chiari syndrome: our experience of 71 patients. J Gastroenterol Hepatol. 2000;15:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 54] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Kage M, Arakawa M, Kojiro M, Okuda K. Histopathology of membranous obstruction of the inferior vena cava in the Budd-Chiari syndrome. Gastroenterology. 1992;102:2081-2090. [PubMed] |
| 118. | Shrestha SM, Okuda K, Uchida T, Maharjan KG, Shrestha S, Joshi BL, Larsson S, Vaidya Y. Endemicity and clinical picture of liver disease due to obstruction of the hepatic portion of the inferior vena cava in Nepal. J Gastroenterol Hepatol. 1996;11:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 77] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 119. | Bayraktar Y, Harmanci O. Etiology and consequences of thrombosis in abdominal vessels. World J Gastroenterol. 2006;12:1165-1174. [PubMed] |
| 120. | Brinson RR, Curtis WD, Schuman BM, Mills LR. Recovery from hepatic vein thrombosis (Budd-Chiari syndrome) complicating ulcerative colitis. Dig Dis Sci. 1988;33:1615-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 121. | Zeitoun G, Escolano S, Hadengue A, Azar N, El Younsi M, Mallet A, Boudet MJ, Hay JM, Erlinger S, Benhamou JP. Outcome of Budd-Chiari syndrome: a multivariate analysis of factors related to survival including surgical portosystemic shunting. Hepatology. 1999;30:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 160] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 122. | Tang TJ, Batts KP, de Groen PC, van Hoek B, Haagsma EB, Hop WC, Janssen HL. The prognostic value of histology in the assessment of patients with Budd-Chiari syndrome. J Hepatol. 2001;35:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 123. | Henderson JM, Warren WD, Millikan WJ, Galloway JR, Kawasaki S, Stahl RL, Hertzler G. Surgical options, hematologic evaluation, and pathologic changes in Budd-Chiari syndrome. Am J Surg. 1990;159:41-48; discussion 48-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 67] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 124. | Okuda H, Yamagata H, Obata H, Iwata H, Sasaki R, Imai F, Okudaira M, Ohbu M, Okuda K. Epidemiological and clinical features of Budd-Chiari syndrome in Japan. J Hepatol. 1995;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 144] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 125. | Darwish Murad S, Valla DC, de Groen PC, Zeitoun G, Haagsma EB, Kuipers EJ, Janssen HL. Pathogenesis and treatment of Budd-Chiari syndrome combined with portal vein thrombosis. Am J Gastroenterol. 2006;101:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 126. | Wadhawan M, Kumar N. Budd-Chiari syndrome. Trop Gastroenterol. 2003;24:3-7. [PubMed] |
| 127. | Bolondi L, Gaiani S, Li Bassi S, Zironi G, Bonino F, Brunetto M, Barbara L. Diagnosis of Budd-Chiari syndrome by pulsed Doppler ultrasound. Gastroenterology. 1991;100:1324-1331. [PubMed] |
| 128. | Gupta S, Barter S, Phillips GW, Gibson RN, Hodgson HJ. Comparison of ultrasonography, computed tomography and 99mTc liver scan in diagnosis of Budd-Chiari syndrome. Gut. 1987;28:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 129. | Millener P, Grant EG, Rose S, Duerinckx A, Schiller VL, Tessler FN, Perrella RR, Ragavendra N. Color Doppler imaging findings in patients with Budd-Chiari syndrome: correlation with venographic findings. AJR Am J Roentgenol. 1993;161:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 130. | Ralls PW, Johnson MB, Radin DR, Boswell WD, Lee KP, Halls JM. Budd-Chiari syndrome: detection with color Doppler sonography. AJR Am J Roentgenol. 1992;159:113-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 131. | Lim JH, Park JH, Auh YH. Membranous obstruction of the inferior vena cava: comparison of findings at sonography, CT, and venography. AJR Am J Roentgenol. 1992;159:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 132. | Mathieu D, Vasile N, Menu Y, Van Beers B, Lorphelin JM, Pringot J. Budd-Chiari syndrome: dynamic CT. Radiology. 1987;165:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 133. | Stark DD, Hahn PF, Trey C, Clouse ME, Ferrucci JT. MRI of the Budd-Chiari syndrome. AJR Am J Roentgenol. 1986;146:1141-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 45] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 134. | Soyer P, Rabenandrasana A, Barge J, Laissy JP, Zeitoun G, Hay JM, Levesque M. MRI of Budd-Chiari syndrome. Abdom Imaging. 1994;19:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 135. | Mitchell MC, Boitnott JK, Kaufman S, Cameron JL, Maddrey WC. Budd-Chiari syndrome: etiology, diagnosis and management. Medicine (Baltimore). 1982;61:199-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 206] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 136. | Wang ZG, Jones RS. Budd-Chiari syndrome. Curr Probl Surg. 1996;33:83-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Millikan WJ, Henderson JM, Sewell CW, Guyton RA, Potts JR, Cranford CA, Cramer AR, Galambos JT, Warren WD. Approach to the spectrum of Budd-Chiari syndrome: which patients require portal decompression. Am J Surg. 1985;149:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 138. | Brearley S, Hawker PC, Dykes PW, Keighley MR. A lethal complication of peripheral vein vasopressin infusion. Hepatogastroenterology. 1985;32:224-225. [PubMed] |
| 139. | Menon KV, Shah V, Kamath PS. The Budd-Chiari syndrome. N Engl J Med. 2004;350:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 326] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 140. | Frank JW, Kamath PS, Stanson AW. Budd-Chiari syndrome: early intervention with angioplasty and thrombolytic therapy. Mayo Clin Proc. 1994;69:877-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 141. | Raju GS, Felver M, Olin JW, Satti SD. Thrombolysis for acute Budd-Chiari syndrome: case report and literature review. Am J Gastroenterol. 1996;91:1262-1263. [PubMed] |
| 142. | Sparano J, Chang J, Trasi S, Bonanno C. Treatment of the Budd-Chiari syndrome with percutaneous transluminal angioplasty. Case report and review of the literature. Am J Med. 1987;82:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 143. | Xu K, He FX, Zhang HG, Zhang XT, Han MJ, Wang CR, Kaneko M, Takahashi M, Okawada T. Budd-Chiari syndrome caused by obstruction of the hepatic inferior vena cava: immediate and 2-year treatment results of transluminal angioplasty and metallic stent placement. Cardiovasc Intervent Radiol. 1996;19:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 144. | Zhang CQ, Fu LN, Xu L, Zhang GQ, Jia T, Liu JY, Qin CY, Zhu JR. Long-term effect of stent placement in 115 patients with Budd-Chiari syndrome. World J Gastroenterol. 2003;9:2587-2591. [PubMed] |
| 145. | Pisani-Ceretti A, Intra M, Prestipino F, Ballarini C, Cordovana A, Santambrogio R, Spina GP. Surgical and radiologic treatment of primary Budd-Chiari syndrome. World J Surg. 1998;22:48-53; discussion 53-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 146. | Witte AM, Kool LJ, Veenendaal R, Lamers CB, van Hoek B. Hepatic vein stenting for Budd-Chiari syndrome. Am J Gastroenterol. 1997;92:498-501. [PubMed] |
| 147. | Janssen HL, Garcia-Pagan JC, Elias E, Mentha G, Hadengue A, Valla DC. Budd-Chiari syndrome: a review by an expert panel. J Hepatol. 2003;38:364-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 330] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 148. | Ryu RK, Durham JD, Krysl J, Shrestha R, Shrestha R, Everson GT, Stephens J, Kam I, Wachs M, Kumpe DA. Role of TIPS as a bridge to hepatic transplantation in Budd-Chiari syndrome. J Vasc Interv Radiol. 1999;10:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Ganger DR, Klapman JB, McDonald V, Matalon TA, Kaur S, Rosenblate H, Kane R, Saker M, Jensen DM. Transjugular intrahepatic portosystemic shunt (TIPS) for Budd-Chiari syndrome or portal vein thrombosis: review of indications and problems. Am J Gastroenterol. 1999;94:603-608. [PubMed] |
| 150. | Senzolo M, Cholongitas EC, Patch D, Burroughs AK. Update on the classification, assessment of prognosis and therapy of Budd-Chiari syndrome. Nat Clin Pract Gastroenterol Hepatol. 2005;2:182-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 151. | Mancuso A, Fung K, Mela M, Tibballs J, Watkinson A, Burroughs AK, Patch D. TIPS for acute and chronic Budd-Chiari syndrome: a single-centre experience. J Hepatol. 2003;38:751-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 152. | Mancuso A, Watkinson A, Tibballs J, Patch D, Burroughs AK. Budd-Chiari syndrome with portal, splenic, and superior mesenteric vein thrombosis treated with TIPS: who dares wins. Gut. 2003;52:438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 153. | Perelló A, García-Pagán JC, Gilabert R, Suárez Y, Moitinho E, Cervantes F, Reverter JC, Escorsell A, Bosch J, Rodés J. TIPS is a useful long-term derivative therapy for patients with Budd-Chiari syndrome uncontrolled by medical therapy. Hepatology. 2002;35:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 154. | Hernández-Guerra M, Turnes J, Rubinstein P, Olliff S, Elias E, Bosch J, García-Pagán JC. PTFE-covered stents improve TIPS patency in Budd-Chiari syndrome. Hepatology. 2004;40:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 155. | Slakey DP, Klein AS, Venbrux AC, Cameron JL. Budd-Chiari syndrome: current management options. Ann Surg. 2001;233:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 156. | Orloff MJ, Daily PO, Orloff SL, Girard B, Orloff MS. A 27-year experience with surgical treatment of Budd-Chiari syndrome. Ann Surg. 2000;232:340-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 157. | Ringe B, Lang H, Oldhafer KJ, Gebel M, Flemming P, Georgii A, Borst HG, Pichlmayr R. Which is the best surgery for Budd-Chiari syndrome: venous decompression or liver transplantation A single-center experience with 50 patients. Hepatology. 1995;21:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 94] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 158. | Eid A, Rahamimov R, Ilan Y, Tur-Kaspa R, Berlatzky Y. Cavoatrial shunt: a graft salvage procedure for suprahepatic caval anastomosis obstruction after liver transplantation. Liver Transpl Surg. 1998;4:239-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 159. | Klein AS, Cameron JL. Diagnosis and management of the Budd-Chiari syndrome. Am J Surg. 1990;160:128-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 160. | Chang CH, Lee MC, Shieh MJ, Chang JP, Lin PJ. Transatrial membranotomy for Budd-Chiari syndrome. Ann Thorac Surg. 1989;48:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 161. | Senning A. Transcaval posterocranial resection of the liver as treatment of the Budd-Chiari syndrome. World J Surg. 1983;7:632-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 162. | Jamieson NV, Williams R, Calne RY. Liver transplantation for Budd-Chiari syndrome, 1976-1990. Ann Chir. 1991;45:362-365. [PubMed] |
| 163. | Srinivasan P, Rela M, Prachalias A, Muiesan P, Portmann B, Mufti GJ, Pagliuca A, O'Grady J, Heaton N. Liver transplantation for Budd-Chiari syndrome. Transplantation. 2002;73:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 164. | Campbell DA, Rolles K, Jamieson N, O'Grady J, Wight D, Williams R, Calne R. Hepatic transplantation with perioperative and long term anticoagulation as treatment for Budd-Chiari syndrome. Surg Gynecol Obstet. 1988;166:511-518. [PubMed] |
| 165. | Moussavian SN, Dincsoy HP, Goodman S, Helm RA, Bozian RC. Severe hyperbilirubinemia and coma in chronic congestive heart failure. Dig Dis Sci. 1982;27:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 166. | Logan RG, Mowry FM, Judge RD. Cardiac failure simulating viral hepatitis. Three cases with serum transaminase levels above 1,000. Ann Intern Med. 1962;56:784-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 167. | Cohen JA, Kaplan MM. Left-sided heart failure presenting as hepatitis. Gastroenterology. 1978;74:583-587. [PubMed] |
| 168. | Nouel O, Henrion J, Bernuau J, Degott C, Rueff B, Benhamou JP. Fulminant hepatic failure due to transient circulatory failure in patients with chronic heart disease. Dig Dis Sci. 1980;25:49-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 39] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 169. | Kisloff B, Schaffer G. Fulminant hepatic failure secondary to congestive heart failure. Am J Dig Dis. 1976;21:895-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 33] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 170. | Dunn GD, Hayes P, Breen KJ, Schenker S. The liver in congestive heart failure: a review. Am J Med Sci. 1973;265:174-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 147] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 171. | Richman SM, Delamn AJ, Grob D. Alterations in indices of liver function in congestive heart failure with particular reference to serum enzymes. Am J Med. 1961;30:211-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 87] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 172. | Jafri SM. Hypercoagulability in heart failure. Semin Thromb Hemost. 1997;23:543-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 173. | Bessman AN, Evans JM. The blood ammonia in congestive heart failure. Am Heart J. 1955;50:715-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 174. | Lowe MD, Harcombe AA, Grace AA, Petch MC. Lesson of the week: restrictive-constrictive heart failure masquerading as liver disease. BMJ. 1999;318:585-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 175. | Denis C, De Kerguennec C, Bernuau J, Beauvais F, Cohen Solal A. Acute hypoxic hepatitis ('liver shock'): still a frequently overlooked cardiological diagnosis. Eur J Heart Fail. 2004;6:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 176. | Runyon BA. Cardiac ascites: a characterization. J Clin Gastroenterol. 1988;10:410-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 177. | Naschitz JE, Slobodin G, Lewis RJ, Zuckerman E, Yeshurun D. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J. 2000;140:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 178. | Ho SS, Brown R, Fitzgibbon B. Hepatocellular carcinoma with cardiac cirrhosis. Med J Aust. 1990;152:553-554. [PubMed] |
| 179. | Cameron J, Oesterle SN, Baldwin JC, Hancock EW. The etiologic spectrum of constrictive pericarditis. Am Heart J. 1987;113:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 146] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 180. | Ling LH, Oh JK, Schaff HV, Danielson GK, Mahoney DW, Seward JB, Tajik AJ. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 347] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 181. | Bertog SC, Thambidorai SK, Parakh K, Schoenhagen P, Ozduran V, Houghtaling PL, Lytle BW, Blackstone EH, Lauer MS, Klein AL. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol. 2004;43:1445-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 182. | Marshall JB, Pola L. Chylous ascites secondary to constrictive pericarditis. A discussion of pathophysiologic mechanisms. Dig Dis Sci. 1982;27:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 183. | Riza Altiparmak M, Avsar S, Yanik S. Chylous ascites and chylothorax due to constrictive pericarditis in a patient undergoing haemodialysis. Neth J Med. 2004;62:59-61. [PubMed] |
| 184. | Solano FX, Young E, Talamo TS, Dekker A. Constrictive pericarditis mimicking Budd-Chiari syndrome. Am J Med. 1986;80:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 185. | Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003;108:1146-1162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 526] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 186. | Talreja DR, Edwards WD, Danielson GK, Schaff HV, Tajik AJ, Tazelaar HD, Breen JF, Oh JK. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 187. | Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 282] [Article Influence: 13.4] [Reference Citation Analysis (0)] |