Published online Feb 7, 2006. doi: 10.3748/wjg.v12.i5.800
Revised: June 28, 2005
Accepted: July 15, 2005
Published online: February 7, 2006
Desmoplastic small round cell tumor (DSRCT) is a rare, highly aggressive malignancy with distinctive histological features: a nesting pattern of cellular growth within dense desmoplastic stroma, occurring in young population with male predominance. The mean survival period is only about 1.5-2.5 years. The tumor has co-expressed epithelial, muscle, and neural markers in immunohistochemical studies. This work reports a 27-year-old man presenting with hematemesis and chronic constipation. Serial studies including endoscopy, upper gastrointestinal series, abdominal computed tomography and barium enema study showed disseminated involvement of visceral organs. The patient underwent aggressive surgery and received postoperative adjuvant chemotherapy consisting of 5-fluorouracil, cyclophosphamide, etoposide, doxorubicin, and cisplatin. He survived without any disease for 20 mo after the surgery. No standard treatment protocol has been established. Aggressive surgery combined with postoperative multi-agent adjuvant chemotherapy is justified not only to relieve symptoms but also to try to improve the outcome in this advanced DSRCT young patient.
- Citation: Chang CC, Hsu JT, Tseng JH, Hwang TL, Chen HM, Jan YY. Combined resection and multi-agent adjuvant chemotherapy for desmoplastic small round cell tumor arising in the abdominal cavity: Report of a case. World J Gastroenterol 2006; 12(5): 800-803
- URL: https://www.wjgnet.com/1007-9327/full/v12/i5/800.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i5.800
Desmoplastic small round cell tumor (DSRCT) is a rare malignancy with highly aggressive behavior spreading widely along the serosal surface. Gerald and Rosai[1] first described the disease in 1989 in terms of distinctive pathologic findings: a nesting pattern of cellular growth within dense desmoplastic stroma, and immunohistochemical co-expression of epithelial, muscle and neural markers[1-3]. A specific chromosome abnormality, t (11;22) (p13;q11 or q12) was identified[4], and DSRCT is believed to be the result of involvement of both Wilms’ tumor suppressor gene and Ewing’s sarcoma gene located on Chromosome 11 and 22, respectively[5]. The tumors usually present in young male individual, as a single mass or multiple masses in the abdominal-pelvic cavity with metastases common to peritoneum, liver and lymphoid tissue[6]. Some cases had presentation sites in the scrotum[6,7], pleural space[6,8,9] and mediastenum[6,10]. Complete resection is generally difficult to obtain due to multiple foci and dissemination. The standard treatment protocol has not been well established. Chemotherapy currently seems to be the best potential treatment. This work reports on a case of DSRCT with no disease for 20 months following complete resection of tumors and postoperative multi-agent adjuvant chemotherapy consisting of 5-fluorouracil (5-FU) and PAVEP (cyclophosphamide, etoposide, doxorubicin and cisplatin).
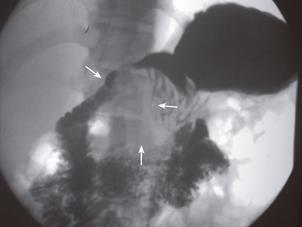
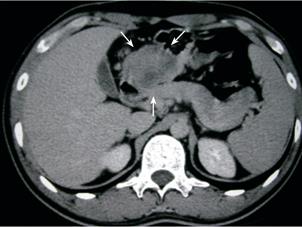
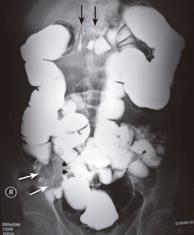
The 27-year-old man had intermittent epigastralgia, abdominal fullness, and chronic constipation for 3 months. He presented sudden onset of hematemesis (500 mL) outside our hospital on September 15, 2003. Physical examination revealed only mild epigastric tenderness. The hemoglobin level was normal. Endoscopy showed esophageal and cardiac ulcers without complete study owing to poor compliance of the patient. Upper gastrointestinal (GI) series demonstrated a mass with external compression at the gastric antrum (Figure 1). Abdominal computed tomography (CT) indicated a 5 cm heterogeneous mass located among the gastric antrum, duodenum loop and pancreatic head (Figure 2). Barium enema study showed external compression of the middle transverse colon, ileocecal valve and ascending colon (Figure 3). Laparoscopic biopsy was performed because multiple intra-abdominal organs were involved. A histopathologic exam of the specimen revealed poorly differentiated carcinoma. The patient was then referred to our hospital for further treatment.
No significant medical or pertinent family history was found. Additionally, no history of exposure to carcinogens such as radiation, tobacco or asbestos was identified. Hematogram showed hemoglobin, 110 g/L and white blood cells (WBC), 11 × 109/L. Biochemistry studies yielded normal results. Tumor markers such as carcinoembryonic antigen and carbohydrate antigen 19-9 were within the normal rage. Laparotomy was performed on October 3, 2003 to relieve the patient’s symptoms. During the operation, the gastric antrum, pancreas, terminal ileum and cecum, sigmoid colon, rectovesicular region and omentum were all involved by tumors. Right hydroureter was also identified due to external compression by the tumor. Subtotal gastrectomy, partial pancreatic resection, total omentectomy, proctocolectomy and J-pouch ileo-anal anastomosis were performed for complete resection of tumors. A double-J stenting was inserted through the ureterostomy by a urologist.
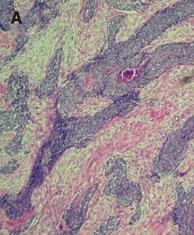
A histopathologic exam of the surgical specimens demonstrated invasive nests of poorly differentiated carcinoma, mainly involving omentum, pancreatic, and gastric, colonic and ileal muscular walls with focal mucosa extension. Large and small foci of tumor cells were observed in fibrous interstitium (Figure 4A). The tumor cells had a small spherical or spheroid nucleus, which was rich in chromatin (Figure 4B). One of 20 peri-colic lymph nodes was positive for metastasis. The section margins were free of tumor. The immunohistochemical tests were positive for desmin (Figure 4C), epithelium membrane antigen and neuron-specific enolase, and focal positive for vimentin, indicating DSRCT.
J-pouch ileo-anal anastomosis leakage with intra-abdominal abscess occurred on postoperative day 7. The patient recovered after treatment by antibiotics and CT-guidance drainage. Postoperative adjuvant chemotherapy with high dose 5-FU (2 600 mg/m2, day 1) was initiated on 24th October, 2003. The patient received altogether eight courses of chemotherapy of 5-FU thereafter. Another chemotherapeutic regimen consisting of PAVEP (cyclophosphamide 300 mg/m2, d 1-3; etoposide 75 mg/m2, day 1-3; doxorubicin 40 mg/m2, day 1; cisplatin 100 mg/m2, day 4) was administered on February 17, 2004. Neutropenic (WBC, 2×109 /L) fever developed one week later even though prophylactic granulocyte colony-stimulating factor (G-CSF) was given the day following chemotherapy. The patient received broad-spectrum antibiotics treatment, and his fever was gradually controlled. He was discharged 12 d later when his WBC returned to normal. Another four chemotherapy courses with PAVEP regimens were completed on May 24, 2004. He survived without any disease 20 mo after the surgery.
DSRCT has a strong male predominance, and the majority arises below the diaphragm with serosal spreading and easily metastasizing to lung, liver, and lymphoid tissues[2,6,11]. The most common site was the pelvis (62%), followed by spreading widely on the peritoneal surface (42%)[12]. Symptoms such as abdominal pain (52.1%), increased abdominal girth (8.4%), and abdominal mass (5.6%) have been reported[14]. Patients also presented with GI or genitourinary obstruction. GI bleeding does not appear to have been reported in the literature. This patient was the first case with DSRCT presented with GI bleeding. The bleeding was associated with tumors directly invading to the gastric mucosa.
The prognosis of DSRCT is dismal with a mean survival of 1.5-2.5 years since the wide spread of the tumor makes radical resection difficult to achieve, and chemotherapeutic agents are only temporarily effective in treating this disease[6,12-15]. Farhat et al [13] recommended that PAVEP should be the first-line drug for treating DSRCT after reviewing 8 cases with complete remission from the treated 60 cases. However, grade four neutropenia occurred in 69% cycles while administering PAVEP regimens despite prophylactic use of G-CSF in 90% of cases in his report[15]. Considering the severe and life-threatening side effects and uncertainty of therapeutic results of PAVEP regimens, other safer and effective chemotherapeutic agents should be considered prior to PAVEP.
5-FU is well-known as a chemotherapeutic agent and a radioactive sensitizer broadly used for GI tract cancers and disseminated intra-abdominal metastatic adenocarcinoma. Gerald et al [2] reported a successful application of 5-FU in treating intra-abdominal DSRCT. Kretschmar et al [6] also reported 60% responsive rate to this tumor. Since DSRCT is a highly aggressive and progressive malignancy, post-operative adjuvant chemotherapy should be initiated as soon as possible. Post-operative anastomotic leakage and right urinary tract obstruction arose in our case. Therefore, 5-FU was applied instead of PAVEP regimen as the first-line drug to treat DSRCT considering the infection problems and the adverse effects of PAVEP regimens such as myelo-suppression and renal toxicity.
DSRCT is a disease so rare that no consistent response to chemotherapy was seen in the literature review[12]. Kurre et al [16] presented another alkylator-based chemotherapy protocol named P6 (cyclophosphamide, doxorubicin, vincristine, ifosfamide, and etoposide), which only improved the progression-free survival without significant survival benefit. Gil et al [12] recommended performing perioperative intraperitoneal chemotherapy when treating DSRCT patients, but no evidence of prolonged survival was observed.
The effect of complete resection of disseminated tumors on survival is still unknown because of the rarity of achieving complete resection at operation. Gil et al [12] reported that the median survival was 20 mo in 4 patients with complete resection of tumors compared with 11 mo in 3 cases without complete resection. Significantly, long-term survivors, including one patient who survived for 101 mo, have been reported when utilizing combined modality treatment comprising surgery and multi-agent chemotherapy. The influence of other salvage therapies, such as immunotherapy or bone marrow ablation, is still undetermined. In this work, the patient underwent complete resection of tumors and received postoperative adjuvant chemotherapy surviving without any disease and without gastrointestinal symptoms 20 mo after surgery, similar to that in Gil’s report[12].
In conclusion, the optimal treatment for DSRCT remains to be determined, indicating the rarity and virulent behavior of the disease. In this advanced DSRCT patient, aggressive surgery combined with postoperative systemic multi-agent adjuvant chemotherapy was justified not only to relieve his symptoms but also to try to improve his outcome. The effectiveness and safety of chemotherapeutic regimens still need to be improved to treat this disease as early as possible postoperatively, especially when patients cannot tolerate the toxicity of alkylator agents after such an aggressive surgery.
S- Editor Wang XL and Guo SY L- Editor Elsevier HK E- Editor Cao L
| 1. | Gerald WL, Rosai J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol. 1989;9:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 293] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991;15:499-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 388] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Ordóñez NG, el-Naggar AK, Ro JY, Silva EG, Mackay B. Intra-abdominal desmoplastic small cell tumor: a light microscopic, immunocytochemical, ultrastructural, and flow cytometric study. Hum Pathol. 1993;24:850-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Biegel JA, Conard K, Brooks JJ. Translocation (11; 22)(p13; q12): primary change in intra-abdominal desmoplastic small round cell tumor. Genes Chromosomes Cancer. 1993;7:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | Ladanyi M, Gerald W. Fusion of the EWS and WT1 genes in the desmoplastic small round cell tumor. Cancer Res. 1994;54:2837-2840. [PubMed] |
| 6. | Kretschmar CS, Colbach C, Bhan I, Crombleholme TM. Desmoplastic small cell tumor: a report of three cases and a review of the literature. J Pediatr Hematol Oncol. 1996;18:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Cummings OW, Ulbright TM, Young RH, Dei Tos AP, Fletcher CD, Hull MT. Desmoplastic small round cell tumors of the paratesticular region. A report of six cases. Am J Surg Pathol. 1997;21:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 97] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Bian Y, Jordan AG, Rupp M, Cohn H, McLaughlin CJ, Miettinen M. Effusion cytology of desmoplastic small round cell tumor of the pleura. A case report. Acta Cytol. 1993;37:77-82. [PubMed] |
| 9. | Parkash V, Gerald WL, Parma A, Miettinen M, Rosai J. Desmoplastic small round cell tumor of the pleura. Am J Surg Pathol. 1995;19:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 112] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Venkateswaran L, Jenkins JJ, Kaste SC, Shurtleff SA, Downing JR, Pappo AS. Disseminated intrathoracic desmoplastic small round-cell tumor: a case report. J Pediatr Hematol Oncol. 1997;19:172-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Leuschner I, Radig K, Harms D. Desmoplastic small round cell tumor. Semin Diagn Pathol. 1996;13:204-212. [PubMed] |
| 12. | Gil A, Gomez Portilla A, Brun EA, Sugarbaker PH. Clinical perspective on desmoplastic small round-cell tumor. Oncology. 2004;67:231-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Farhat F, Culine S, Lhommé C, Duvillard P, Soulié P, Michel G, Terrier-Lacombe MJ, Théodore C, Schreinerova M, Droz JP. Desmoplastic small round cell tumors: results of a four-drug chemotherapy regimen in five adult patients. Cancer. 1996;77:1363-1366. [PubMed] |
| 14. | Takahira K, Ohi S, Fujii N, Matsuura Y, Sano M, Hanai H, Kaneko E. Intra-abdominal desmoplastic small round cell tumor (IDSRT). J Gastroenterol. 2000;35:712-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Lippe P, Berardi R, Cappelletti C, Massacesi C, Mattioli R, Latini L, Cellerino R. Desmoplastic small round cell tumour: a description of two cases and review of the literature. Oncology. 2003;64:14-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kurre P, Felgenhauer JL, Miser JS, Patterson K, Hawkins DS. Successful dose-intensive treatment of desmoplastic small round cell tumor in three children. J Pediatr Hematol Oncol. 2000;22:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |