Published online Mar 7, 2006. doi: 10.3748/wjg.v12.i9.1439
Revised: May 1, 2005
Accepted: October 10, 2005
Published online: March 7, 2006
AIM: To study the effect of retrorsine on mouse he-patocyte proliferation.
METHODS: Mice and rats were treated respectively with two injections of retrorsine (as retrosine-treated group) or saline (as non-treated group) at 2 wk intervals. They received a single injection of carbon tetrachloride (CCl4) 4 wk later. On d 0, 1, 2, 3, 4, 6, 15 after CCl4 administration, the animals were killed and their livers were excised. Hematoxylin and eosin (HE) staining and Ki-67 antibody immunohistochemical analysis of liver samples were used to evaluate the pathological changes and hepatocyte proliferation.
RESULTS: In rats treated with retrorsine and CCl4, the liver displayed obvious megalocytosis, proliferation of mild bile duct, small hepatocyte-forming nodule, which were not found in liver samples from non-treated group. However, in mice treated with retrorsine combined with CCl4, the liver displayed hepatocyte degeneration and necrosis in perivenous areas. There was no obvious difference between retrorsine-treated group and non-treated group. Ki-67 immunohistochemical analysis showed that in rats treated with retrorsine, the positive hepatocytes mainly found in small hepatocyte nodules, were obviously less than those in non-treated group. The mice treated with retrorsine showed that the number of Ki-67 positive hepatocytes was very high and more than that in non-treated group.
CONCLUSION: Retrorsine has no effect on mouse hepatocyte proliferation.
- Citation: Zhou XF, Wang Q, Chu JX, Liu AL. Effects of retrorsine on mouse hepatocyte proliferation after liver injury. World J Gastroenterol 2006; 12(9): 1439-1442
- URL: https://www.wjgnet.com/1007-9327/full/v12/i9/1439.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i9.1439
Hepatocyte transplantation can not only treat liver degenerative disorders and other serious liver injuries, but also replace liver transplantation[1,2]. Before clinical application, it is necessary to use an animal model to test whether exogenous liver cells can integrate and grow in the recipient liver. Observations in humans and other vertebrates demonstrated that native liver cells have a very high regenerative potential and outgrow the exogenous cells[3]. Therefore, in animal models for hepatocyte transplantation, it is very important to inhibit the proliferation of native hepatocytes. It has been reported that retrorsine, a member of the pyrrolizidine alkaloid (PAs) family can impair the proliferative capacity of mature hepatocytes. Retrorsine-induced block-ade is in G1/S, late “S” and /or “G2/M” phase of cell cycle[4-6]. Laconi et al.[4] reported that syngeneic transplantation of hepatocytes in liver of dipeptidyl-peptidase type IV-deficient (DPPIV-) rats treated with retrorsine could achieve 95% chimerism and restore its normal function. Although retrorsine has been used in rat model, there are very few reports on its use to create a mouse model for hepatocyte transplantation[7]. In this study, we investigated the effect of retrorsine on mouse mature hepatocytes. The results indicate that retrosine cannot inhibit mouse hepatocyte proliferation after liver injury.
Male C57BL/6J mice (6 wk) and male F344 rats (5 wk) were purchased from National Rodent Laboratory Animal Resources, Shanghai Branch, China. They were maintained in a 12 h light/dark cycle and fed with standard food and water ad libitum. All animals received humane care and study protocols complied with guidelines of Shanghai Second Medical University.
Retrorsine (Sigma-Aldrich) was added to distilled water at 10 mg/mL and titrated to pH 2.5 with 1 mol/L HCl to dissolve it completely. The solution was neutralized using 1 mol/L NaOH. Subsequently NaCl was added. The final concentration was 5 mg/mL retrorsine and 0.15 mol/L NaCl, pH 7.0. The working solution was used immediately after preparation.
Carbon tetrachloride (CCl4) was diluted 1:10 using sterile mineral oil and maintained in a rubber plug-sealed glass tube. Ki-67 (Clone SP6) rabbit monoclonal antibody, a cell proliferation marker[8] was purchased from Lab Vision.
After one week of acclimatization, the mice and rats were randomly divided into two groups and received two intraperitoneal injections of retrorsine (70 mg/kg for mice[7] and 30 mg/kg for rats[4,6] (as retrosine-treated group)) or saline (as non-treated group) at 2-week interval. Four weeks after the second injection, diluted CCl4 was respectively injected into mice and rats, ip 5 mL/kg[7]. Day 0 was set just before CCl4 injection. On days 1, 2, 3, 4, 6, and 15 after CCl4 administration, 3-5 animals from each group were killed. The liver of animals was excised and fixed in 40 g/L formaldehyde for the following study.
Samples were dehydrated in alcohol and embedded in paraffin. Sections were cut at 5 µm thickness. For pathological analysis, the liver sections were stained with hematoxylin and eosin (HE) according to the standard procedures.
For Ki-67 immunohistochemical staining, antigen retrieval was carried out by incubating slides in antigen retrieval buffer (0.01 mol/L citrated buffer, pH 6.0) at 95 °C for 30 min. The slides were incubated with the primary antibody, Ki-67,at 4°C overnight. The secondary antibody used was anti-rabbit conjugated with horseradish peroxidase (Jackson Immunoresearch). 3,3-diaminobenzidine tetrahydro-chloride containing 0.1g/L hydrogen peroxide was used as a substrate. The proportion of Ki-67 positive hepatocytes was counted from at least 2000 cells from serial fields for each sample under microscope with 20× magnifications.
Data were expressed as mean±SE. Sigmaplot 2001 and SAS for windows 6.12 softwares were used for data analysis and plot. The significance of variances was found to be appropriate by Student’s t- test or χ2 test.
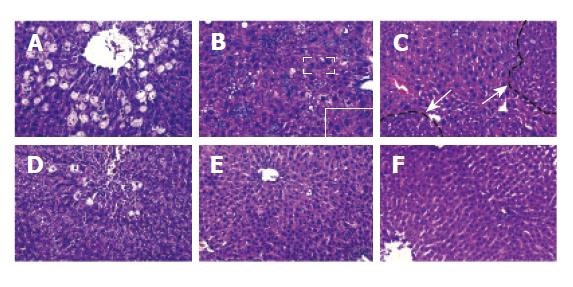
Before CCl4 administration, liver morphology had no obvious change both in mice and in rats treated with retrorsine. After injecting CCl4, much more severe hepatocyte balloon degeneration and necrosis in perivenous areas were found on day 1 in retrorsine-treated group than in non-treated group. Some megalocytosis, mild bile duct proliferation and small hepatocyte proliferation-formed nodules occurred in retrosine-treated group but not in non-treated group (Figure 1).
Livers of both retrorsine-treated and non-treated groups showed necrosis in perivenous areas on day 2 after CCl4 injection. After then hepatocytes in periportal areas began to proliferate, mitotic figures of liver cells could be found. Such a pathological phenomenon might be due to destruction of CCl4 in liver[9,10]. There were no megalocytosis and other pathological changes in rats. On the 15th day, hepatic parenchyma in both groups became normal (Figure 2).
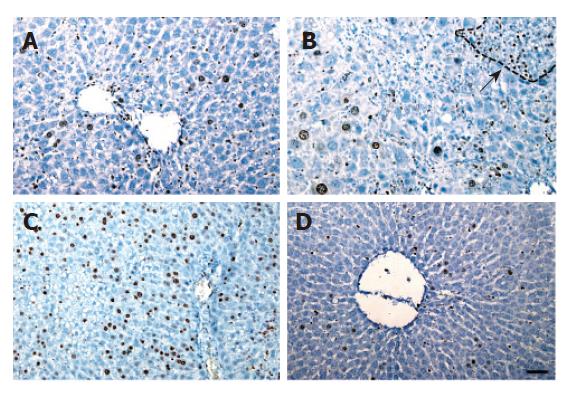
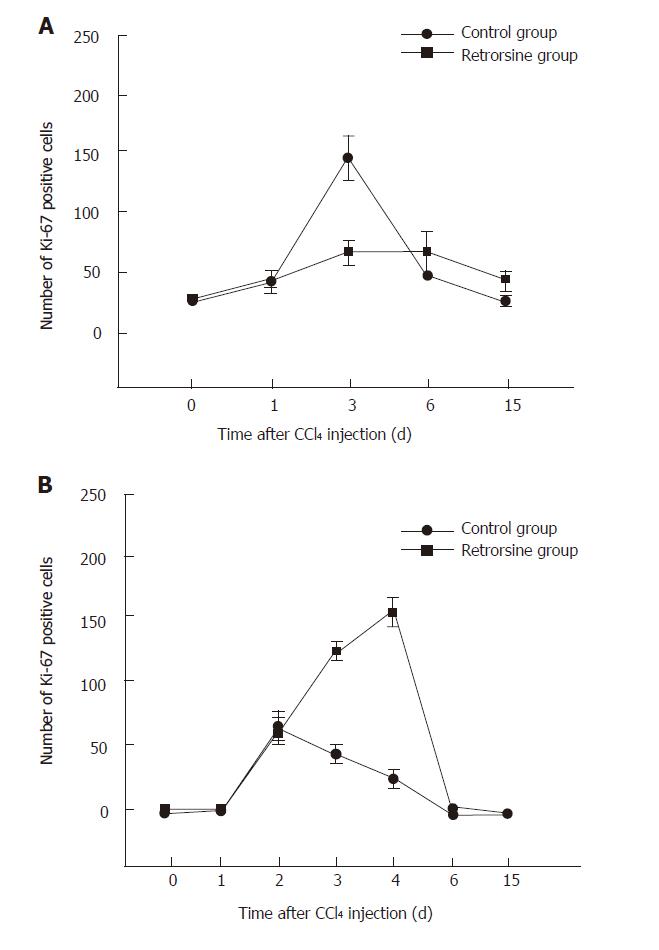
Before CCl4 injection, a small number of Ki-67 positive hepatocytes appeared in mice and rats treated with retrorsine. For the rats, the number of positive hepatocytes was increased slowly, reached the peak on day 6 in retrorsine-treated group and most of positive cells were small hepatocytes in nodules. In non-treated group, the maximum number of positive hepatocytes appeared on day 3 (Figures 3 and 5A).
The maximum number of positive hepatocytes was observed on day 4 in retrorsine-treated group of mice and almost all the positive cells were from mature hepatocytes. The maximum number of positive cells appeared on the 2nd day in non-treated group. Both groups showed a similar proliferation pattern (Figures 4 and 5B).
The present study described the comparative pathological changes and kinetic of hepatocyte proliferation in mice and rats treated with retrorsine and CCl4. Guo et al [7] reported that two doses of retrorsine (70 mg/kg at 2 wk interval) could be tolerated by > 90% of mice. If the dosage is over 70 mg/kg, the mortality rate of animals would increase. We used this dosage in our experiments and tested ethanol or water as a solvent for retrosine and treated mice with the same dosage (70 mg/kg). The survival rate was 85% (22/26) and 84% (27/32), respectively. No statistically significant difference was displayed between them (χ2 = 0.001, P = 0.980), suggesting that only water can be used as a solvent for retrosine.
The rats treated with retrorsine and CCl4 showed megalocytosis, mild bile duct proliferation and small hepatocyte proliferation-formed nodules. This phen-omenon has been described by many authors[4,6,12-15]. But in mice treated with the same protocol, we did not find similar pathological changes. Guo et al[7] studied liver repopulation after cell transplantation in mice treated with retrorsine and CCl4, and found that there are no liver pathological changes.
Ki-67 immunohistochemical analysis showed that proliferation of hepatocytes in rats treated with retrorsine was blocked. Avril et al[16] have reported similar results. Laconi et al[6] reported that BrdU labeling index of rats treated with retrorsine combined with partial hepatectomy is also significantly lower than that in non-treated group.
The number of Ki-67 positive hepatocytes in mice treated with the same protocol was higher than that in non-treated group. Guo et al[7] reported that the number of proliferating liver cells (detected by proliferating cell nuclear antigen, PCNA) in mice treated with CCl4 alone is > 60 %. Our result is consistent with theirs. Since they did not show hepatocyte proliferation after retrorsine treatment combined with CCl4, we considered that after liver injury mouse hepatocyte proliferation might not inhibited by retrorsine. Since Ki-67 positive hepatocytes in mice treated with retrorsine combined with CCl4 was higher than that in non-treated group in our study, it is possible that retrorsine might increase the sensitivity of mouse liver to CCl4 injury because the cell proliferation response is always dependent on the extent of liver injury.
Retrorsine has long been known for its ability to block hepatocyte division[4-6]. Megalocytosis results from replicating hepatocytes which are blocked after DNA synthesis and prior to mitotic division, thus resulting in a large number of cells with enlarged nuclei (megalocytosis)[4,6,17]. Our results demonstrated megalocytosis was only present in livers of rats but not in livers of mice treated with retrorsine, indicating that the effect of retrorsine on mice is different from that on rats. Significant species difference in susceptibility to PAs intoxication has been reported, which is mainly due to the variations in balance between the formation of toxic metabolites and detoxification pathways[18-20]. Although both mice and rats belong to murine, they might have a different process of metabolism or detoxification of PAs in their livers, which may lead to resistance of mice to retrorsine. Moreover, rats receiving CCl4 2 wk after the second injection of retrorsine displayed higher mortality rate than those receiving CCl4 4 wk after the second injection of retrorsine (data not shown). There was no obvious difference in the mortality rate and other physiological indices in mice receiving CCl4 2 wk or 4 wk after the second injection of retrorsine, which proved our hypothesis that retrorsine could seriously injure rat liver but not mouse liver.
As reported by Guo et al[7], when hepatocytes are transplanted into mice treated with retrorsine alone, the chimerism rate of exogenous liver cells is less than 1%. It was reported that the chimerism rate of rats treated with retrorsine alone could reach 95%[14]. It is possible that the effect of retrorsine on suppressing proliferation of mouse liver cells is limited in decreasing the chimerism rate. Therefore, retrorsine has no effect on mouse hepatocytes.
The authors thank Dr. Aditi Karandikar for reading the manuscript, Yong Lu and Nan Zheng for their assistance in handling rats and mice, and Fan Tang for her technical assistance.
Co-first authors: Xiao-Fei Zhou and Qian Wang
S- Editor Wang J L- Editor Wang XL E- Editor Wu M
| 1. | Fausto N. Liver regeneration. J Hepatol. 2000;32:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 898] [Cited by in RCA: 911] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 2. | Ouyang EC, Wu CH, Walton C, Promrat K, Wu GY. Transplantation of human hepatocytes into tolerized genetically immunocompetent rats. World J Gastroenterol. 2001;7:324-330. [PubMed] |
| 3. | Overturf K, al-Dhalimy M, Ou CN, Finegold M, Grompe M. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Pathol. 1997;151:1273-1280. [PubMed] |
| 4. | Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 296] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Samuel A, Jago MV. Localization in the cell cycle of the antimitotic action of the pyrrolizidine alkaloid, lasiocarpine and of its metabolite, dehydroheliotridine. Chem Biol Interact. 1975;10:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Picard C, Lambotte L, Starkel P, Sempoux C, Saliez A, Van Den Berge V, de Saeger C, Horsmans Y. Retrorsine: a kinetic study of its influence on rat liver regeneration in the portal branch ligation model. J Hepatol. 2003;39:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Guo D, Fu T, Nelson JA, Superina RA, Soriano HE. Liver repopulation after cell transplantation in mice treated with retrorsine and carbon tetrachloride. Transplantation. 2002;73:1818-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Birner P, Ritzi M, Musahl C, Knippers R, Gerdes J, Voigtländer T, Budka H, Hainfellner JA. Immunohistochemical detection of cell growth fraction in formalin-fixed and paraffin-embedded murine tissue. Am J Pathol. 2001;158:1991-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Gupta S, Rajvanshi P, Aragona E, Lee CD, Yerneni PR, Burk RD. Transplanted hepatocytes proliferate differently after CCl4 treatment and hepatocyte growth factor infusion. Am J Physiol. 1999;276:G629-G638. [PubMed] |
| 10. | Pilichos C, Perrea D, Demonakou M, Preza A, Donta I. Management of carbon tetrachloride-induced acute liver injury in rats by syngeneic hepatocyte transplantation in spleen and peritoneal cavity. World J Gastroenterol. 2004;10:2099-2102. [PubMed] |
| 11. | Wei HS, Li DG, Lu HM, Zhan YT, Wang ZR, Huang X, Zhang J, Cheng JL, Xu QF. Effects of AT1 receptor antagonist, losartan, on rat hepatic fibrosis induced by CCl(4). World J Gastroenterol. 2000;6:540-545. [PubMed] |
| 12. | Dahlke MH, Popp FC, Bahlmann FH, Aselmann H, Jäger MD, Neipp M, Piso P, Klempnauer J, Schlitt HJ. Liver regeneration in a retrorsine/CCl4-induced acute liver failure model: do bone marrow-derived cells contribute. J Hepatol. 2003;39:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Laconi S, Curreli F, Diana S, Pasciu D, De Filippo G, Sarma DS, Pani P, Laconi E. Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatol. 1999;31:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Gordon GJ, Coleman WB, Hixson DC, Grisham JW. Liver regeneration in rats with retrorsine-induced hepatocellular injury proceeds through a novel cellular response. Am J Pathol. 2000;156:607-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 130] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Gordon GJ, Coleman WB, Grisham JW. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am J Pathol. 2000;157:771-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 98] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Avril A, Pichard V, Bralet MP, Ferry N. Mature hepatocytes are the source of small hepatocyte-like progenitor cells in the retrorsine model of liver injury. J Hepatol. 2004;41:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E. Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol. 2001;158:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Fu PP, Xia QS, Lin G, Chou MW. Genotoxic pyrrolizidine alkaloids-methanisms leading to DNA adduct formation and tumorigenicity. Int J Mol Sci. 2002;3:948-964 doi: 10.3390/i3090948. |
| 19. | Huan JY, Miranda CL, Buhler DR, Cheeke PR. Species differences in the hepatic microsomal enzyme metabolism of the pyrrolizidine alkaloids. Toxicol Lett. 1998;99:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Chojkier M. Hepatic sinusoidal-obstruction syndrome: toxicity of pyrrolizidine alkaloids. J Hepatol. 2003;39:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |