Published online Feb 21, 2006. doi: 10.3748/wjg.v12.i7.1105
Revised: October 6, 2005
Accepted: October 26, 2005
Published online: February 21, 2006
AIM: To asses the expression of myeloid dendritic cells (CD11c+) subset during acute HCV hepatitis and its possible involvement in natural history of the infection.
METHODS: We enrolled 11 patients with acute hepatitis C (AHC) (Group A), 10 patients with acute hepatitis A (AHA) (as infective control-Group B) and 10 healthy donors (group C) in this study. All patients underwent selective flow cytometry gating strategies to assess the peripheral number of the myeloid dendritic cells (mDCs) to understand the possible role and differences during acute hepatitis.
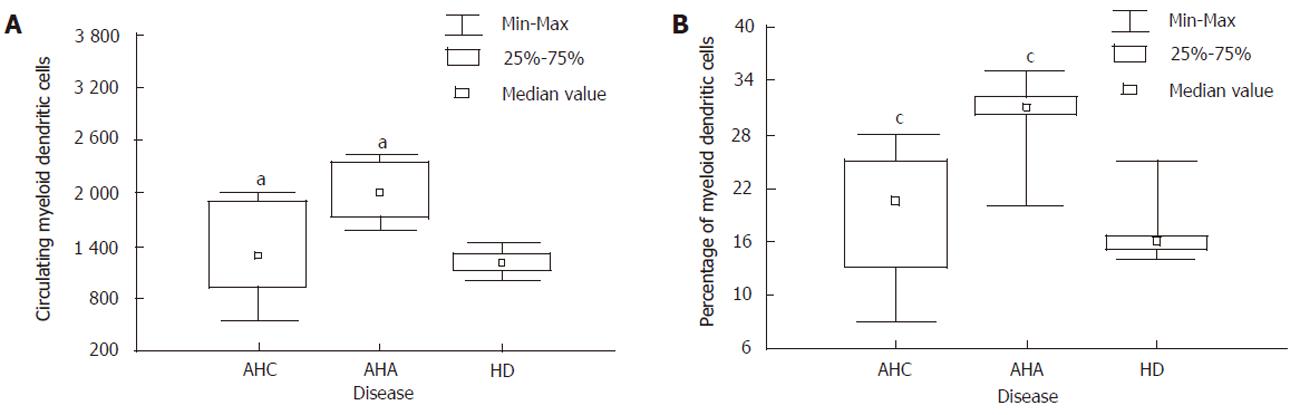
RESULTS: Eight of 11 patients with acute HCV hepatitis did not show any increase of mDCs compared to healthy individuals, while a significant decrease of mDCs was found in absolute cell count (z = -2.37; P < 0.05) and percentage (z= -2.30; P < 0.05) as compared with AHA. On the contrary, The remaining three patients of the group A had a higher mDCs number and percentage as occur in group B. Interestingly, after six months, those patients did not show any increase of mDCs subset were chronically infected. while the three subjects with an increase of peripheral mDCs, as in HAV acute infection, resolved the illness.
CONCLUSION: The lack of increase of mDCs during acute hepatitis C might be an important factor involved in chronicization of the infection.
- Citation: Perrella A, Atripaldi L, Bellopede P, Patarino T, Sbreglia C, Tarantino G, Sorrentino P, Conca P, Ruggiero L, Perrella O. Flow cytometry assay of myeloid dendritic cells (mDCs) in peripheral blood during acute hepatitis C: Possible pathogenetic mechanisms. World J Gastroenterol 2006; 12(7): 1105-1109
- URL: https://www.wjgnet.com/1007-9327/full/v12/i7/1105.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i7.1105
Hepatitis C virus (HCV) infection represents one of the most important causes of chronic viral hepatitis, liver disease, and hepatocellular carcinoma[1]. Many mechanisms have been proposed to account for the ability of HCV to elude the host defense and progress towards chronic disease: the high rate of the mutation as well as the ability of the virus to influence T helper cell subsets during the acute infection. Indeed, an early Th2 predominant immune response seems to influence the natural history of the infection[2,3]. Even if, several research studies have focused attention on the T lymphocytes (CD4+ and CD8+) activity in acute and chronic hepatitis C[4,5], however, the frequency of the cells involved in the presentation of the antigen (APCs) and so in the immune response activation is not fully elucidated. Among these subsets, the dendritic cells (DCs) have one of the most important roles in the host defense, but few data are available on these cells during the acute hepatitis C. That subset, divided in plasmacytoid DCs (pDCs) and myeloid DCs (mDCs), expresses high levels of MHC class I and II antigens, as well as costimulatory molecules, playing an essential role in triggering primary immune response[6]. Particularly, the mDCs are involved in Th1 network activation[7], that is fundamental to clear HCV infection[2,3]. The aim of the present study was to use flow cytometry as a tool to enumerate mature mDCs in peripheral blood of patients with acute HCV infection and consequently to asses the ability to present the antigen and activate the immune response against the virus as well as its possible influence on disease progression. Thus, we presented, for the first time to our knowledge, a study on the frequency of peripheral myeloid dendritic cells (mDCs) (CD11c+) in two hepatitides due to RNA hepatotropic virus characterized by a common natural immune system pathway[8-10]: one HCV which frequently leads to chronicity; and the other HAV which normally leads to a self-limited infection with a complete resolution, thus as control population, we investigated healthy individuals.
Following the approval of our Local Ethical Committee, the study was carried out on 31 subjects, who were divided into three groups. Group A: 11 patients with acute C hepatitis; Group B: 10 patients with acute A hepatitis; Group C: 10 normal individuals as controls. All patients had been admitted to our Infectious Disease Emergency Unit during the symptomatic phase, at least after one week from the first symptoms. Regarding to the group A, the criteria for diagnosis of acute hepatitis C were: (1) at admittance, HCV-RNA positive and anti-HCV negative or documented HCV-RNA negativity before admission otherwise seroconversion from anti-HCV negative to positive ELISA test; (2) negative clinical history of autoimmune disease, chronic viral hepatitis or other viral infections, including HIV; (3) serum alanine aminotransferase (ALT) levels at least 10 × upper limit of normal value (ULN < 40 IU/mL); and (4) absence of alcohol or drug abuse. Of note that our hospital is a reference center for infectious disease, moreover, most of our patients were ex-prisoner and so they underwent to periodical control.
All HAV-infected patients were negative for other chronic viral hepatitis (HBV and HCV), alcohol/drug abuse, other associated autoimmune disorders or infectious disease.
Since none of patients of group A underwent IFN treatment, they were evaluated at time 0 (T0) and after at least six months Time 6 (T6) following their laboratory parameters to asses a possible progression towards chronic infection (positive HCV-RNA qualitative assay and hypertransaminasis) or its resolution, as well as their mDCs frequencies.
Blood samples were collected from all patients on admittance to our unit, at the beginning of clinical symptoms (T0). All biochemical and viral assays were performed at our centralized laboratory. The HCV-RNA qualitative and quantitative assays were performed using the Amplicor Roche system with the cut-off values set to 50 and 600 UI/mL, respectively.
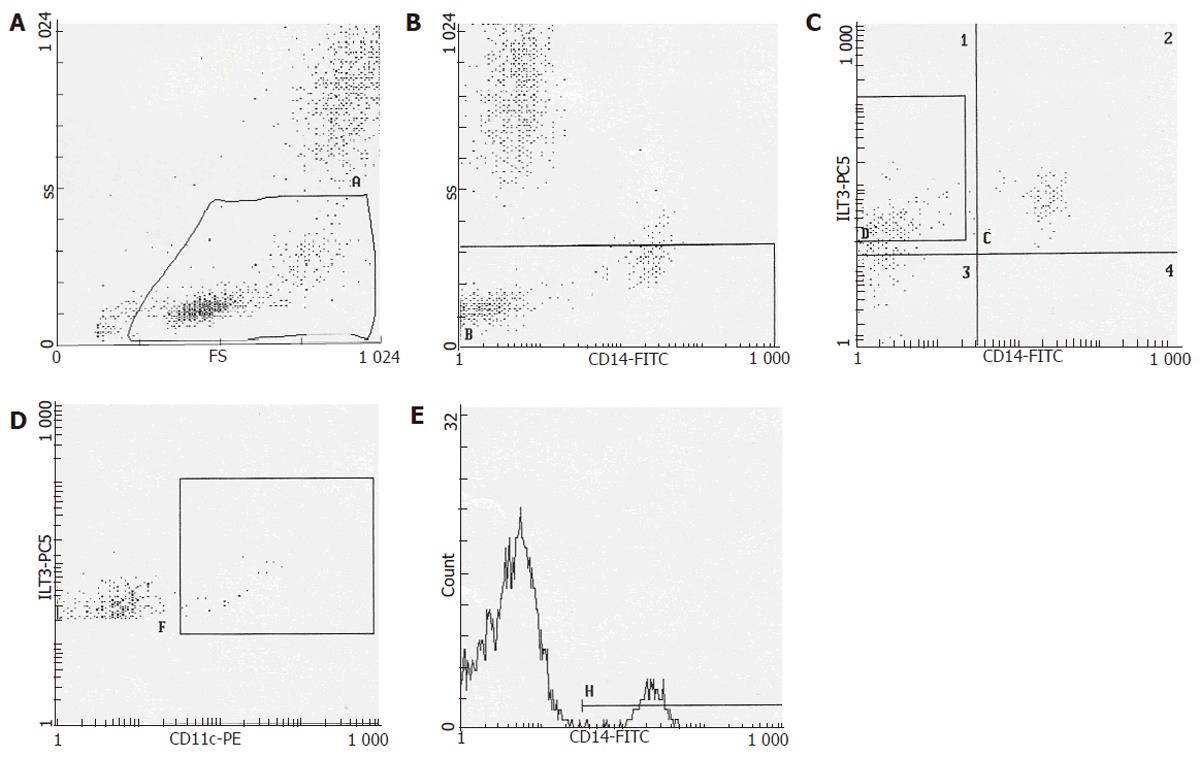
The mDCs assessment was executed at our immunological laboratory by a three-color flow cytometry using a Beckman Coulter Profile system. The mDCs assay involved the use of anti-ILT3, anti-CD14 and anti-CD11c monoclonal antibodies (mAb) labelled with the the fluorochromes -PC5,-FITC,-PE, applying a particular gate strategy, according to the criteria of Beckman Coulter. All monoclonal antibodies were applied following the manufacturer’s instructions and on the basis of our previous experiences. Briefly, after the cells were gated using a side scatter profile to exclude granulocyte and possible debris, the cells were further gated using CD14+ mAb marker to select a population of monocytes and pro-myeloid dendritic cells, such marker is not expressed on plasmocytoid cells and so its usage gives us the ability to early select the mDCs[11]. In addition, the cells were further gated using ILT3-mAb to obtain purified mDCs population[12]. Finally, a third gate was applied using CD11c to obtain the only mature mDCs (CD11c+)[13] (Figure 1). Coupled to this cytometry analysis, we also evaluated the total CD4+ T cells to asses the possible correlation between T lymphocytes and mDCs.
Considering the small number of patients, we used non-parametric test. Spearman test was applied particularly to asses possible correlation, and on the other hand, U Mann-Whitney test (two tailed) was performed to evaluate difference among the groups. The results were considered statistical significant at P < 0.05.
Table 1 shows the descriptive analysis of the standard laboratory, virological and immunological parameters. In group A, nine patients were genotype 1b, while the other two had genotype 2a/2c. The mean value of mDCs in the normal donors was 1350 cells, representing about the 15% of the ILT3 cells. Eight of 11 patients with acute hepatitis C showed to have a lower absolute cell count percentage of peripheral blood mDCs when compared to acute hepatitis A (Mann-Whitney U test; z = -2.37, P < 0.05 and z = -2.30, P <0.05) (Figure 2), while they did not display any statistical significant differences with respect to group C (healthy individuals), thereby suggesting the lack of enrollment in peripheral blood of this dendritic cell subset during the acute infection. The remaining 3 patients (3/11; two with genotype 2a/2c and one genotype 1) showed an increase of mDCs presenting an absolute cell count and percentage analogous to patients with acute hepatitis A (Table 1). After six months, patients who at T0 showed an absolute number and percentage of mDCs like healthy individuals had positive HCV-RNA qualitative assay, hypertransaminases (2 time upper limit normal value, 92 IU/mL) and also preserved the same frequency of mDCs (Table 1). Of note that 3/11 of patients who showed an increase of mDCs were found to be negative for hypertransaminasemia and HCV-RNA after 9 months when they came back our department. Finally, a strong positive correlation (Spearman’s test; r=0.90, P<0.05) between the mDCs and CD4+ was found in group B, while it was not demonstrated in group A. Noteworthy, no correlation was pointed out among HCV-RNA, transaminases and the mDCs both at T0 and T6, suggesting the lack of a direct involvement in liver necrosis of viral cycle.
| Parameters | A-T0 | A-T6 | A-res | B | C |
| Age (yr, mean±SD) | 42±2 | 42±3 | 46±5 | 35±3 | 32±5 |
| Gender | |||||
| Male (n = 9) | 8/11 | 8/11 | 1/11 | 9/10 | 6/10 |
| Female (n = 2) | 0/11 | 0/11 | 2/11 | 1/10 | 4/10 |
| ALT level (<40 U/L) | 1 325±360 | 92±12 | 1 620±220 | 1 328±325 | <40 |
| AST level (<40 U/L) | 817±435 | 84±9 | 942±160 | 996±73 | <40 |
| HCV-RNA (IU/mL, mean±SD) | 239±2 x10^3 | + | 198±3 x10^3 | - | - |
| HBsAg | - | - | - | - | - |
| Genotype | |||||
| 1 | 8/11 | 8/11 | 1/11 | n.a. | n.a. |
| 2a/2c | 0/11 | 0/11 | 2/11 | n.a. | n.a. |
| CD4+ | 993±333 | 850±240 | 850±240 | 558±167 | 922±345 |
| CD11c+% | 19±9% | 17±7% | 26±8% | 30±5% | 15±4 % |
| CD11c+ cells | 1 220±558 | 1 312±345 | 1 760±232 | 1 980±311 | 1 350±200 |
| Mode of infection | |||||
| Sexual transmission | 1/11 | 0 | |||
| Unclear | 8/11 | 0 | |||
| Surgical procedures | 2/11 | 0 | |||
| Alimentary | 0 | 10/10 | |||
mDCS have an important role during the infection[6,14], therefore, one of the most vital strategies of the virus could be to inhibit or interfere with their function. Indeed, some viruses can evolve to present strategies to diminish the circulating dendritic cells evading the immune surveillance. Previous studies reported a decrease of DCs during different viral infectious disease and most recently in chronic hepatitis C[14,15]. Particularly during chronic HCV infection, it has also been shown that the virus is able to slow down the DCs by direct infection[15]. Nevertheless, it is unknown if the virus has influence on the circulating DCs during acute infection too. Although the present investigation was carried out on a small group of patients, however, this simple gate strategy in flow cytometry is probably the first to show that patients who demonstrated to have the same absolute number and percentage of mDCs as healthy individuals evolved towards a chronic disease, while those having an increase of mDCs, such as in HAV, were not chronically infected. Even if the number of patients resolving the infection is too small to be suitable for any statistical test, it is clear considering the flow cytometry data that this subset has a possible connection to the pathogenesis of a persistent infection. In this regard, the results relatively to the increase of mDCs during a self-limited RNA viral hepatitis like HAV, seem to confirm the hypothesis of a role for those peripheral blood professional APCs subset in the resolution of the viral infection, probably enrolling CD4+ T cells as the correlation suggested. The mechanisms hiden behind these events, through which HCV could participate in decreasing mDCS in peripheral blood, are still unclear. Nevertheless, it has recently been shown the ability of HCV to interfere with TLR 3 activity during the immune system response[16], since it has an important role in mDCs life cycle and function[6,9,10,17]. Thus, the lack of expansion occurring in patients during acute HCV infection by different ways could represent an important additional skill of the virus to elude the first line of host defense represented by the innate immunity. Although our study did not asses the direct viral infection of DCs, it clearly documents that HCV is able to evolve a kind of strategy to boost its survival by preventing the increase of mDCs during the acute infection in patients evolving in chronic hepatitis. Of note that mDCs are able to activate a Th1 immune network, and that it is well known to be necessary to clear HCV[4,5,18], so their inactivation might have a great influence on host defense during acute infection.
In conclusion, we speculate that during acute hepatitis C, the virus might prevent the activation of circulating myeloid dendritic cells (ILT3/CD11c+) conditioning the natural history of the hepatitis. Thus, an early assessment by means of flow cytometry of mDCs using our gate strategy could give useful suggestions about the possible progression of infection, thereby proposing the early enrolment of the patients in IFN-treatment, particularly for the genotype 1. Supplementary studies, using flow cytometry assay coupled to functional analysis as well as assaying the possible variation of mDCs peripheral blood expression after interferon plus ribavirin treatment, should be carried out on a wider population. In fact, this subset could be a possible target for an immunotherapy to ameliorate the antigen presenting function and so the immune response
The authors thank Dr. Ragai Mitry for his precious help in the evaluation of our manuscript.
S- Editor Wang J L- Editor Kumar M E- Editor Bai SH
| 1. | Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 2. | Eckels DD, Zhou H, Bian TH, Wang H. Identification of antigenic escape variants in an immunodominant epitope of hepatitis C virus. Int Immunol. 1999;11:577-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Chang KM, Rehermann B, McHutchison JG, Pasquinelli C, Southwood S, Sette A, Chisari FV. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997;100:2376-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 241] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 4. | Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 925] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 5. | Tsai SL, Liaw YF, Chen MH, Huang CY, Kuo GC. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology. 1997;25:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 274] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1461] [Cited by in RCA: 1396] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 7. | Klagge IM, Abt M, Fries B, Schneider-Schaulies S. Impact of measles virus dendritic-cell infection on Th-cell polarization in vitro. J Gen Virol. 2004;85:3239-3247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2734] [Cited by in RCA: 2864] [Article Influence: 136.4] [Reference Citation Analysis (0)] |
| 9. | Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2469] [Cited by in RCA: 2553] [Article Influence: 121.6] [Reference Citation Analysis (0)] |
| 10. | Finberg RW, Kurt-Jones EA. Viruses and Toll-like receptors. Microbes Infect. 2004;6:1356-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Nguyen XD, Eichler H, Dugrillon A, Piechaczek C, Braun M, Klüter H. Flow cytometric analysis of T cell proliferation in a mixed lymphocyte reaction with dendritic cells. J Immunol Methods. 2003;275:57-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Cella M, Döhring C, Samaridis J, Dessing M, Brockhaus M, Lanzavecchia A, Colonna M. A novel inhibitory receptor (ILT3) expressed on monocytes, macrophages, and dendritic cells involved in antigen processing. J Exp Med. 1997;185:1743-1751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 348] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | O'Doherty U, Peng M, Gezelter S, Swiggard WJ, Betjes M, Bhardwaj N, Steinman RM. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487-493. [PubMed] |
| 14. | Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737-745. [PubMed] |
| 15. | Goutagny N, Fatmi A, De Ledinghen V, Penin F, Couzigou P, Inchauspé G, Bain C. Evidence of viral replication in circulating dendritic cells during hepatitis C virus infection. J Infect Dis. 2003;187:1951-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 119] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Li K, Foy E, Ferreon JC, Nakamura M, Ferreon AC, Ikeda M, Ray SC, Gale M, Lemon SM. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005;102:2992-2997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 817] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 17. | Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol. 2003;171:3154-3162. [PubMed] |
| 18. | Ward S, Lauer G, Isba R, Walker B, Klenerman P. Cellular immune responses against hepatitis C virus: the evidence base 2002. Clin Exp Immunol. 2002;128:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |