Published online Dec 21, 2006. doi: 10.3748/wjg.v12.i47.7649
Revised: October 1, 2006
Accepted: October 9, 2006
Published online: December 21, 2006
AIM: To construct an expression plasmid encoding human wild-type midkine (MK) and enhanced green fluorescence protein (EGFP) fusion protein (MK-EGFP), and to analyze the subcellular localization of MK in different carcinoma cell lines.
METHODS: Two kinds of MK coding sequences with or without signal peptide were cloned into plasmid pEGFP-N2, and the recombinant plasmids constructed were introduced into HepG2, MCF7 and DU145 cells, respectively, by transfection. With the help of laser scanning confocal microscopy, the expression and subcellular localization of MK-GFP fusion protein could be detected.
RESULTS: Compared with the GFP control, in which fluorescence was detected diffusely over the entire cell body except in the nucleolus, both kinds of fusion protein MK-GFP were localized exclusively to the nucleus and accumulated in the nucleolus in the three kinds of cancer cell lines.
CONCLUSION: This study reveals the specific nucleolar translocation independent of signal peptide, which may be involved in the mechanism that MK works. It provides valuable evidence for further study on the functions of MK in nucleus and its possible mechanisms, in which ribosomal RNA transcription and ribosome assembly are involved.
- Citation: Dai LC, Xu DY, Yao X, Min LS, Zhao N, Xu BY, Xu ZP, Lu YL. Construction of a fusion protein expression vector MK-EGFP and its subcellular localization in different carcinoma cell lines. World J Gastroenterol 2006; 12(47): 7649-7653
- URL: https://www.wjgnet.com/1007-9327/full/v12/i47/7649.htm
- DOI: https://dx.doi.org/10.3748/wjg.v12.i47.7649
Midkine (MK) is a 13 kDa protein originally found to be a secretory heparin-binding growth factor that is involved in cell growth, migration and survival. However, a number of subsequent studies showed that it is expressed at high levels in a variety of human carcinomas[1-5], indicating that it may play important roles in carcinogenesis. Recent studies demonstrated that MK can promote the growth of Wilm’s tumor cells and fibroblasts[6,7], the transformation of NIH3T3 cells[8], anti-apoptotic activity of Wilm’s tumor cells treated with cisplatin[9], and induction of a strong angiogenic response in rabbit corneal assay[10]. All of these suggest the multifunction of MK in carcinogenesis, cell growth, differentiation and apoptosis, and the study of its functional mechanism might lead to a new approach for cancer therapy including gastrointestinal tract cancers, since a number of recent studies have demonstrated that MK is not only highly expressed in gastrointestinal cancers, but also modulates biological phenotypes for gastric cancer growth and metastasis[2,11-13]. Moreover, it has been shown that MK could serve as a marker for the diagnosis and treatment of gastrointestinal cancers[14,15].
In addition, several subcellular localization studies have demonstrated that MK localizes in nucleus[16-18], such as in the nucleolus of PHA-activated peripheral blood lymphocytes[19], indicating that MK may serve as a transcriptional factor, or is involved in nuclear function. Nuclear transcriptional factors have been known to play roles in cell growth, differentiation, apoptosis and carcinogenesis[20-22]; however, whether MK plays roles in these functions is yet to be elucidated. In the present study, we found that MK was localized to the nucleolus of HepG2, MCF7 and DU145 cells by using MK-green fluorescence protein (GFP) fusion proteins as tracking molecules. The results showed that both MKs with or without signal peptide were exclusively localized to the nucleus and accumulated in the nucleolus of all the three carcinoma cells, while the fluorescence of GFP control was detected all over the cell except the nucleolus. Our findings may provide valuable evidence for further studies on the functions of MK in nucleus and its mechanisms, in which ribosomal RNA transcription and ribosome assembly might be involved.
The following three cell lines were obtained from American Type Culture Collection (ATCC, M anassas, VA): hepatoma cell line (HepG2), prostate carcinoma cell line (DU145) and breast cancer cell line (MCF7). They were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone Laboratories, Logan, UT) supplemented with 10% fetal bovine serum (FBS, Hyclone Laboratories, Logan, UT) at 37°C, with 5% humidified CO2 and passaged every 3 d by trypsinization.
Total RNA was extracted from the 12-wk abortive fetal liver (provided by our own hospital) with RNeasy mini kit (Qiagen Corp), and full-length human MK gene with or without signal peptide was obtained by reverse transcription polymerase chain reaction (RT-PCR). The upstream primer of MK gene without the signal peptide is 5’-AAAGAAAGATAAGGTGAAGAAGGGCGG-3’, which has the EcoRI site and the downstream primer is 5’-GGGATCCGGTCCTTTCCCTTCCCTTTCTTG-3’, which has the BamHI site. The upstream primer of MK gene with signal peptide is 5’-GGAATTCATGCAGCACCGAGGCTTCCT-3’, which has the EcoRI site and the downstream primer is the same with the former. After PCR amplification, the resulting fragments were digested with EcoRI and BamHI and identified by electrophoresis and sequenced by ABI 3700 (Sangon Bioengineering Company, Shanghai). Ethical approval for the use of fetal liver tissue was obtained.
Two MK gene fragments with or without signal peptide were separately inserted into pEGFP-N2 plasmids (enhanced green fluorescent protein N-terminal protein fusion vector, Clontech Laboratories, Palo Alto, CA), and the recombinant plasmids were used to transform E.coli DH 5α and the product was amplified in LB medium, Qiaprep® Spin Plasmid MINIPREP KIT (Qiagen, Hilden, Germany). After that, two recombinant plasmids pEGFP-MKS (EGFP fused with MK with signal peptide) and pEGFP-MKN (EGFP fused with MK without signal peptide) were obtained. Transient transfections were performed with Effectene Transfection Reagent (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Cells were plated at a density of 1 × 105 cells per well on a 35 mm dish 24 h before transfection and were transfected with 0.4 μg of pEGFP-MKS, pEGFP-MKN and pEGFP-N2, respectively.
The cells in microplates were collected into 1.5 mL Eppendorf tubes on ice, centrifuged at 2000 r/min for 5 min at 4°C, resuspended in 100 μL cell lysis buffer, and incubated on ice for 1 h. The lysates were centrifuged at 12 000 r/min for 10 min at 4°C; the supernatants were collected and stored at -20°C for electrophoresis. Proteins in conditioned media were separated by electrophoresis on 13% SDS-PAGE gels and transferred electrophoretically onto nitrocellulose membranes. After being blocked for 2 h with 5% skim milk, blots were incubated with 1:10 diluted rabbit antihuman MK (Biovendor Laboratory Medicine, Inc.) and 1:5000 diluted β-actin (Santa Cruze, CA, USA) at room temperature for 2 h. After being washed three times with TBST, the membrane was separately incubated with donkey anti-rabbit MK (Santa Cruze, USA) at a dilution of 1:5000 and anti-mouse GAPDH (Santa Cruze, CA, USA) at a dilution of 1:5000. Finally, the protein bands were visualized with an ECL kit (Pierce, USA). Quantitative analysis of the blots was performed with an imaging densitometer. Beta-actin was used as a control.
Ten hours after transfection, the cells were observed dynamically with laser scanning confocal microscopy (Leica, Heidelberg, Germany) for live imaging and the live images were obtained using a 40 × 1 NA oil immersion objective.
To visualize the subcellular localization of MK, HepG2 cells were washed three times with PBS, fixed with 4% paraformaldehyde (PFA) in PBS for 30 min at 4°C and permeabilized with 0.1% Triton X-100. After blocked with 3% bovine serum albumin (BSA) in PBS at room temperature for 1 h, the cells were stained with 1:100 diluted goat anti-MK antibody (Santa Cruz, CA, USA) at 4°C overnight. The treated cells were incubated with 1:100 diluted FITC-conjugated mouse anti-goat antibody (Vector Laboratories, Ltd, Peterborough, England) at 20°C for 45 min. Finally, they were extensively washed with PBS and examined with a fluorescence microscope.
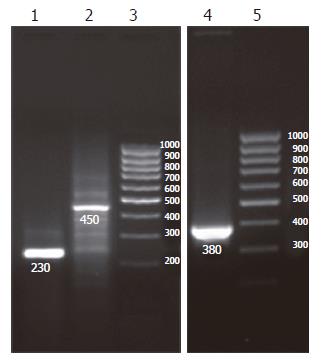
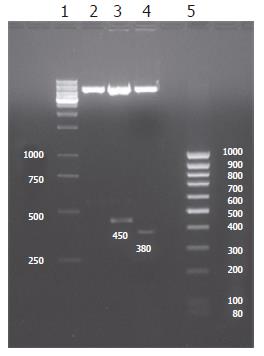
Two expected fragments of 450 bp (MKS) and 380 bp (MKN) were clearly shown in 1.5% agarose gel electrophoresis by RT-PCR and restriction endonucleases (EcoRI and BamHI) digestion identification, suggesting that the desired products were obtained (Figures 1 and 2). Subsequent sequence analysis also proved that the obtained fragments were exactly the same as the sequence of MK gene from GenBank.
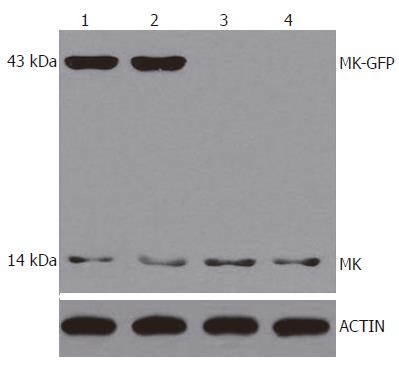
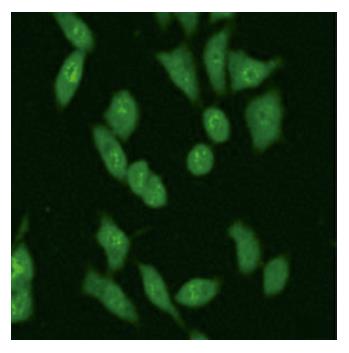
To examine whether the MK-GFP fusion proteins were expressed in the cells transfected with pEGFP-MKN and pEGFP-MKS, 24 h after transfection, Western blotting and immunostaining assay were performed. The results revealed that MK-GFP fusion proteins were highly expressed in all the cells transfected with pEGFP-MKS and pEGFP-MKN in comparison with the low levels of native MK proteins expressed by tumor cells themselves (Figure 3, Lanes 1 and 2), whereas no signals were seen in the cells transfected with pEGFP-N2 (Figure 3, Lane 3) and with no plasmid cell controls (Figure 3, Lane 4). By immunostaining, MK was clearly detectable in the nucleoli in HepG2 cells (Figure 4), thus providing evidence for subcellular localization of MK by laser scanning confocal microscopy.
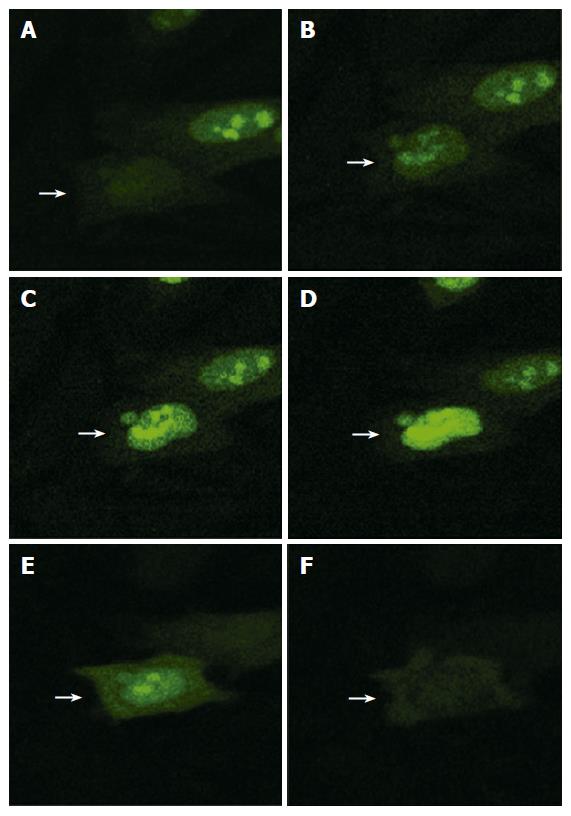
Dynamic subcellular localization process of fusion protein MK-GFP was observed by laser scanning confocal microscopy at different time points using the fusion protein MKN-GFP in HepG2 cells. The results showed that 11 h after transfection, the fusion protein MKN-GFP began to appear in the nucleus where it accumulated in the nucleolus (Figure 5). At 16 h 40 min time point, it was degraded in the nucleolus and finally disappeared.
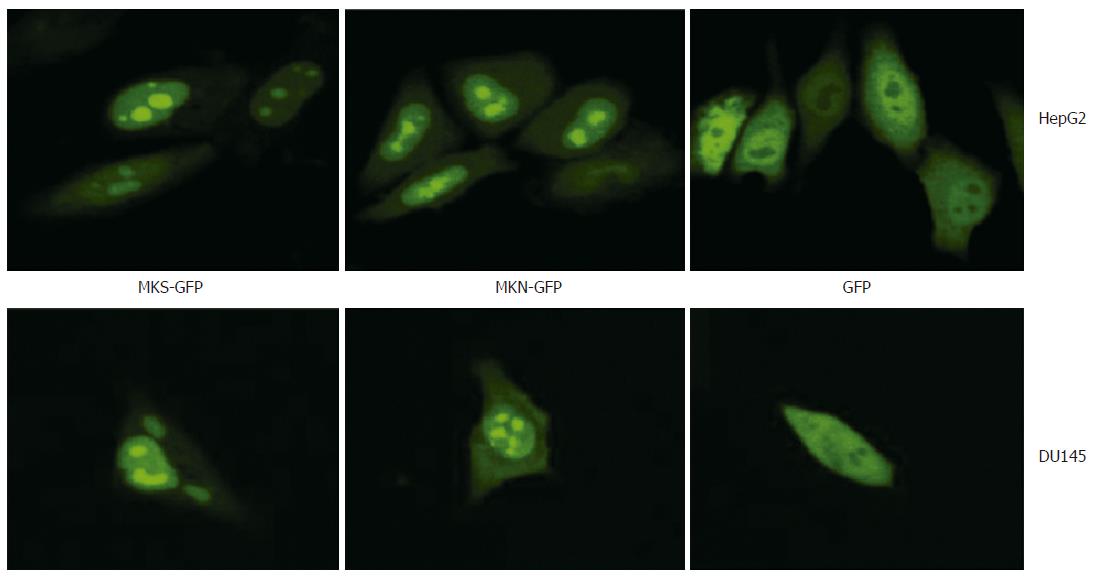
The effect of signal peptide on MK localization was carefully observed in HepG2, DU145 and MCF7 cell lines, respectively. The results showed that 24 h after transfection, both fusion proteins MKS-GFP and MKN-GFP in the two cell lines, HepG2 and DU145, were localized exclusively to the nucleus and accumulated in the nucleolus, while GFP control was detected diffusely over the entire cell body except in the nucleolus, indicating that the signal peptide in pEGFP-MKS did not participate in the nuclear and nucleolar localization (Figure 6). Similar results were obtained in MCF7 cell line (data not shown).
GFP protein is stable in vivo and has been fused to the C or N terminus of many cellular and extracellular proteins without a loss of activity, thereby permitting the tagging of proteins for gene regulation analysis, protein localization, or specific organelle labeling[23]. Proteins below 40 kDa, such as GFP, without any specific location signal can diffuse freely through the nuclear pore complex, and thus GFP can be used as a perfect positive control to detect the localization information of MK. In our study, based on the findings that GFP had no effect on the subcellular localization of the fusion protein, we confirmed that it was MK protein that drove the fusion protein into the nucleus, and to be exclusively localized in the nucleolus.
Localization of proteins in subcellular structures is important for the study of their functions. Nuclear located proteins usually act as transcription factors involved in the regulation of cell proliferation, differentiation and apoptosis. The localization of MK to the nucleolus of three carcinoma cell lines suggests that MK may act as a nuclear factor associated with carcinogenesis. In addition, considering the functions of nucleolus, MK may be involved in the transcription of rRNAs and its subsequent manipulation in cellular proliferation and other biological activities.
Nuclear translocation is mainly mediated by signal mechanisms[24], in which nuclear localization signal (NLS) helps extra-nuclear proteins bind with certain carriers to be transported through the nuclear pore complex and enter the nucleolus[25,26]. However, we have revealed similar distributions of both MKN-GFP and MKS-GFP fusion proteins in the HepG2 and DU145 cell lines, indicating that there is no such kind of a “signal” in the peptides of MK. Therefore, further research is needed to detect the possible “NLS” that leads MK to enter the nucleolus and to elucidate the precise translocation mechanism.
MK (Midkine, MK) is a 13 kDa protein originally found to be a secretory heparin-binding growth factor that is involved in cell growth, migration and survival. Subsequent studies have suggested that it may play important roles in carcinogenesis. MK has been shown to be localized in the nucleus or cytosol.
Owing to the multifunction of MK in carcinogenesis, cell growth, differentiation and apoptosis, the study of its functional mechanism might lead to a new approach for cancer therapy. Further studies on the functions of MK in carcinoma cells and its possible mechanisms are important for understanding the mechanism by which MK works.
In this study, we analyzed the subcellular localization of MK in different carcinoma cell lines by using MK-GFP fusion proteins as tracking molecules. The results showed that both MK with or without signal peptide were exclusively localized to the nucleus and accumulated in the nucleolus of all the three carcinoma cell lines, while the fluorescence of GFP control was detected all over the cell except the nucleolus.
The results provide valuable evidence for further study on the functions of MK in nucleus and its mechanisms, in which ribosomal RNA transcription and ribosome assembly are involved.
NLS: nuclear localization signal which helps extra-nuclear proteins bind with certain carriers to be transported through the nuclear pore complex and enter the nucleus.
In this manuscript by Dai et al., the authors report the subcellular localization of MK in different carcinoma cell lines. They employed an approach based on the visualization of a fluorescent fusion protein of EGFP with two different variants of MK, with or without signal peptide. The authors observed nuclear localization and nucleolar accumulation of the fusion proteins, regardless of the presence or absence of signal peptide. Their findings may be important for the study of the mechanism(s) of nuclear import of MK, and shed new light on the function of this protein.
S- Editor Wang J L- Editor Zhu LH E- Editor Bi L
| 1. | Koide N, Hada H, Shinji T, Ujike K, Hirasaki S, Yumoto Y, Hanafusa T, Kadomatsu K, Muramatsu H, Muramatsu T. Expression of the midkine gene in human hepatocellular carcinomas. Hepatogastroenterology. 1999;46:3189-3196. [PubMed] |
| 2. | Aridome K, Tsutsui J, Takao S, Kadomatsu K, Ozawa M, Aikou T, Muramatsu T. Increased midkine gene expression in human gastrointestinal cancers. Jpn J Cancer Res. 1995;86:655-661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 123] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Garver RI, Radford DM, Donis-Keller H, Wick MR, Milner PG. Midkine and pleiotrophin expression in normal and malignant breast tissue. Cancer. 1994;74:1584-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Ye C, Qi M, Fan QW, Ito K, Akiyama S, Kasai Y, Matsuyama M, Muramatsu T, Kadomatsu K. Expression of midkine in the early stage of carcinogenesis in human colorectal cancer. Br J Cancer. 1999;79:179-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 69] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | O'Brien T, Cranston D, Fuggle S, Bicknell R, Harris AL. The angiogenic factor midkine is expressed in bladder cancer, and overexpression correlates with a poor outcome in patients with invasive cancers. Cancer Res. 1996;56:2515-2518. [PubMed] |
| 6. | Muramatsu H, Shirahama H, Yonezawa S, Maruta H, Muramatsu T. Midkine, a retinoic acid-inducible growth/differentiation factor: immunochemical evidence for the function and distribution. Dev Biol. 1993;159:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 179] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Muramatsu H, Muramatsu T. Purification of recombinant midkine and examination of its biological activities: functional comparison of new heparin binding factors. Biochem Biophys Res Commun. 1991;177:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 157] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Kadomatsu K, Hagihara M, Akhter S, Fan QW, Muramatsu H, Muramatsu T. Midkine induces the transformation of NIH3T3 cells. Br J Cancer. 1997;75:354-359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Qi M, Ikematsu S, Ichihara-Tanaka K, Sakuma S, Muramatsu T, Kadomatsu K. Midkine rescues Wilms' tumor cells from cisplatin-induced apoptosis: regulation of Bcl-2 expression by Midkine. J Biochem. 2000;127:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Choudhuri R, Zhang HT, Donnini S, Ziche M, Bicknell R. An angiogenic role for the neurokines midkine and pleiotrophin in tumorigenesis. Cancer Res. 1997;57:1814-1819. [PubMed] |
| 11. | Rha SY, Noh SH, Kwak HJ, Wellstein A, Kim JH, Roh JK, Min JS, Kim BS, Chung HC. Comparison of biological phenotypes according to midkine expression in gastric cancer cells and their autocrine activities could be modulated by pentosan polysulfate. Cancer Lett. 1997;118:37-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Ikematsu S, Yano A, Aridome K, Kikuchi M, Kumai H, Nagano H, Okamoto K, Oda M, Sakuma S, Aikou T. Serum midkine levels are increased in patients with various types of carcinomas. Br J Cancer. 2000;83:701-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Rha SY, Noh SH, Kim TS, Yoo NC, Roh JK, Min JS, Kim BS. Modulation of biological phenotypes for tumor growth and metastasis by target-specific biological inhibitors in gastric cancer. Int J Mol Med. 1999;4:203-212. [PubMed] |
| 14. | Aridome K, Takao S, Kaname T, Kadomatsu K, Natsugoe S, Kijima F, Aikou T, Muramatsu T. Truncated midkine as a marker of diagnosis and detection of nodal metastases in gastrointestinal carcinomas. Br J Cancer. 1998;78:472-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Ono HA, Davydova JG, Adachi Y, Takayama K, Barker SD, Reynolds PN, Krasnykh VN, Kunisaki C, Shimada H, Curiel DT. Promoter-controlled infectivity-enhanced conditionally replicative adenoviral vectors for the treatment of gastric cancer. J Gastroenterol. 2005;40:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Salama RH, Muramatsu H, Zou K, Inui T, Kimura T, Muramatsu T. Midkine binds to 37-kDa laminin binding protein precursor, leading to nuclear transport of the complex. Exp Cell Res. 2001;270:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Shibata Y, Muramatsu T, Hirai M, Inui T, Kimura T, Saito H, McCormick LM, Bu G, Kadomatsu K. Nuclear targeting by the growth factor midkine. Mol Cell Biol. 2002;22:6788-6796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 109] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 18. | Suzuki N, Shibata Y, Urano T, Murohara T, Muramatsu T, Kadomatsu K. Proteasomal degradation of the nuclear targeting growth factor midkine. J Biol Chem. 2004;279:17785-17791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Callebaut C, Nisole S, Briand JP, Krust B, Hovanessian AG. Inhibition of HIV infection by the cytokine midkine. Virology. 2001;281:248-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Bellas RE, FitzGerald MJ, Fausto N, Sonenshein GE. Inhibition of NF-kappa B activity induces apoptosis in murine hepatocytes. Am J Pathol. 1997;151:891-896. [PubMed] |
| 21. | Heyninck K, Kreike MM, Beyaert R. Structure-function analysis of the A20-binding inhibitor of NF-kappa B activation, ABIN-1. FEBS Lett. 2003;536:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Nair A, Venkatraman M, Maliekal TT, Nair B, Karunagaran D. NF-kappaB is constitutively activated in high-grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003;22:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Lorang JM, Tuori RP, Martinez JP, Sawyer TL, Redman RS, Rollins JA, Wolpert TJ, Johnson KB, Rodriguez RJ, Dickman MB. Green fluorescent protein is lighting up fungal biology. Appl Environ Microbiol. 2001;67:1987-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 196] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Imamoto N. Diversity in nucleocytoplasmic transport pathways. Cell Struct Funct. 2000;25:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Jans DA, Xiao CY, Lam MH. Nuclear targeting signal recognition: a key control point in nuclear transport. Bioessays. 2000;22:532-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Jans DA, Hassan G. Nuclear targeting by growth factors, cytokines, and their receptors: a role in signaling. Bioessays. 1998;20:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |