Published online Mar 14, 2007. doi: 10.3748/wjg.v13.i10.1618
Revised: January 25, 2007
Accepted: February 25, 2007
Published online: March 14, 2007
It is controversial whether steroid therapy should be continued to prevent the recurrence of autoimmune hepatitis (AIH) in patients who have undergone liver transplantation (LTx) due to AIH. We report a case of recurrent autoimmune hepatitis after LTx despite a persistently normal range of alanine aminotransferase (ALT). A 50-year-old woman was admitted to our hospital because of jaundice and severe liver dysfunction, where she was diagnosed with liver failure due to AIH. Steroid therapy was not effective enough and the patient received living-donor LTx in 1999. Following the operation, the level of ALT was maintained within a normal range and anti-nuclear antibody (ANA) became negative, however, the serum level of IgG gradually elevated and ANA became positive, while platelets decreased. A liver biopsy performed 6 years after LTx showed histological findings of AIH and she was diagnosed with recurrent AIH. A recurrence of AIH may occur after LTx even if the level of ALT remains within a normal range. We consider that a protocol liver biopsy should be performed in patients who undergo LTx due to AIH to decide the indication for steroid therapy.
- Citation: Yao H, Michitaka K, Tokumoto Y, Murata Y, Mashiba T, Abe M, Hiasa Y, Horiike N, Onji M. Recurrence of autoimmune hepatitis after liver transplantation without elevation of alanine aminotransferase. World J Gastroenterol 2007; 13(10): 1618-1621
- URL: https://www.wjgnet.com/1007-9327/full/v13/i10/1618.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i10.1618
Autoimmune hepatitis (AIH) is characterized by the existence of autoantibodies, liver dysfunction and active hepatitis with infiltration of lymphocytes and plasma cells in the liver, and occurs mainly in females[1-3]. Steroid therapy is the first choice of treatment and most patients respond well, though some patients with AIH progress to chronic or acute liver failure[4]. Liver transplantation (LTx) is a therapeutic option for those patients, as the prognosis following LTx is relatively good (a 5-year survival rate of 80%-90%)[5]. However, some studies have reported that recurrent AIH ranges from 20% to 42% after LTx[6-10]. Such recurrence is usually accompanied with an elevation of alanine aminotransferase (ALT), though that does not occur in all cases[6]. Herein, we report a patient with AIH recurrence after living-donor LTx whose ALT level remained within a normal range.
A 50-year-old woman was presented with general fatigue and jaundice, and admitted to a local hospital on January 10, 1999. Because of a severely abnormal liver function test, the patient was then transferred to our hospital. Based on physical findings and laboratory data (total bilirubin 11.1 mg/dL, ALT 1145 IU/L, prothrombin time 35%, anti-nuclear antibody: anti-nuclear antibody (ANA) 1:160, IgG 3600 mg/dL), she was diagnosed with acute AIH. Steroid therapy including pulse therapy was initially employed, however, the patient did not respond well and her general state became worse. We assessed her condition as indicating LTx.
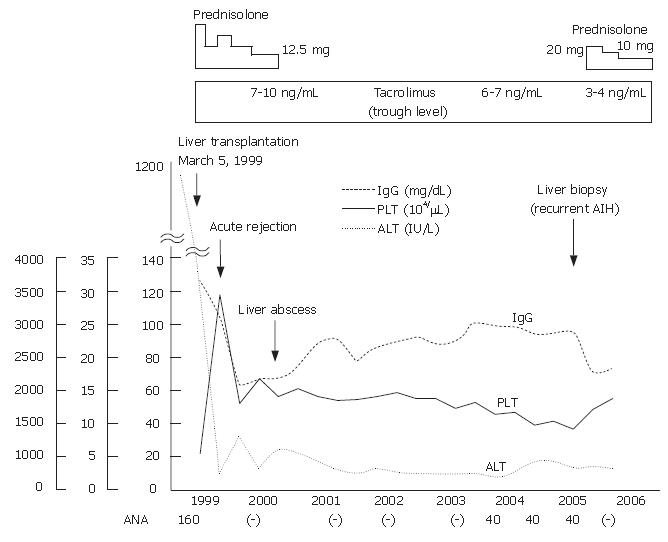
The living-donor LTx operation was performed on March 5, 1999 using the right lobe donated by the patient’s elder sister, who had no history of autoimmune diseases, with a normal range of liver function tests, and was negative for the anti-nuclear antibody. We previously reported the clinical course from onset to just after LTx of this case[11]. The patient experienced episodes of transient mild acute rejection and a liver abscess at 3 mo and 1 year, respectively, following LTx, which were resolved by immunosuppression against the rejection and administration of antibiotics and discontinuation of prednisolone (PSL) for the liver abscess. Tacrolimus was continued for immunosuppression, with the trough level kept at 6-10 ng/mL. Her clinical course after LTx is shown in Figure 1.
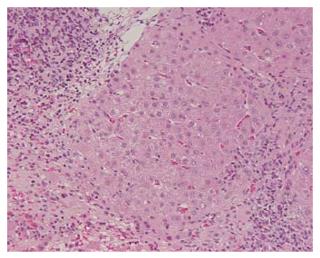
Steroid therapy was discontinued in 2000 and the serum levels of aspartate aminotransferase (AST), ALT, ALP, and γ-GTP remained within normal ranges. ANA remained negative following LTx, however, became positive in 2003. Although the patient had no subjective symptoms, she was admitted to our hospital again in March, 2005 for further examinations, because of elevated serum IgG and decreased platelet count. The laboratory data obtained upon admission were: AST 32 IU/L, ALT 14 IU/L, platelet 9.3 × 104/μL, PA-IgG 67.5 ng/107C, IgG 2720 mg/dL, ANA 1:40, and anti-smooth muscle antibody 1:40 with other autoantibodies negative (Table 1). Various viral markers, including hepatitis B surface antigen, anti-hepatitis B core antibody, and anti-hepatitis C virus antibody, were negative, while hepatitis B virus DNA, hepatitis C virus RNA, and hepatitis G virus RNA were undetectable by polymerase chain reaction assays. There was no history of alcohol abuse and no drug toxicity. Moderate splenomegaly was demonstrated by a computed tomography examination. Histopathological findings of the liver showed infiltrations of lymphocytes and plasma cells in the portal area, and a mild necroinflammatory change in the parenchyma (Figure 2). Bridging fibrosis was also observed. Acute or chronic rejection, such as biliary or vascular lesions, was not found. The patient was diagnosed histologically as chronic hepatitis at stage 3 and grade 1 (CH F3/G1) due to AIH. Based on the histological changes and clinical course, she was diagnosed with a recurrence of AIH.
| CBC | Renal function | ||
| WBC | 4400/μL | Na | 140 mEq/L |
| RBC | 3.72 × 106/μL | K | 3.9 mEq/L |
| Hb | 11.9 g/dL | Cl | 106 mEq/L |
| Ht | 36% | BUN | 21 mg/dL |
| Platelet | 9.3 × 104/μL | CRE | 0.7 mg/dL |
| ESR | 60 mm/h | UA | 4.6 mg/dL |
| Coagulation test | HbA1c | 5.00% | |
| Prothrombin time | 80.40% | Urinalysis | |
| Bleeding time | 5.0 min | Protein | (-) |
| Blood chemistry | Blood | (-) | |
| Total protein | 8.1 g/dL | Sugar | (-) |
| Albumin | 3.9 g/dL | Urobilinogen | (±) |
| Globulin | 4.2 g/dL | Serological test | |
| Total bilirubin | 1.1 mg/dL | HBs Ag | (-) |
| Direct bilirubin | 0.1 mg/dL | Anti-HBc | (-) |
| AST | 32 IU/L | Anti-HCV | (-) |
| ALT | 14 IU/L | ANA | × 40 |
| LDH | 182 IU/L | (Diffuse pattern) | |
| ALP | 210 IU/L | AMA | (-) |
| γ-GTP | 25 IU/L | ASMA | (-) |
| LAP | 54 IU/L | Anti-LKM-1 | (-) |
| CH.E | 135 IU/L | LE test | (-) |
| ZTT | 19 U | IgG | 2720 mg/dL |
| TTT | 6 U | IgA | 259 mg/dL |
| Total Cholesterol | 161 mg/dL | IgM | 94 mg/dL |
| ICG R15 | 27% | PA IgG | 67.5 ng/107C |
| K-ICG | 0.094 | Anti-platelet antibody | (-) |
| HLA-DR | DR4 DR5 | ||
| Tacrolimus | 5.2 ng/mL |
We prescribed prednisolone (PSL) at 20 mg/d plus tacrolimus, which maintained a trough level of 3-4 ng/mL. PSL daily dosage tapered gradually to 10 mg. Upon admission, the IgG level was 2720 mg/dL, while after treatment it was reduced to 2100 mg/dL and ANA became negative. ESR, ALT and platelets were 60 mm/h, 14 IU/L, and 9.3 × 104/μL, respectively before treatment and became 22 mm/h, 13 IU/L and 14.5 × 104/μL respectively thereafter.
Quality of life and graft survival rates are generally satisfactory in patients with end-stage liver failure following LTx, however, recurrence is possible in patients who develop various liver diseases, including AIH. The criteria used to diagnose a recurrence of AIH are based on increased serum transaminase levels, positivity of autoantibodies, marked hypergammaglobulinemia with elevation of serum IgG, portal lymphocytic and plasmacytic infiltration, interface hepatitis, and steroid dependency, while appropriate investigations are also necessary to exclude other possible causes of liver disease, such as rejection, viral infection, drug-induced liver disease, or biliary tract problems[1,6,12,13]. The present patient fulfilled those criteria, except for a normal ALT range, and was diagnosed with recurrent AIH based on the histological findings. A few reports have described some asymptomatic patients who underwent liver biopsies and showed abnormalities compatible with recurrent AIH without biochemical evidence of hepatitis[6,10]. It has also been reported that classic clinical, laboratory, immuno-serological and histological manifestations can be absent in patients with recurrent AIH[13,14].
It has been reported that the discontinuation of steroid administration may increase the risk of AIH recurrence[7]. However, long-term steroid therapy can cause multiple side-effects, and reintroduction of steroids was effective against recurrent AIH[7]. Successful outcomes in patients who underwent LTx due to AIH and withdrew from steroids have been reported by some centers[7,15]. Therefore, it remains controversial whether steroids should be continuously administrated following LTx or only reintroduced after recurrence.
In the present case, a gradual decrease in the number of platelets was observed. It is well known that platelet level is related to the degree of portal hypertension caused by advanced liver diseases. It was suspected that the decrease in platelet count in the present patient was caused by hypersplenism due to advanced fibrosis of the liver caused by the recurrence of AIH, because the liver biopsy findings showed advanced fibrosis (F3). It is important to monitor the platelets count even if the ALT level remains normal. In addition, increase in serum IgG and ESR were observed in our patient, which may be markers suggestive of AIH recurrence, though they are not specific.
Our findings confirmed that recurrent AIH should not be excluded even if the level of ALT remains normal after LTx in patients with AIH. We consider that a protocol liver biopsy should be performed in those cases.
The authors express their appreciation to Dr. Hiroto Egawa of the Department of Transplant Surgery, Kyoto University Graduate School of Medicine, for his advice regarding therapy.
S- Editor Liu Y L- Editor Ma JY E- Editor Che YB
| 1. | Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2003] [Cited by in RCA: 1986] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 2. | Onji M, Nonaka T, Horiike N, Moriwaki H, Muto Y, Ohta Y. Present status of autoimmune hepatitis in Japan. Gastroenterol Jpn. 1993;28 Suppl 4:134-138. [PubMed] |
| 3. | Toda G, Zeniya M, Watanabe F, Imawari M, Kiyosawa K, Nishioka M, Tsuji T, Omata M. Present status of autoimmune hepatitis in Japan--correlating the characteristics with international criteria in an area with a high rate of HCV infection. Japanese National Study Group of Autoimmune Hepatitis. J Hepatol. 1997;26:1207-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Abe M, Hiasa Y, Masumoto T, Kumagi T, Akbar SM, Ninomiya T, Matsui H, Michitaka K, Horiike N, Onji M. Clinical characteristics of autoimmune hepatitis with histological features of acute hepatitis. Hepatol Res. 2001;21:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006;354:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 604] [Article Influence: 31.8] [Reference Citation Analysis (1)] |
| 6. | Ratziu V, Samuel D, Sebagh M, Farges O, Saliba F, Ichai P, Farahmand H, Gigou M, Féray C, Reynès M. Long-term follow-up after liver transplantation for autoimmune hepatitis: evidence of recurrence of primary disease. J Hepatol. 1999;30:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Narumi S, Hakamada K, Sasaki M, Freise CE, Stock PG, Roberts JP, Ascher NL. Liver transplantation for autoimmune hepatitis: rejection and recurrence. Transplant Proc. 1999;31:1955-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Reich DJ, Fiel I, Guarrera JV, Emre S, Guy SR, Schwartz ME, Miller CM, Sheiner PA. Liver transplantation for autoimmune hepatitis. Hepatology. 2000;32:693-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 108] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Ayata G, Gordon FD, Lewis WD, Pomfret E, Pomposelli JJ, Jenkins RL, Khettry U. Liver transplantation for autoimmune hepatitis: a long-term pathologic study. Hepatology. 2000;32:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Prados E, Cuervas-Mons V, de la Mata M, Fraga E, Rimola A, Prieto M, Clemente G, Vicente E, Casanovas T, Fabrega E. Outcome of autoimmune hepatitis after liver transplantation. Transplantation. 1998;66:1645-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Kawai K, Michitaka K, Miyauchi S, Sano M, Abe M, Ninomiya T, Matsuura B, Masumoto T, Akbar SM, Horiike N. Acute-onset autoimmune hepatitis treated with living donor-liver transplantation. Intern Med. 2003;42:158-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Manns MP, Bahr MJ. Recurrent autoimmune hepatitis after liver transplantation-when non-self becomes self. Hepatology. 2000;32:868-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Duclos-Vallée JC, Sebagh M, Rifai K, Johanet C, Ballot E, Guettier C, Karam V, Hurtova M, Feray C, Reynes M. A 10 year follow up study of patients transplanted for autoimmune hepatitis: histological recurrence precedes clinical and biochemical recurrence. Gut. 2003;52:893-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | González-Koch A, Czaja AJ, Carpenter HA, Roberts SK, Charlton MR, Porayko MK, Rosen CB, Wiesner RH. Recurrent autoimmune hepatitis after orthotopic liver transplantation. Liver Transpl. 2001;7:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 114] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Heffron TG, Smallwood GA, Oakley B, Pillen T, Welch D, Martinez E, Romero R, Stieber AC. Autoimmune hepatitis following liver transplantation: relationship to recurrent disease and steroid weaning. Transplant Proc. 2002;34:3311-3312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |