Published online Feb 21, 2006. doi: 10.3748/wjg.v12.i7.1071
Revised: July 2, 2005
Accepted: August 26, 2005
Published online: February 21, 2006
AIM: To examine the effect of Eubacterium limosum (E.limosum) on colonic epithelial cell line in vitro, and to evaluate the effect of E.limosum on experimental colitis.
METHODS: E.limosum was inoculated anaerobically and its metabolites were obtained. The growth stimulatory effect of the E.limosum metabolites on T84 cells was evaluated by SUDH activity, and the anti-inflammatory effect by IL-6 production. The change in mRNA of toll like receptor 4 (TLR4) was evaluated by real time PCR. Colitis was induced by feeding BALB/C mice with 2.0% dextran sodium sulfate. These mice received either 5% lyophilized E.limosum (n = 7) or control diet (n = 7). Seven days after colitis induction, clinical and histological scores, colon length, and cecal organic acid levels were determined.
RESULTS: The E.limosum produced butyrate, acetate, propionate, and lactate at 0.25, 1.0, 0.025 and 0.07 mmol/L, respectively in medium. At this concentration, each acid had no growth stimulating activity on T84 cells; however, when these acids were mixed together at the above levels, it showed significantly high activity than control. Except for lactate, these acids significantly attenuated IL-6 production at just 0.1 mmol/L. In addition, under TNF-α stimulation, butyrate attenuated the production of TLR4 mRNA. The treatment with E.limosum significantly attenuated clinical and histological scores of colitis with an increase of cecal butyrate levels, compared with the control group.
CONCLUSION: E.limosum can ameliorate experimental colonic inflammation. In part, the metabolite of E.limosum, butyrate, increases mucosal integrity and shows anti-inflammatory action modulation of mucosal defense system via TLR4.
- Citation: Kanauchi O, Fukuda M, Matsumoto Y, Ishii S, Ozawa T, Shimizu M, Mitsuyama K, Andoh A. Eubacterium limosum ameliorates experimental colitis and metabolite of microbe attenuates colonic inflammatory action with increase of mucosal integrity. World J Gastroenterol 2006; 12(7): 1071-1077
- URL: https://www.wjgnet.com/1007-9327/full/v12/i7/1071.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i7.1071
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn's disease, is a chronic and aggressive idiopathic inflammatory disorder of the gastrointestinal tract.[1] The detailed mechanism and causes of IBD are not fully understood; however, abundant experimental observations suggest that luminal bacteria (microflora) play a crucial role in the pathogenesis of IBD.[2,3,4] In experimental colitis models, colitis has not occurred without the presence of intestinal flora. This indicates that microflora are likely to induce colonic inflammation and its recurrence.[5] Recently, therapeutic approaches to modifying microflora have been attempted by the use of antibiotics, probiotics, and prebiotics.[4,6,7]
Germinated barley foodstuff (GBF), which consists mainly of dietary fiber and glutamine (Gln)-rich protein, is a prebiotic product for UC patients.[8] In our previous studies, GBF exhibited preventive and therapeutic effects in experimental colitis models, especially an improvement of severe bloody diarrhea and attenuation of colonic mucosal damage, accompanied by the modulation of microflora.[9] In colitis models, GBF or its fiber fraction significantly increased cecal butyrate production, and GBF significantly increased the number of butyrate producing Eubacterium limosum (E. limosum), with a decrease in colonic pH.[9,10] As a result, in this colitis model, GBF was considered to be efficiently fermented and converted by E. limosum into short-chain fatty acids (SCFAs), including butyrate.[8,9,11]
Furthermore, a clinical trial of GBF showed that this treatment can benefit UC patients by increasing the level of stool butyrate. This clinical efficacy was also associated with an increase in the number of Bifidobacterium and E. limosum, both of which are regarded as beneficial bacteria, in the stool.[12,13] However, the mechanism of the therapeutic effect of GBF and E. limosum has been incompletely understood. The detailed metabolism of E. limosum in gastrointestinal tract is also little understood, differing from Lactobacillus or Bifidobacterium as representative probiotics.[8] The net viability in human gastrointestinal tract and resistance to gastric or bile acids were not clear in detail, and these difficulties might be caused by the obligate anaerobic character. The only distinct effect of E. limosum is increase of butyrate production in ulcerative colitis[14] and experimental colitis.[12] Thereupon, we pointed out the major metabolites of E. limosum (short chain fatty acids including butyrate) and the minute role in colonic epithelium. Although this study is preliminary, we focus on the role of E.limosum in vivo and its metabolites on colonic epithelium in vitro.
E. limosum (JCM6421 Riken Bioresource Center, Saitama, Japan) was inoculated with modified GAM medium (Nissui Pharmaceutical Co., Tokyo, Japan) anaerobically at 37 °C. AnaeroPack (Mitsubishi Gas Chemical Co,. Tokyo, Japan) was used to obtain the anaerobic condition. The E. limosum and supernatant were separated by centrifugation and filtration, sequentially lyophilized. The number of E. limosum after lyophilization was of the same order as before being cultured (109 CFU/1 g). The organic acid content of the supernatant (residual medium) was determined by HPLC using the method described in our previous study.[9] Briefly, the supernatant was filtered through a membrane filter and separated with an ion-exclusion column (Shodex Rspak KC-811, 8 mmID × 30 cm long, Showa Denko.Co., Ltd., Tokyo, Japan). Organic acids were detected using bromothymol blue as the post-column reagent. The column temperature was 60 °C, and the mobile phase was a 0.75 mmol/L sulfuric acid solution (flow rate : 0.8 mL/ min).
The methods for maintenance of T84 (ATCC CCL-248, Rockville, MD) cells in culture were as previously described in other studies.[15,16] Briefly, T84 cells were grown in monolayers in a 1:1 mixture of Dulbecco’s modified Eagle's Medium/Nutrient Mixture F - 12 Ham (Sigma St. Louis MO, USA) supplemented with, 50 U/mL penicillin, 50 μg/mL streptomycin and 5% fetal bovine serum. Confluent monolayers were subcultured by trypsinization with 0.5% trypsin and 5.3 mmol/L EDTA in Ca2+ and Mg2+ free phosphate-buffered saline.
For the determination of cell growth activity, succinate dehydrogenase (SUDH) activity was evaluated. Since SUDH was reported to be well related to the proliferative and viability activity[17], 6 × 104 cells were plated onto each well of 96-well plates and the cells were treated with the respective mediums for 24 h, Cell Counting Kit-8 (Dojin Chemicals Co., Ltd. Kumamoto Japan) was used, then SUDH activity was measured as per the manufacturer's instructions.[17] For the determination of IL-6, cells were plated onto each well of 48-well plates. The cells were grown to confluence in cell calture dishes. They were treated with TNF-alpha (50ng/mL; Funakoshi Co., Tokyo, Japan) for another 24 h and the supernatants were harvested for measurement of IL-6, IL-6 ELISA (Genzyme Cambridge MA) as per the manufacturer's instructions.
For the determination of expression of TLR4 mRNA, T84 cells were plated into each well of 60mm × 15mm cell culture dishes. The cells were grown to confluence in cell calture dishes. They were treated with TNF-alpha (25ng/mL; Funakoshi Co., Tokyo, Japan) with sodium butyrate (0, 0.5, 1.0, and 2.0 mmol/L) for another 6 hrs. Then total RNA extraction was carried out by RNeasy Mini Kit (QIAGEN-JAPAN KK Tokyo) and RT-PCR was performed (Applied Biosystems 7300 real time PCR system, Applied Biosystem-Japan Tokyo Japan). RT-PCR was carried out with specific primers and probes for TLR4 receptor components and housekeeping gene, beta-actin (Taqman gene expression assay, Applied Biosystem-Japan Tokyo Japan). The primer pairs used for amplification of TLR4 were (5'-3') CAG AGT TTC CTG CAA TGG ATC A and GCT TAT CTG AAG GTG TTG CAC AT. TaqMan MGB probes (labeled with fluorescent reporter dye 6FAM) were used for analysis of TLR4 (CGT TCA ACT TCC ACC AAG AGC TGC CT). Respective data were shown after normalized to housekeeping gene (beta-actin).
Fourteen 8-wk-old female BALB/c mice were purchased from Charles River Japan (Kanagawa, Japan). The mice were housed individually in cages in a room kept at 20 to 25°C and 40 to 70% relative humidity with a 12 h lighting cycle (conventional conditions).[12] They were allowed free access to diet and drinking water. First, the 14 mice were fed laboratory chow for 1 wk during their acclimatization period. They were then divided into two groups of 7: a 5% E. limosum group (E.limisum group) and a cellulose-diet control group (control group). The total volume of protein and dietary fiber in both diets was adjusted to 14.6% protein and 3.0 % dietary fiber.[19,12] The compositions of the two diets are shown in Table 1.
| CE | E. limosum | |
| (g/kg diet) | ||
| Casein | 146.0 | 100.0 |
| Vitamin mixture | 10.0 | 10.0 |
| Mineral mixture | 35.0 | 35.0 |
| Choline chloride | 2.0 | 2.0 |
| Cellulose | 30.0 | |
| E. limosum | 50.0 | |
| Corn oil | 50.0 | 50.0 |
| DSS | 20.0 | 20.0 |
| Corn starch | 707.0 | 733.0 |
| (without DSS) | (727.0) | (753.0) |
After one week of pre-feeding, the mice weighing 20 to 22 g in the two groups were given 2% dextran sodium sulfate (mol wt, 5000; Wako Pure Chemical Co., Osaka, Japan) in their diet to induce colitis. The animals were sacrificed at d 7, and the colon and cecal contents were removed. This animal experiment was approved by the local ethical committee.
In all the mice, the body weight, occult blood, and rectal bleeding, as well as stool consistency were monitored daily. The presence of blood in feces was evaluated using a hemoccult test kit (Beckman Coulter, Fullerton, CA). The disease activity index was determined by scoring changes in weight, hemoccult positivity, gross bleeding, and stool consistency, described in our previous study.12) Briefly, we used five grades of weight loss (0: no loss or weight gain, 1: 1 to 5%, 2: 5 to 10%, 3: 10 to 20%, 4: loss of more than 20%), three grades of stool consistency (0: normal, 2: loose, 4: diarrhea) and three grades of occult blood (0: normal, 2: occult blood positive, 4: gross bleeding). And disease activity score was sum of above parameters.
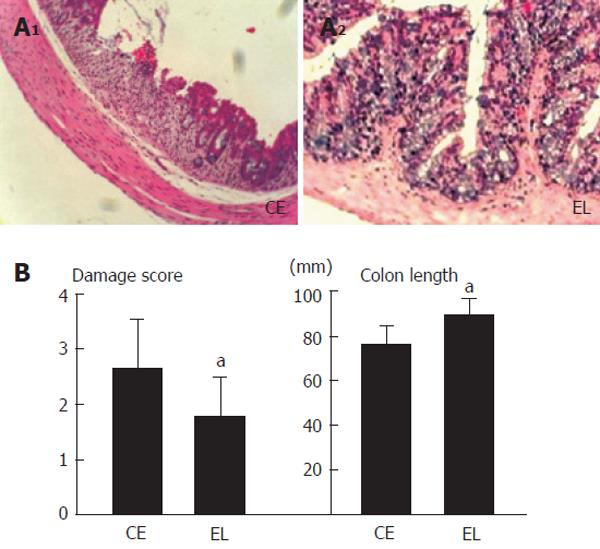
The entire colon was removed, and its length was measured. After removal, the colon was immediately divided into three segments (proximal: 1cm from ileo-cecal valve, middle, and distal: 1.5cm from anal columns), and a part of each segment was fixed in 10% neutral buffered formalin. After fixation, the specimens were stained with hematoxylin and eosin.[12] Six sections from each segment were scored for colitis severity (crypt scores) based on the method of Cooper et al by two blind examiners.[20] Briefly, this scoring method is: grade 0, intact crypt; grade 1, cystic dilatation of crypts; grade 2, loss of the basal one-third of the crypt; grade 3, loss of the basal two-thirds of the crypt; grade 4, loss of entire crypts with the surface epithelium remaining intact; grade 5, loss of both the entire crypt and surface epithelium.[10,12] The average damage score was shown in Figure 5.
The cecal contents were removed immediately after the dissection. Because the cecal contents of the mice were very small, we had to gather all the samples in the two groups. Organic acids were measured by the HPLC methods described in Experiment 1.[9]
Results are expressed as means ± SD. Differences in the disease activity were evaluated by chi-square analysis. The Student t test (two-tailed) was used to test all other comparisons. Significance was accepted at P < 0.05.
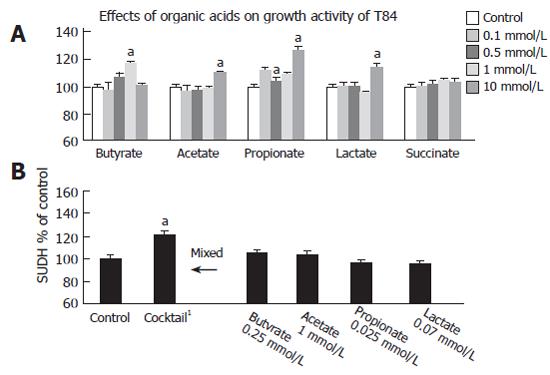
The effects of organic acids on growth activity of T84 are shown in Figure 1A and B. Figure 1A shows the effects of sodium-butyrate, sodium-acetate, sodium-propionate, sodium-lactate, and sodium-succinate at different levels (0, 0.1 mmol/L, 0.5 mmol/L, 1 mmol/L, and 10 mmol/L) on the growth activity of T84 as determined by SUDH activity. Butyrate significantly increased the growth activity of T84 at 1 mmol/L. Acetate and lactate promoted a significantly high growth activity at just 10 mmol/L. With regards to the propionate, at 0.1 and 10 mmol/L, significant increases were observed. There were no statistical differences in succinate supplementation at all dose levels.
In our previous study, E. limosum produced butyrate (2.5 mmol/L), acetate (10 mmol/L), propionate (0.25 mmol/L), and lactate (0.7 mmol/L) in the medium and the addition of 10 % E.limosum supernatant to T84 significantly increased SUDH activity.[21] Therefore, the organic acid mixture adjusted to that of 10 % of E. limosum supernatant (butyrate (0.25 mmol/L), acetate (1 mmol/L), propionate (0.025 mmol/L), and lactate (0.07 mmol/L) was simultaneously added as a cocktail to T84. This cocktail significantly increased the growth activity, as shown in Figure 1 B.
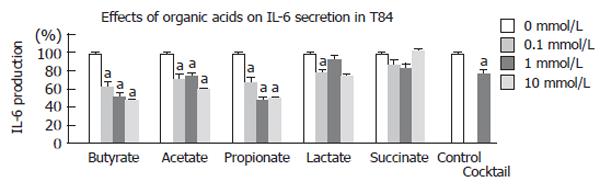
Three kinds of short-chain fatty acids (butyrate, acetate, propionate) promoted a significantly decrease of IL-6 production in a dose-dependant manner. Lactate only showed a decrease of IL-6 production at 0.1 mmol/L, and succinate had no anti-inflammatory effects at any dose level. (Figure 2). In this experiment, the cocktail also significantly attenuated IL-6 production (77 % of control, P < 0.05). However, it does not seem the synergistic effects as in growth stimulatory effect.
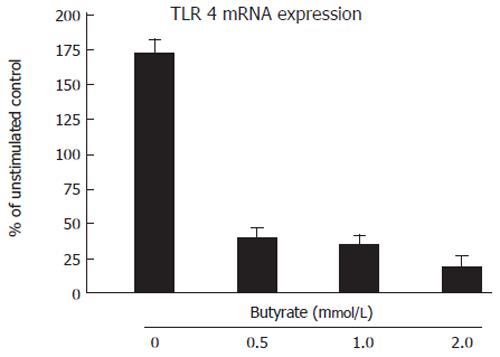
Figure 3 showed the effect of butyrate on expression of TLR4 mRNA in T84. Interestingly, butyrate attenuated the expression of TLR4 mRNA at low-level 0.5 mmol/L.
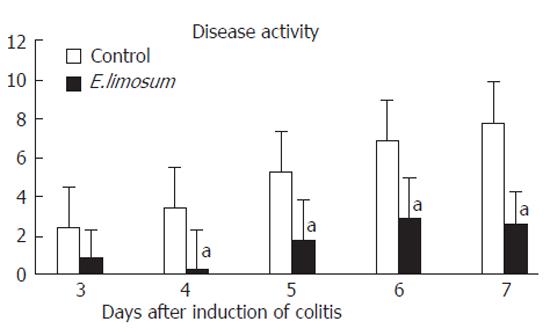
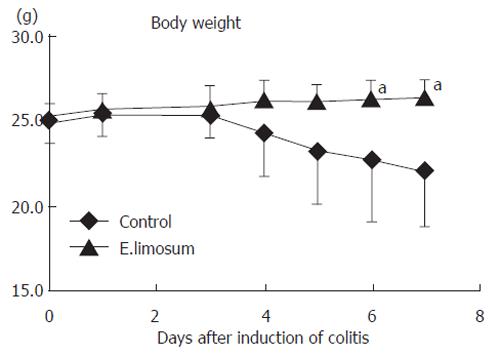
The change in body weight after initiation of colitis is shown in Figure 4. The E.limisum group showed a significant retention of body weight on d 6 and 7, compared with the control group. The change in disease activity of both dietary groups is shown in Figure 5. Prior to the change in body weight, the disease activity on d 4 was significantly attenuated in the E.limisum group. The organic acid contents in both dietary groups are shown in Table 2. This data is for pooled samples (n = 7 in respective groups), so statistical analysis was not possible; however, the level of each organic acid dramatically increased.
| Succinate | Lactate | Acetate | Propionate | n-Butyrate | |
| (µmol / g content) | |||||
| CE | 0.689 ± 0.572 | 3.211 ± 2.373 | 17.105 ± 9.653 | 2.144 ± 0.881 | 1.644 ± 0.294 |
| EL | 1.624 ± 0.439 | 7.445 ± 2.882 | 38.106 ± 4.258 | 7.168 ± 1.000 | 2.523 ± 0.552 |
Figure 6 A shows representative histological photographs of colonic mucosa in both groups (magnification X 50). The colon length and damage scores of the groups are shown in Figure 6B. The E.limisum diet significantly attenuated the mucosal damage and shortening of colon length compared to those in the control group.
IBD is considered to be a dysfunctional response of the mucosal immune system to luminal antigens and commensal microbiota in genetically susceptible individuals. Therefore, it is a rationally therapeutic strategy to eliminate the pathogenic bacterial component.[22,23] It has already been reported that manipulation of microflora by antibiotics, probiotics, and prebiotics was effective in IBD treatment. Antibiotic therapy is recognized to have an essential role in IBD. For example, metronidazole decreases perianal complications in Crohn's disease patients and ciprofloxacin reduces the presence of Clostridium difficle, which is an aggravating bacteria in IBD, and improves clinical symptoms.[24] With regards to the probiotic therapy, it is also considered a rational therapeutic option in IBD. Recent studies have shown that restoring the microbial balance using probiotics might contribute to the normalization of microflora and the attenuation of symptoms in IBD.[4] Prebiotic administration can also modulate the microflora by increasing the populations of beneficial bacteria and thereby quantitatively changing the composition of the microflora.[25] These alterations may act beneficially, in part by causing a luminal induction of short-chain fatty acids (SCFAs), which are the major probiotic metabolites, are important nutrients for the intestine, and induce an acidic environment. Especially, butyrate plays a trophic role as a first nutrient for colonocytes.[12,26] It has also been reported to act as an anti-inflammatory agent by inactivating transcriptional factor (NF-κB or STAT3).[12]
GBF stimulated growth of Bifidobacterium species and E. limosum and production of its metabolites butyrate and other short-chain fatty acids. Four-week administration of 20 to 30 g of GBF to patients with mild to moderate UC decreased clinical and endoscopic evidence of inflammation in a trial. This treatment increased fecal density of Bifidobacterium species and E. limosum.[8] A longer term (24 wk) open-label study reached similar conclusions.[13,27] In our previous study, the metabolites of E. limosum obtained as the supernatant of the medium after inoculation increased the cell growth rate as determined by SUDH activity and attenuated the production of IL-6 by TNF-alpha stimulation in T84.[21] However, the detailed role of E. limosum metabolites on mucosa has not been determined.
In this study, the role of the main composition of metabolites, a mixture of organic acids, on mucosa was examined. Except for succinate, the organic acids we used significantly increased the growth activity of T84. Interestingly, the cocktail containing acids at low levels, which had no stimulatory effects, significantly increased the growth rate compared to that in the control. The epithelium may require the coexistence of multiple organic acids to stimulate its growth. The cocktail significantly attenuated IL-6 production as well as the production of all short-chain fatty acids. Compared with a single acid, multiple, coexisting organic acids may increase the barrier function and anti-inflammatory effects on inflammatory colonic mucosa at a relatively low level. In the future, we need to evaluate the detailed mechanism of organic acids on mucosa, while considering the influence of the other, soluble unknown factors in metabolites.
In this study, a powder of E. limosum was administered to experimental colitis model, and an E.limisum diet significantly prevented body-weight loss and disease activity compared with the control group. Also, the E.limisum diet dramatically increased the levels of 3 kinds of short chain fatty acids and 2 organic acids. As described in the method section, we could not evaluate the microbial data in cecal content, because its volume was very low. In addition, it is still not clear that the net viability in gastrointestinal tract and resistance to gastric or bile acids was derived from its obligate anaerobic character. It is speculated that the number of dietary E. limosum changed (decreased) day by day, in the diet since this bacterium is an obligate anaerobe and may have been adversely affected by ambient oxygen exposure during dietary administration. Care was excersiced in the preparation of sufficient E. limosum due to its slow growth rate and culture yields. Additional microbiological data is required to determine precise growth requirements of this microbiota.
However, the present data may suggest that E. limosum might be able to reproduce in the gastrointestinal tract, although the obligate anaerobe E. limosum was treated under aerobic conditions for use in as an animal diet for a relatively long time. The observed increase of cecal organic acid may strongly support this conjecture.
Microbiota itself contains many kinds of chemical component (peptide-glycan, enzyme, nucleic acid, protein, lipids, and so on). It is very difficult to identify the active component responsible for the anti-inflammatory effect in E. limosum. At first, we pointed out its metabolites, especially butyrate in this study. As shown in a previous study, butyrate demonstrated unique characteristics in colitic rats fed GBF[9]
The monolayer of intestinal epithelial cells (IEC) plays a role in sensing the external gastrointestinal environment. Disruption or breakdown in the signaling function of IEC is important in the development of IBD.[28] It was reported that IEC recognized pathogen or bacteria is based on the conserved signature molecules in microbes (microbe or pathogen-associated molecular patterns; PAMPs).[28,29] PAMPs are found in lipopolysaccharides (LPS) of gram-negative bacteria, and lipoteichoic acids (LTA) of gram-positive bacteria. One of pattern recognition receptor families mediated the response of immune cells to PAMP is TLR family. TLR4 and TLR2, which recognize LPS, peptideglycan and LTA, respectively were well studied. In this study, we investigated the role of the major metabolites butyrate by E. limosum on the mucosal innate immune response via TLR4. It is very difficult to isolate the cell wall components of E. limosum and to evaluate the respective components, since these components might be modified or hydrolyzed by other microflora in gastrointestinal tract, so that, first of all, we applied the metabolites butyrate.
Remarkably butyrate decreased the expression of TLR4 mRNA level with decrease of IL-6 production in T84 cell line. Since a detailed mechanism is still unclear, we needed to evaluate of the role of butyrate in different concentrations or cell lines. However, it was considered that butyrate had an innate immune modulatory effect.
With regards to the cell wall fraction of E. limosum, we do not have experimental data, and a detailed study will be needed, however, an unidentified peptideglycan or a CpG-DNA fraction derived from E. limosum may contribute to the colonic mucosal immune response via TLR 2 or 9.[30] In our previous preliminary study, GBF peculiarly decreased the number of CD4+ lymphocytes in the colonic intrarepithelial layer and lamina propria in RAG2-/- CD45RB high-transfer colitis mice, together with an increase of cecal butyrate content.[31] It might also be considered that an increase of butyrate production is evidence of the presence of modulating microflora in this chronic colitis model. Considering the above results, the effects of GBF on colitis could be partly caused by a change in the mucosal immune system via modulation of microflora and its metabolitic organic acids, especially butyrate production.
In this paper, we described the effects of the special bacteria (E. limosum) and its metabolites on colitis and colonic epithelium. In research regarding the pathogenesis of IBD and medical therapeutic approaches to IBD, we may have to consider the role of microflora and the interaction between epithelium and bacteria in the intestinal tract in more detail. Although these strategies hold great promise and appear to be useful in certain settings, more studies for example, the relationship between mucosa and microflora and its metabolites will be needed. This therapy may provide a new nutraceutical approach to IBD treatment.
S- Editor Guo SY L- Editor Pravda J E- Editor Liu WF
| 1. | Podolsky DK, Fiocchi C. In: Inflammatory Bowel Disease, 5th Ed. Kirsner, J.B. W.B. 2000;191-207. |
| 2. | Shanahan F. Immunology. Therapeutic manipulation of gut flora. Science. 2000;289:1311-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 53] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Gionchetti P, Rizzello F, Venturi A, Campieri M. Probiotics in infective diarrhoea and inflammatory bowel diseases. J Gastroenterol Hepatol. 2000;15:489-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 718] [Article Influence: 34.2] [Reference Citation Analysis (1)] |
| 5. | Dianda L, Hanby AM, Wright NA, Sebesteny A, Hayday AC, Owen MJ. T cell receptor-alpha beta-deficient mice fail to develop colitis in the absence of a microbial environment. Am J Pathol. 1997;150:91-97. [PubMed] |
| 6. | Cong Y, Konrad A, Iqbal N, Elson CO. Probiotics and immune regulation of inflammatory bowel diseases. Curr Drug Targets Inflamm Allergy. 2003;2:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Guarner F, Malagelada JR. Gut flora in health and disease. Lancet. 2003;361:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2113] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 8. | Kanauchi O, Mitsuyama K, Araki Y, Andoh A. Modification of intestinal flora in the treatment of inflammatory bowel disease. Curr Pharm Des. 2003;9:333-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Araki Y, Andoh A, Koyama S, Fujiyama Y, Kanauchi O, Bamba T. Effects of germinated barley foodstuff on microflora and short chain fatty acid production in dextran sulfate sodium-induced colitis in rats. Biosci Biotechnol Biochem. 2000;64:1794-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Kanauchi O, Iwanaga T, Andoh A, Araki Y, Nakamura T, Mitsuyama K, Suzuki A, Hibi T, Bamba T. Dietary fiber fraction of germinated barley foodstuff attenuated mucosal damage and diarrhea, and accelerated the repair of the colonic mucosa in an experimental colitis. J Gastroenterol Hepatol. 2001;16:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Kanauchi O, Fujiyama Y, Mitsuyama K, Araki Y, Ishii T, Nakamura T, Hitomi Y, Agata K, Saiki T, Andoh A. Increased growth of Bifidobacterium and Eubacterium by germinated barley foodstuff, accompanied by enhanced butyrate production in healthy volunteers. Int J Mol Med. 1999;3:175-179. [PubMed] |
| 12. | Kanauchi O, Serizawa I, Araki Y, Suzuki A, Andoh A, Fujiyama Y, Mitsuyama K, Takaki K, Toyonaga A, Sata M. Germinated barley foodstuff, a prebiotic product, ameliorates inflammation of colitis through modulation of the enteric environment. J Gastroenterol. 2003;38:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Hanai H, Kanauchi O, Mitsuyama K, Andoh A, Takeuchi K, Takayuki I, Araki Y, Fujiyama Y, Toyonaga A, Sata M. Germinated barley foodstuff prolongs remission in patients with ulcerative colitis. Int J Mol Med. 2004;13:643-647. [PubMed] |
| 14. | Mitsuyama K, Saiki T, Kanauchi O, Iwanaga T, Tomiyasu N, Nishiyama T, Tateishi H, Shirachi A, Ide M, Suzuki A. Treatment of ulcerative colitis with germinated barley foodstuff feeding: a pilot study. Aliment Pharmacol Ther. 1998;12:1225-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406-1415. [PubMed] |
| 16. | Weymer A, Huott P, Liu W, McRoberts JA, Dharmsathaphorn K. Chloride secretory mechanism induced by prostaglandin E1 in a colonic epithelial cell line. J Clin Invest. 1985;76:1828-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K. A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet. Biol Pharm Bull. 1996;19:1518-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 537] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 18. | Abreu MT, Arnold ET, Thomas LS, Gonsky R, Zhou Y, Hu B, Arditi M. TLR4 and MD-2 expression is regulated by immune-mediated signals in human intestinal epithelial cells. J Biol Chem. 2002;277:20431-20437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 19. | Kanauchi O, Agata K. Protein, and dietary fiber-rich new foodstuff from brewer's spent grain increased excretion of feces and jejunum mucosal protein content in rats. Biosci Biotechnol Biochem. 1997;61:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. [PubMed] |
| 21. | Kanauchi O, Matsumoto Y, and Andoh A. Effects of prebiotics on colonic environment and mucosal barrier function in inflammatory bowel disease. J. JSMUFF (In Japanese). 2004;2:93-98. |
| 22. | Sartor RB. In: Sartor RB, Sandbom WJ, eds. Kirsner's inflammatory bowel diseases. 6th Ed. 2003;138-162. |
| 23. | Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2693] [Cited by in RCA: 2747] [Article Influence: 119.4] [Reference Citation Analysis (2)] |
| 24. | Linskens RK, Huijsdens XW, Savelkoul PH, Vandenbroucke-Grauls CM, Meuwissen SG. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol Suppl. 2001;29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401-1412. [PubMed] |
| 26. | Babakissa C, Colomb V, Andrieux C, Cherbuy C, Vaugelade P, Bernard F, Popot F, Corriol O, Ricour C, Duée PH. Luminal fermentation and colonocyte metabolism in a rat model of enteral nutrition. Dig Dis Sci. 2003;48:1339-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Kanauchi O, Mitsuyama K, Homma T, Takahama K, Fujiyama Y, Andoh A, Araki Y, Suga T, Hibi T, Naganuma M. Treatment of ulcerative colitis patients by long-term administration of germinated barley foodstuff: multi-center open trial. Int J Mol Med. 2003;12:701-704. [PubMed] |
| 28. | Singh JC, Cruickshank SM, Newton DJ, Wakenshaw L, Graham A, Lan J, Lodge JP, Felsburg PJ, Carding SR. Toll-like receptor-mediated responses of primary intestinal epithelial cells during the development of colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G514-G524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 29. | Ortega-Cava CF, Ishihara S, Rumi MA, Kawashima K, Ishimura N, Kazumori H, Udagawa J, Kadowaki Y, Kinoshita Y. Strategic compartmentalization of Toll-like receptor 4 in the mouse gut. J Immunol. 2003;170:3977-3985. [PubMed] |
| 30. | Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 595] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 31. | Kanauchi O, Suga T, Tochihara M, Hibi T, Naganuma M, Homma T, Asakura H, Nakano H, Takahama K, Fujiyama Y. Treatment of ulcerative colitis by feeding with germinated barley foodstuff: first report of a multicenter open control trial. J Gastroenterol. 2002;37 Suppl 14:67-72. [PubMed] |