Published online Mar 14, 2007. doi: 10.3748/wjg.v13.i10.1575
Revised: December 1, 2006
Accepted: December 15, 2007
Published online: March 14, 2007
AIM: To investigate the effect of a new oral preparation, highly concentrated in fish cartilage, in a group of inflammatory bowel diseases (IBD) patients with chronic iron deficient anemia.
METHODS: In an open label pilot study, we supple-mented a group of 25 patients (11 with Crohn’s disease and 14 with ulcerative colitis) in stable clinical conditions and chronic anemia with a food supplement which does not contain iron but contains a standardized fraction of fish cartilage glycosaminoglycans and a mixture of antioxidants (Captafer Medestea, Turin, Italy). Patients received 500 mg, twice a day during meals, for at least 4 mo. Patients were suggested to maintain their alimentary habit. At time 0 and after 2 and 4 mo, emocrome, sideremia and ferritin were examined. Paired data were analyzed with Student’s t test.
RESULTS: Three patients relapsed during the study (2 in the 3rd mo, 1 in the 4th mo), two patients were lost to follow up and two patients dropped out (1 for orticaria, 1 for gastric burning). Of the remaining 18 patients, levels of serum iron started to rapidly increase within the 2nd mo of treatment, P < 0.05), whereas serum ferritin and hemoglobin needed a longer period to significantly improve their serum levels (mo 4) P < 0.05. The product was safe, easy to administer and well tolerated by patients.
CONCLUSION: These data suggest a potential new treatment for IBD patients with iron deficiency chronic anemia and warrant further larger controlled studies.
- Citation: Belluzzi A, Roda G, Tonon F, Soleti A, Caponi A, Tuci A, Roda A, Roda E. A new iron free treatment with oral fish cartilage polysaccharide for iron deficiency chronic anemia in inflammatory bowel diseases: A pilot study. World J Gastroenterol 2007; 13(10): 1575-1578
- URL: https://www.wjgnet.com/1007-9327/full/v13/i10/1575.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i10.1575
Inflammatory bowel diseases (IBD) represent chronic recurrent diseases of the gut with a typical negative balance of iron that leads to anemia.
Iron deficiency anemia affects approximately 30% of patients with IBD. The symptoms correlated with iron deficiency are very common in IBD and microcytic and hypocromic anemia has a significant impact on the quality of life of IBD affected patients[1-3].
Anemia from iron deficiency is due to inadequate intake or the loss of iron, whereas in chronic inflammatory conditions, anemia is also the result of decreased erythropoiesis, secondary to increase of proinflammatory cytokines, reactive oxygen metabolites and nitric oxide.
Patients with IBD are treated commonly for their disease, which can ameliorate the iron deficiency and the anemia. Oral iron supplements may have a negative effect on the inflammatory activity of the disease. Studies in animal models suggest that iron may increase the activity of the disease through activation of the NFkb pathway of inflammation[4]; moreover, iron supplementation may cause alterations in the immune function affecting the inflammatory state by increasing the level of inflammation itself, modulating the function of macrophages and Th1 cells[5]. Finally in animal models of ulcerative colitis (UC) and Crohn disease (CD) it has been shown that the iron not absorbed can reach the ileum and the colon increasing, at the site of ulceration, the oxidative stress and enhancing pro-inflammatory cytokines[6,7]. An alternative approach to treat iron deficient anemia in IBD patients is to improve bioavailability of the iron normally introduced from the diet.
A pilot study of a food supplement (Captafer trade mark) containing selected fish cartilage and a mixture of antioxidants (Table 1) has been recently tested in healthy women with iron deficiency at reproductive age, free from estroprogestinic therapy. A significant elevation in serum iron and ferritin levels after 30 and 60 d of treatment was noted, in contrast with the inefficacy of the placebo[8].
| Twe tablets composition | % RDA | Per 100 g | |
| Energy | 1.3 Kcal 5.5 KJ | 83 Kcal 347 kJ | |
| Proteins (N × 6.25) | 0.19 g | 11.9 g | |
| Carboydrates | 0.01 g | 0.8 g | |
| Fat | 0.05 g | 3.5 g | |
| Vitamin C | 90 mg | 150 | 5.62 g |
| Vitamin E | 30 mg | 300 | 1.87 g |
| Folic acid | 150 mcg | 75 | 0.01 g |
| Zinc | 15 mg | 100 | 0.93 g |
| Cupper | 1.2 mg | 0.07 g | |
| Selected fish cartilage | 800 mg | 50 g |
Our work focused on the understanding of the role of this food supplement in iron deficiency and chronic anemia that characterized patients with IBD. With this aim in an open pilot study, we selected a group of patients with IBD in clinical remission with chronic anemia and low levels of serum iron and ferritin. The bioavailability of iron was ascertained by analyzing three variables: serum iron, hemoglobin and serum ferritin.
The study was an open-label, pilot clinical trial approved by the local ethical committee of the University of Bologna, S.Orsola Hospital. Twenty-five patients with IBD (11 Crohn’s disease, 14 ulcerative colitis) were selected from our registered labeled archive. Patient characteristics at the time of enrollment are listed in Table 2. Patients in clinical remission, according to CDAI for CD and Simple Clinical Colitis Activity Index for UC, taking mesalamine 2.4 g/d for preventing relapse and having mycrocytic and hypocromic anemia were considered eligible for this study. Disease localization in patients with CD was: 6 in the ileocolon, 3 in the ileum, and 2 in the colon. The treatment consisted of a supplementation of 500 mg of Captafer™ twice a day during meals for at least 4 mo. The composition of Captafer tablet is shown in Table 1.
| Characteristics | Crohn’s disease | Ulcerative colitis |
| Age (yr) | 20-60 ± 12 | 30-70 ± 15 |
| Sex (M/F) | 9/2 | 8/6 |
| Simple clinical colitis activity index | 3-6 ± 1 | |
| Score on Crohn’s disease activity index | 59-120 ± 20 |
Patients were suggested to maintain their alimentary habit throughout all the study period. An hemocrome, sideremia and ferritin evaluation plus a normal screening laboratory exam was performed at time 0 (before the beginning of the treatment), and after 2 and 4 mo, respectively. Patients who had a relapse of the disease during the period of the treatment, who had adverse reactions, or who did not make all the blood evaluations were excluded from the final statistical analysis.
The serum iron level (μg/mL), ferritin level (ng/mL) and hemoglobin value (g/dL) were analyzed and compared with Student’s t test for paired data. Three groups were considered: the baseline group (time 0), the mo 2 group and the mo 4 group. The three parameters (serum iron, serum ferritin and hemoglobin) were compared by pairing the baseline group with the mo 2 and mo 4 groups, respectively. Data were expressed for each group as geometric mean ± SD. A P value < 0.05 was considered to be statistically significant.
Eighteen of the 25 patients who began the treatment completed the study.
Reasons for not completing the study were: relapse of the disease (3/25), loss to follow up (2/25) and adverse reactions (2/25). Two patients relapsed during the third month and one in the fourth month of treatment. Of the patients who dropped out, one developed orticaria and one complained a gastric burning. The occurrence of these events was not linked to the treatment or was independent events. Ten of the patients who completed the study were affected by ulcerative colitis and eight had Crohn’s disease.
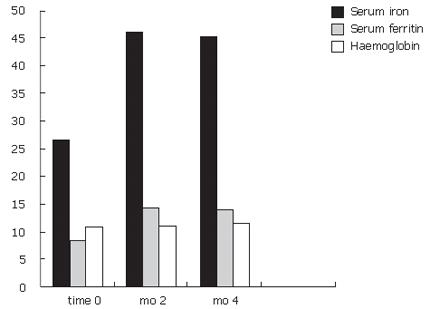
The results are shown in Table 3. With regard to serum iron levels, after two months of treatment, a significant increase was seen(P < 0.05). The increase was around 1.722 fold of baseline values (serum iron: baseline value 26.7 μg/dL; mo 2, 46 μg/dL). With regard to serum ferritin and hemoglobin, a trend towards increased value at mo 2 was noted (Figure 1).
Statistical analysis of the results obtained after 4 mo of treatment were consistent with the purpose of this study. There was a significant increase in serum iron, ferritin and hemoglobin (P < 0.05). The serum iron increased 1.66 fold, serum ferritin increased 1.65 fold and the hemoglobin increased 1.035 fold after 4 mo of treatment. Data analysis did not show any difference between UC and CD patients or between CD subgroups, according to disease localization. The product was safe, easy to administer and patients tolerated the treatment for all the period.
Dietary iron occurs as haeme and non-haeme iron (mostly from plants). The non-haeme iron is transformed to the ferric state in the presence of oxygen. After reduction to the ferrous state, iron is transferred to enterocytes by the apical transporter divalent metal transporter 1. If iron stores are replete, iron is trapped in ferritin and lost in the intestinal lumen when the cell desquamates.
In inflammatory bowel diseases, iron impairment may be due to several factors: poor absorption due to the activity of the disease localized in the small intestine or to the surgical resection, chronic loss of blood due to chronic intestinal bleeding and an inflammatory environment determined by the presence of proinflammatory and inflammatory cytokines[9-14]. In IBD the iron is lost because of the presence of chronic bleeding from the gut, which amount exceeds the iron that may be absorbed from the diet. Although iron absorption may be affected in Crohn’s disease due to the localization of the disease, several treatments have been introduced to restore iron depletion.
As it is reported by large studies the efficacy of oral iron is limited by poor absorption, intolerance and induction of oxidative stress at the site of bowel inflammation[4-6].
Another therapeutical option is the treatment with iron i.v.[15], particularly iron sucrose together with erythropoietin; this combination is effective but its clinical use is limited by high cost and difficulty of administration[16].
In this study we proposed an alternative and potential new treatment for iron deficient anemia in inflammatory bowel disease using a new food supplement with high content of fish cartilage that was well tolerated and without major adverse reactions. The only previous clinical experience was in healthy women with iron deficiency with a substantial enhancement of blood values[8].
In a recent paper it has been shown that the carbo-hydratic component of the fish muscle is involved in determining a better bioavailability of iron by increasing absorption of iron. Huh et al[17] used an in vitro model to confirm and analyze the fish meat factors that enhance the non-haeme iron bioavailability, showing a 5 fold increase in iron uptake. They evidenced that low-molecular-weight carbohydrates of muscle tissue obtained from fish are responsible for increasing non-haeme iron uptake in caco-2 cells. These carbohydrates may be represented by oligosaccharides originating from glycosaminoglycans in the extracellular matrix of muscle tissue. This finding well correlates with our results. Our preparation is composed of glycosaminoglycans in a greater percentage, which may be involved in the enhancement of non-haeme iron absorption.
In our study, a significant increase in serum iron was seen after 2 mo of therapy, and a significant increase in serum iron, serum ferritin and hemoglobin was noted at the end of the treatment. These preliminary data seem to offer a new and alternative approach to treat iron deficiency in patients with IBD, because of the efficacies tested, absence of major adverse effects, and easy administration. A placebo controlled trial is needed to confirm the potential beneficial effects of this preparation.
To Medestea, Via Ribes 5 Colleretto Giacosa (TO) Italy for supplying the product Captafer.
S- Editor Liu Y L- Editor Zhu LH E- Editor Che YB
| 1. | Bartels U, Pedersen NS, Jarnum S. Iron absorption and serum ferritin in chronic inflammatory bowel disease. Scand J Gastroenterol. 1978;13:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Cronin CC, Shanahan F. Anemia in patients with chronic inflammatory bowel disease. Am J Gastroenterol. 2001;96:2296-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Dejaco C, Gasche C. Anemia in chronic inflammatory intestinal disease: an often underestimated problem. Dtsch Med Wochenschr. 2002;127:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Kadiiska MB, Burkitt MJ, Xiang QH, Mason RP. Iron supplementation generates hydroxyl radical in vivo. An ESR spin-trapping investigation. J Clin Invest. 1995;96:1653-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 91] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Oldenburg B, van Berge Henegouwen GP, Rennick D, Van Asbeck BS, Koningsberger JC. Iron supplementation affects the production of pro-inflammatory cytokines in IL-10 deficient mice. Eur J Clin Invest. 2000;30:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Reifen R, Matas Z, Zeidel L, Berkovitch Z, Bujanover Y. Iron supplementation may aggravate inflammatory status of colitis in a rat model. Dig Dis Sci. 2000;45:394-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Weiss G, Bogdan C, Hentze MW. Pathways for the regulation of macrophage iron metabolism by the anti-inflammatory cytokines IL-4 and IL-13. J Immunol. 1997;158:420-425. [PubMed] |
| 8. | Rondanelli M, Opizzi A, Andreoni L, Trotti R. Effect of treatment with food supplementation in women with iron deficiency: double bind, randomised, placebo-controlled trial. 2007;in press. |
| 9. | Faquin WC, Schneider TJ, Goldberg MA. Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood. 1992;79:1987-1994. [PubMed] |
| 10. | Gasche C, Lomer MC, Cavill I, Weiss G. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 348] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Gasché C, Reinisch W, Lochs H, Parsaei B, Bakos S, Wyatt J, Fueger GF, Gangl A. Anemia in Crohn's disease. Importance of inadequate erythropoietin production and iron deficiency. Dig Dis Sci. 1994;39:1930-1934. [PubMed] |
| 12. | Lomer MC, Kodjabashia K, Hutchinson C, Greenfield SM, Thompson RP, Powell JJ. Intake of dietary iron is low in patients with Crohn's disease: a case-control study. Br J Nutr. 2004;91:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Oldenburg B, Koningsberger JC, Van Berge Henegouwen GP, Van Asbeck BS, Marx JJ. Iron and inflammatory bowel disease. Aliment Pharmacol Ther. 2001;15:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Sears DA. Anemia of chronic disease. Med Clin North Am. 1992;76:567-579. [PubMed] |
| 15. | KENT H. Physical medicine and rehabilitation as a hospital service. Am Pract Dig Treat. 1954;5:477-480. [PubMed] |
| 16. | Schröder O, Mickisch O, Seidler U, de Weerth A, Dignass AU, Herfarth H, Reinshagen M, Schreiber S, Junge U, Schrott M. Intravenous iron sucrose versus oral iron supplementation for the treatment of iron deficiency anemia in patients with inflammatory bowel disease--a randomized, controlled, open-label, multicenter study. Am J Gastroenterol. 2005;100:2503-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |